Introduction
Lung cancer is the leading cause of cancer-related
death worldwide (1). In Taiwan,
~16,000 patients were diagnosed with lung cancer and 10,000
patients died in 2020. Advanced-stage cancer accounts for more than
half of lung cancer cases (2). Late
diagnosis leads to low 5-year survival rate (26.5% for lung cancer
in Taiwan in 2018) (3).
Driver mutations in non-small cell lung cancer
(NSCLC) have been found, revolutionizing cancer treatment.
Epidermal growth factor receptor gene (EGFR) mutation and
anaplastic lymphoma kinase gene (ALK) rearrangement are key driving
mutations in NSCLC (4,5). The distribution of activating EGFR
mutations in lung cancer varies by area and ethnicity (6). EGFR and ALK mutations are commonly
found in non- or light smokers, patients with adenocarcinoma and
Asian patients (7).
Tyrosine kinase inhibitors (TKIs) to block the EGFR
or ALK pathway yield lower toxicity, higher tumor response rate and
a longer progression-free survival (PFS) than chemotherapy
(8). Previous investigations have
found greater prevalence of brain metastasis in cases of lung
cancer with EGFR and ALK mutations than those without driver
mutation (9,10). As a result, substantial drug
penetration across the blood-brain barrier is required for the new
generation of TKI designs. In the FLAURA trial, PFS and overall
survival (OS) were significantly superior in patients receiving the
third-generation TKI osimertinib, than those receiving the
first-generation TKIs gefitinib and erlotinib (PFS, 18.9 vs. 10.2
months; OS, 38.6 vs. 31.8 months) (11,12).
In the ALEX trial, the alectinib group had greater PFS than the
crizotinib group (34.8 vs. 10.9 months) (13). For these reasons, the newer
generations of TKIs used in patients with EGFR mutation, such as
osimertinib, and those used in patients with ALK rearrangement
(alectinib, brigatinib and lorlatinib) have been recommended as
first-line therapy for advanced lung cancer by European Society for
Medical Oncology and National Comprehensive Cancer Network
guidelines (14,15).
The aforementioned clinical trials in cancer
treatment have primarily focused on therapeutic efficacy in the
case of individual gene alterations. However, less information has
been reported about the impact of different driver genes on cancer
survival (11–13). Few studies have addressed this issue
and they did not include newer TKIs, such as osimertinib or
lorlatinib (16,17). Therefore, the present retrospective
study aimed to investigate the clinical outcome and characteristics
of patients with advanced-stage NSCLC with different EGFR and ALK
mutation status.
Materials and methods
Study participants
In the present retrospective observational study,
the 628 patients (325 female and 303 male; age, >20 years old)
were diagnosed with advanced NSCLC at Changhua Christian Hospital,
a tertiary medical center of Changhua in Taiwan between August 2011
and January 2021. Clinical characteristics, treatment plans,
laboratory analyses, imaging reports, cancer driver mutation, and
survival status were compiled from electronic medical records. The
inclusion criteria were as follows: i) Histologically diagnosed
non-squamous NSCLC; ii) documented mutation status of EGFR and ALK;
iii) cancer stage IIIB to IVB according to the 8th edition American
Joint Committee on Cancer definition (18) and iv) receiving systemic therapy for
>30 days. The exclusion criteria were as follows: i) Patients
under the age of 20 years and ii) concomitant EGFR and ALK mutation
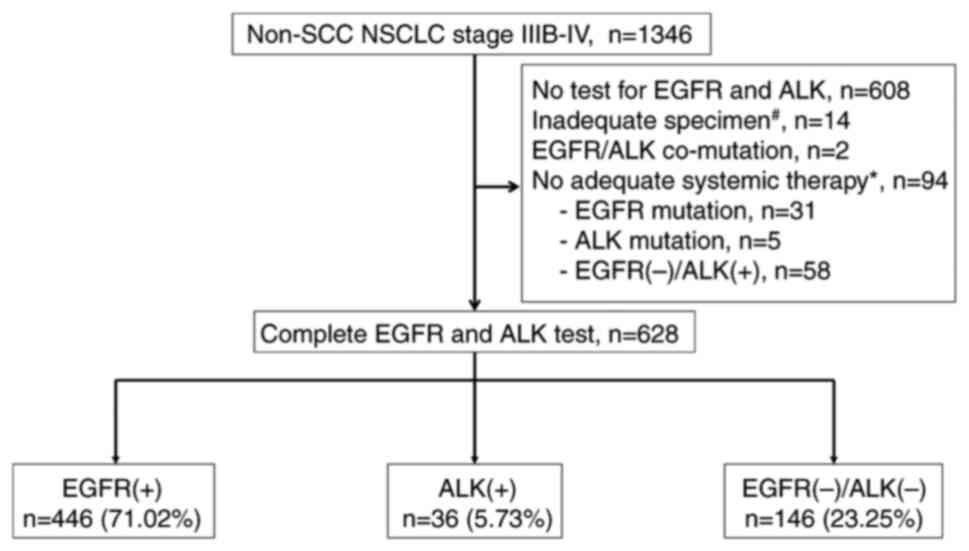
in the initial diagnosis. A total of 1,346 patients with
non-squamous NSCLC were included in the screening. Finally, 628
patients meeting the study criteria were divided into three groups
(EGFR, ALK and WT) according to status of EGFR mutation and ALK
rearrangement (Fig. 1). Patients
who did not have an EGFR mutation in the PCR or an ALK
rearrangement in the tissue immunohistochemistry (IHC) staining
were labeled as WT for the analysis.
EGFR and ALK analysis
Tissue specimens from tumor biopsy, surgical
resection or cell block of pleural effusion were routinely fixed
with 10% neutral buffered formalin for 24–48 h at room temperature
and then embedded in paraffin. Formalin-fixed paraffin-embedded
tissue (FFPET) was cut at 5 µm thickness for the quantitative PCR
of EGFR and at 4 µm thickness for the IHC stain of ALK.
For PCR analysis, genomic DNA was extracted from
FFPET by using a Cobas® DNA Sample Preparation kit for
DNA extraction (cat. no. 05985536190, Roche Diagnostics Operations
Inc.) (19). PCR amplification and
detection of TaqMan probe were performed in the region of EGFR
exons 18–21 by using an automated Cobas® EGFR Mutation
Test v2 (cat. no. 07248563190, Roche Diagnostics Operations Inc.)
and in vitro diagnostic software. Table SI lists the genes that could be
identified in the Cobas® platform. Positive EGFR
mutation was defined as the presence of sensitive mutations for
EGFR TKIs.
For ALK IHC, slides were stained immediately after
FFPET was cut, as antigenicity of cut tissue sections may diminish
over time. Solutions and kits of ALK IHC stain were all supplied by
Roche. For antigen retrieval, Cell Conditioning 1 (cat. no.
950-124; Roche Diagnostics Operations Inc.) at 100°C for 92 min was
applied (20). Reaction Buffer
Concentrate (10X; cat. no. 950-300; Roche Diagnostics Operations
Inc.) was used for washing and rehydration and blocking at 37°C for
5 min. Quenching step was performed using OV PEROX IHBTR (3%
hydrogen peroxide solution) for 4 min. Anti-ALK D5F3 rabbit
monoclonal primary antibody (cat. no. 1011879; Roche Diagnostics
Operations Inc.) was incubated at 37°C for 16 min. OV HQ UNIV LINKR
secondary antibody was incubated for 12 min. OV DAB (cat. no.
760-700; Roche Molecular Systems, Inc.) was used for chromogen
detection and hematoxylin II (cat. no. 790-2208; Roche Diagnostics
Operations Inc.) was used as counterstain for 4 min. The whole
stain procedure was automated by the Roche Ventana BenchMark ULTRA
platform with Ventana system software 12.3 (Roche Diagnostics
Operations Inc.) according to the manufacturer's instructions.
Finally, light microscope was used to visualize ALK IHC stain.
Staining elements on normal pulmonary tissue and inflammatory cells
were excluded from analysis. Positive detection of ALK was defined
as strong granular cytoplasmic staining in tumor cells.
Statistical analysis
Categorical data are presented as a number and
percentage; continuous variables are presented as the mean ±
standard deviation. Continuous variables within groups were
compared using one-way ANOVA followed by post hoc test of Scheffé.
Categorical data were compared using Pearson's χ2 test.
Prognostic factors for lung cancer survival were identified using
Cox proportional regression model with backward elimination. The
Kaplan-Meier curve was used to estimate the survival rate over time
and differences were compared by log-rank test. Data were analyzed
using IBM SPSS Statistics for Windows (Version 26; IBM Corp.).
Survival curves were plotted with RStudio (2022.12.0+353,
posit.co/products/open-source/rstudio/). P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient characteristics
The study population consisted of 628 patients, with
a mean age of 66.55±12.56 years and 52% female. EGFR mutation was
found in 446 (71.02%) patients, ALK rearrangement in 36 (5.73%) and
WT in the remaining 146 (23.25%) patients. A total of 548 (87.3%)
patients had died. The mean follow-up time was 26.50±22.24
months.
Most patients in EGFR and ALK groups were female
(EGFR, 56.28; ALK, 63.89; WT, 34.93%), never smoked (EGFR, 83.33;
ALK, 88.57; WT, 71.23%) or had lower smoking pack-years (EGFR,
11.06±25.76; ALK, 5.34±18.38; WT, 27±38.01) and had less chronic
obstructive pulmonary disease comorbidity (EGFR, 27.13; ALK, 13.89;
WT, 38.36%; Table I). Compared with
ALK and WT groups, patients in EGFR group were older and possessed
a higher proportion of stage IVB (EGFR, 56.28; ALK, 50.00; WT,
47.26%).
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Characteristic | EGFR (n=446) | ALK (n=36) | Wild-type
(n=146) | P-value |
|---|
| Age, years | 67.64±12.45 | 61.97±13.84 | 64.36±12.10 | 0.020 |
| Sex (%) |
|
|
| <0.001 |
|
Female | 251 (56.28) | 23 (63.89) | 51 (34.93) |
|
|
Male | 195 (43.72) | 13 (36.11) | 95 (65.07) |
|
| BMI, kg/m | 23.24±3.58 | 22.88±3.07 | 23.17±3.97 | 0.845 |
| Smoking status
(%) |
|
|
| 0.004 |
|
Never | 370 (83.33) | 31 (88.57) | 104 (71.23) |
|
|
Former | 11 (2.48) | 0 (0.00) | 2 (1.37) |
|
|
Current | 63 (14.19) | 4 (11.43) | 40 (27.4) |
|
| Smoking
pack-years | 11.06±25.76 | 5.34±18.38 | 27±38.01 | <0.001 |
| ECOG (%) |
|
|
| 0.440 |
|
0-1 | 353 (84.86) | 32 (88.89) | 127 (88.81) |
|
| ≥2 | 63 (15.14) | 4 (11.11) | 16 (11.19) |
|
| Hypertension
(%) | 31 (6.95) | 1 (2.78) | 9 (6.16) | 0.609 |
| Arrythmia (%) | 53 (11.88) | 4 (11.11) | 9 (6.16) | 0.147 |
| DM (%) | 124 (27.80) | 12 (33.33) | 38 (26.03) | 0.678 |
| Hyperlipidemia
(%) | 93 (20.85) | 9 (25.00) | 34 (23.29) | 0.727 |
| COPD (%) | 121 (27.13) | 5 (13.89) | 56 (38.36) | 0.004 |
| CKD (%) | 78 (17.49) | 7 (19.44) | 31 (21.23) | 0.592 |
| Pathology (%) |
|
|
| 0.370 |
|
Adenocarcinoma | 434 (97.31) | 33 (91.67) | 140 (95.89) |
|
|
Adenosquamous carcinoma | 6 (1.35) | 1 (2.78) | 3 (2.05) |
|
|
Carcinoma, NOS | 6 (1.35) | 2 (5.56) | 3 (2.05) |
|
| Tumor diameter,
cm | 4.57±2.07 | 4.24±2.01 | 4.54±2.25 | 0.693 |
| Stage (%) |
|
|
| <0.001 |
|
IIIB | 17 (3.81) | 3 (8.33) | 15 (10.27) |
|
|
IIC | 8 (1.79) | 4 (11.11) | 9 (6.16) |
|
|
IVA | 170 (38.12) | 11 (30.56) | 53 (36.3) |
|
|
IVB | 251 (56.28) | 18 (50.00) | 69 (47.26) |
|
Distant metastasis of NSCLC with
different genetic mutations
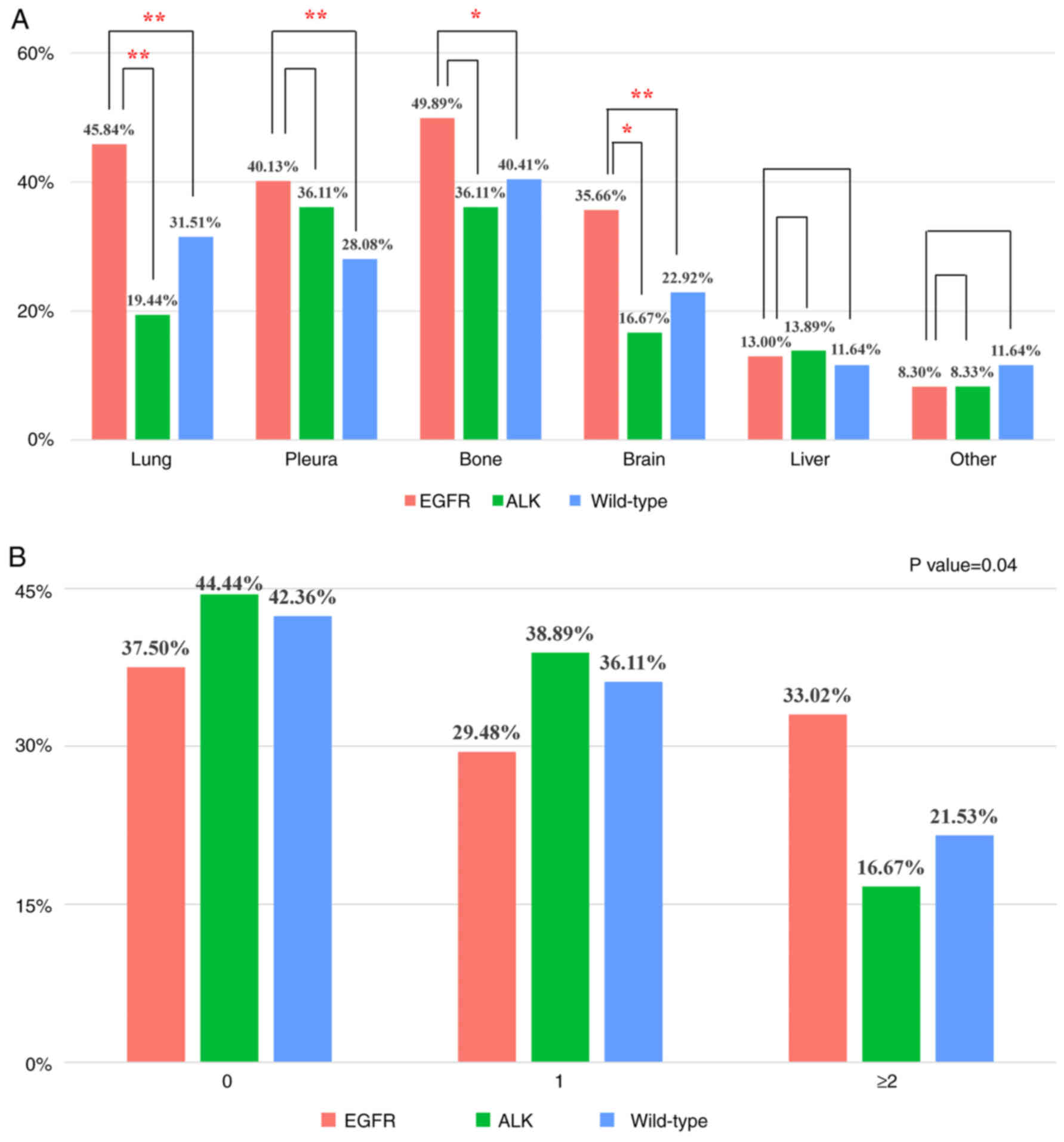
EGFR group more commonly developed metastases in the
lung (EGFR, 45.84; WT, 31.51%), pleura (EGFR, 40.13; WT, 28.08%),
bone (EGFR, 49.89; WT, 40.41%) and brain (EGFR, 35.66; WT, 22.92%)
than WT group (Fig. 2A). Compared
with the ALK group, EGFR group more commonly exhibited lung (EGFR,
45.84; ALK, 19.44%) and brain (EGFR, 35.66; ALK, 16.67%)
metastases. Patients with EGFR mutation were more likely to have
multiple extrapulmonary organ metastases than those with ALK
rearrangement and WT group (EGFR, 33.02; ALK, 16.67; WT, 21.53%;
Fig. 2B).
Treatment of patients with advanced
lung cancer
TKIs were the predominant treatments for the EGFR
and ALK groups, whereas chemotherapy was used for patients in the
WT group (Table II). EGFR group
had the highest proportion of patients receiving geftinib as
first-line therapy and the ALK group had the highest proportion
receiving treatment with crizotinib. Some patients with driver
mutations did not receive chemotherapy (EGFR, 45.29%; ALK, 33.33%;
Table II). In the WT group, 41.78%
of patients did not receive second-line chemotherapy when disease
progressed, due to potential side effects or deteriorating
health.
 | Table II.Systemic treatment of patients with
advanced non-small cell lung cancer. |
Table II.
Systemic treatment of patients with
advanced non-small cell lung cancer.
| Treatment (%) | EGFR (n=446) | ALK (n=36) | Wild-type
(n=146) | P-value |
|---|
| Chemotherapy | 244 (54.71) | 24 (66.67) | 141 (96.58) |
<0.001a |
| Line of
chemotherapy |
|
|
|
<0.001a |
| 1 | 119 (26.68) | 12 (33.33) | 61 (41.78) |
|
| 2 | 66 (14.80) | 5 (13.89) | 41 (28.08) |
|
| ≥3 | 59 (13.23) | 7 (19.44) | 39 (26.71) |
|
| EGFR TKI | 444 (99.55) | 9 (25.00) | 60 (41.10) |
<0.001a |
|
Geftinib | 270 (60.54) | 5 (13.89) | 23 (15.75) |
<0.001a |
|
Erlotnib | 179 (40.13) | 4 (11.11) | 44 (30.14) | 0.001b |
|
Afatinib | 97 (21.75) | 0 (0.00) | 2 (1.37) |
<0.001a |
|
Osimertinib | 39 (8.74) | 0 (0.00) | 3 (2.05) | 0.005b |
| ALK TKI | 0 (0.00) | 33 (91.67) | 6 (4.11) |
<0.001a |
|
Crizotinib | 0 (0.00) | 25 (69.44) | 6 (4.11) |
<0.001a |
|
Ceritinib | 0 (0.00) | 10 (27.78) | 0 (0.00) |
<0.001a |
|
Alectinib | 0 (0.00) | 14 (38.89) | 1 (0.68) |
<0.001a |
|
Brigatinib | 0 (0.00) | 3 (8.33) | 0 (0.00) |
<0.001a |
|
Lorlatinib | 0 (0.00) | 4 (11.11) | 1 (0.68) |
<0.001a |
Survival analysis
In the Cox-regression analysis with backward
elimination, driver mutations of EGFR and ALK, lower smoking
pack-years, younger age, better performance status (Eastern
Cooperative Oncology Group score, 0–1) (21), no pleural metastasis and fewer
extrapulmonary metastases led to significantly better OS (Table III). The hazard ratio (HR) for ALK
rearrangement (0.545, 95% CI 0.325–0.913) was lower than for EGFR
mutation (0.764, 95% CI 0.593–0.985) compared with WT. ALK group
had a significantly longer OS time than the WT group (HR, 0.487,
95% CI 0.301–0.787). No significant difference was observed between
the EGFR and WT groups (HR, 0.91, 95% CI 0.728–1.138). This was
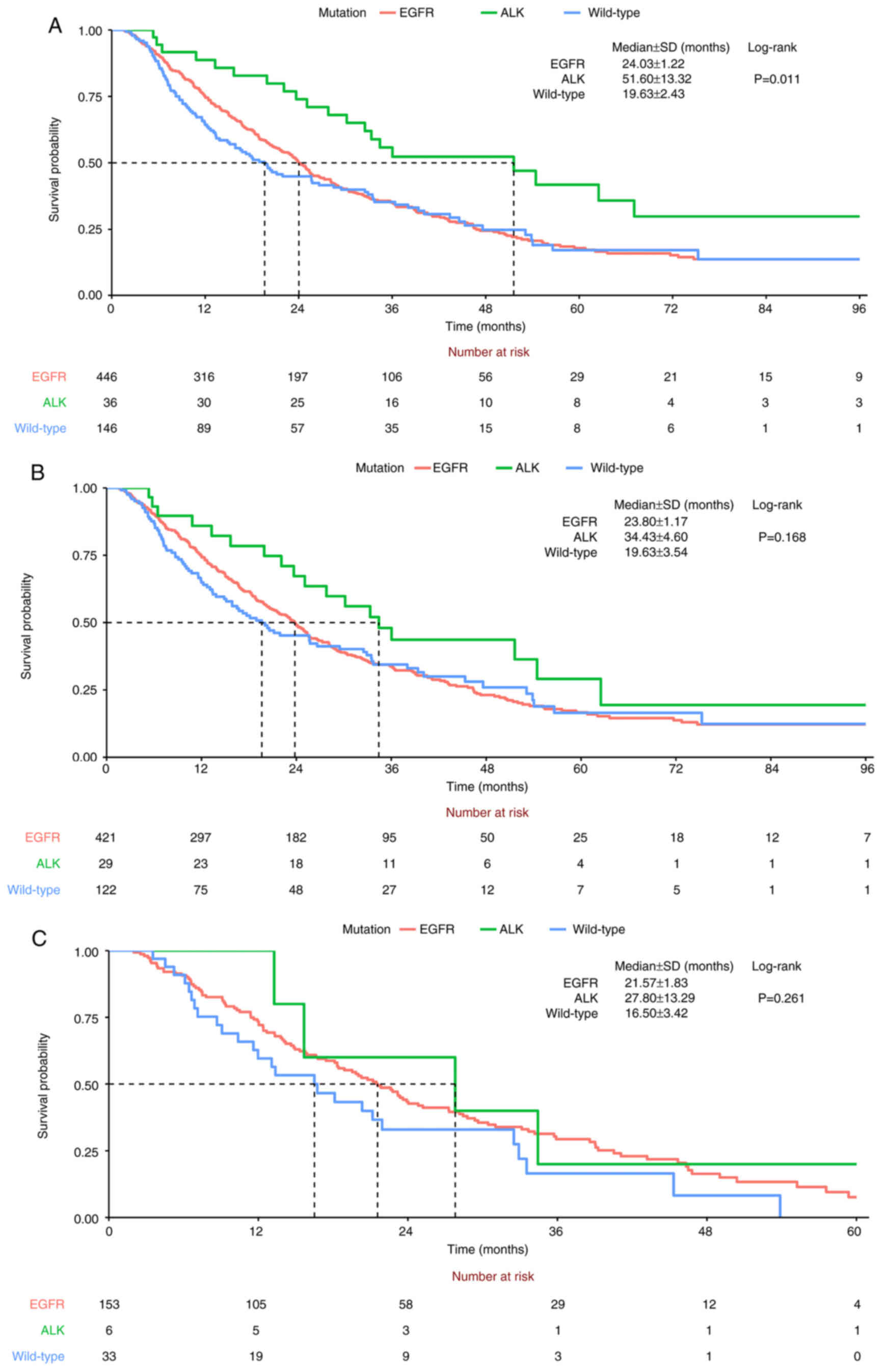
consistent with Kaplan-Meier survival analysis (Fig. 3A). Patients with ALK rearrangement
had the longest median OS, followed by those with EGFR mutation,
then WT group (ALK, 51.6±13.32; EGFR, 24.03±1.22; WT, 19.63±2.43
months). 19 deletion (19-Del) and L858R were key mutations in the
EGFR driver gene (Fig. S1A). The
19-Del and L858R EGFR mutations were included in the survival
analysis (Fig. S1B). ALK group
still had the longest median survival time, followed by 19-Del and
L858R EGFR mutations. The prognosis was worst for the WT group. The
order of median survival time in the ALK, EGFR and WT groups was
not changed in the subgroup analysis for stage IV NSCLC (ALK,
34.43±4.60; EGFR, 23.80±1.17; WT, 19.63±3.54 months; Fig. 3B) and brain metastasis (ALK,
27.80±13.29; EGFR, 21.57±1.83; WT, 16.50±3.42 months; Fig. 3C) but the difference didn't reach
the statistical significance.
 | Table III.Survival analysis by multivariate
Cox-regression model with backward elimination. |
Table III.
Survival analysis by multivariate
Cox-regression model with backward elimination.
|
| Multivariate
analysis | Multivariate
analysis with backward elimination |
|---|
|
|
|
|
|---|
| Characteristic | aHR (95% CI) | P-value | aHR (95% CI) | P-value |
|---|
| Group |
|
|
|
|
|
Wild-type | 1.000 |
| 1.000 |
|
|
EGFR | 0.910
(0.728–1.138) | 0.409 | 0.764
(0.593–0.985) | 0.038a |
|
ALK | 0.487
(0.301–0.787) | 0.003a | 0.545
(0.325–0.913) | 0.021a |
| Smoking | 1.022
(0.989–1.056) | 0.198 | 1.004
(1–1.007) | 0.044a |
| Age | 1.019
(1.011–1.027) |
<0.001b | 1.016
(1.007–1.026) | 0.001a |
| BMI | 0.973
(0.946–0.100) | 0.046a | |
|
| ECOG |
|
|
|
|
|
0-1 | 1.000 |
| 1.000 |
|
|
2-4 | 2.032
(1.571–2.629) |
<0.001b | 1.795
(1.328–2.427) |
<0.001b |
| Arrythmia | 1.598
(1.200–2.126) | 0.001a | |
|
| CKD | 1.400
(1.154–1.699) | 0.001a |
|
|
| Tumor size | 1.060
(1.015–1.108) | 0.009a |
|
|
| Lung
metastasis | 1.121
(0.927–1.357) | 0.239 |
|
|
| Pleural
metastasis | 1.146
(0.943–1.391) | 0.17 | 1.260
(1.006–1.578) | 0.045a |
| EPMS |
|
|
|
|
| 0 | 1.000 |
| 1.000 |
|
| 1 | 1.397
(1.107–1.765) | 0.005 a | 1.358
(1.045–1.763) | 0.022a |
| ≥2 | 1.880
(1.481–2.386) |
<0.001b | 1.840
(1.4–2.42) |
<0.001b |
Discussion
The present study retrospectively analyzed patients
with non-squamous NSCLC in the advanced stage. Patients with ALK
rearrangement had a better clinical outcome than those with EGFR
mutation and WT. Those with ALK rearrangement exhibited
significantly longer OS than those with EGFR mutation (51.6±13.32
vs. 24.03±1.22 months). Among the patients with brain metastasis, a
longer median OS was observed in ALK than in EGFR group. Driver
mutations in EGFR and ALK, lower smoking pack-years, younger age,
better performance status (ECOG 0–1), no pleural metastasis and
fewer extrapulmonary metastasis were key prognostic factors. To the
best of our knowledge, the present study is the largest to assess
real-world outcomes in patients with advanced NSCLC according to
driver mutation status.
High prevalence of EGFR and ALK mutations (71.02 and
5.73%, respectively) were observed. In previous studies, both
mutations were frequently detected in never or light smokers,
patients with adenocarcinoma and Asian patients (6,7).
Graham et al (6) found
incidence of EGFR mutations in NSCLC varies by area. In Asia, India
and South Korea have the lowest EGFR mutation rates (both 29.1%).
The highest EGFR mutation rate (54.9%) is in Taiwan (Fig. S2). Here, all of the enrolled
patients were Taiwanese. In the initial screening, patients with
squamous cell carcinoma were excluded. More WT patients who did not
receive appropriate treatment were excluded compared with the EGFR
and ALK groups. These reasons could explain the high EGFR mutation
rate.
More lung and pleural metastases were observed in
the EGFR than in the WT group. No significant difference was
observed between the ALK and WT groups. Patients with EGFR mutation
more commonly presented with lung metastasis than those with the
ALK rearrangement. Previous studies reported the same metastatic
distribution according to mutation status (22,23).
EGFR-mutated NSCLC was associated with an increase in lung
metastases. Mendoza et al (23) found that there was a lower frequency
of lung metastasis for ALK-rearranged NSCLC than EGFR mutation and
WT. Besides, our study found that EGFR group more commonly had
brain metastasis than ALK rearrangement and WT in the initial
diagnosis. It deserved our attention before planning cancer
therapy.
Bone, brain, liver and adrenal gland are well-known
as common extrapulmonary metastatic sites (EPMSs) (23,24). A
total of 40% of patients with NSCLC with EPMS have ≥2
extrapulmonary metastases in the initial diagnosis (24). Here, bone and brain metastases were
the most common. Up to 48% of patients with EPMS had ≥2 distant
metastatic sites. EGFR group exhibited more extrapulmonary
metastases than the ALK and WT groups. These observations indicated
metastatic sites in lung cancer are affected by the type of driver
mutation.
Lynch et al (25) found that NSCLC responsiveness to TKI
is mainly related to specific mutations in the EGFR gene, rather
than EGFR overexpression. Molecular therapy has been extensively
used in patients with lung cancer and driver mutations (14,15).
More potent next-generation TKIs have been successively developed
(26,27). FLAURA trial demonstrated superior
PFS and OS in patients with EGFR-mutated NSCLC receiving the
third-generation TKI osimertinib compared with those receiving the
first-generation TKIs gefitinib and erlotinib (PFS, 18.9 vs. 10.2;
OS, 38.6 vs. 31.8 months, respectively) (11,12).
For ALK-rearranged NSCLC, a longer PFS was observed in treatment
with second- than first-generation TKI (brigatinib vs. crizotinib,
24 vs. 11.1 months in ALTA-1L trial; alectinib vs. crizotinib, 34.8
vs. 10.4 months in ALEX trial) (13,28).
The ALEX trial also demonstrated superior OS following first-line
treatment with alectinib than crizotinib (5-year OS, 62.5 with
alectinib and 45.5% with crizotinib) (13). For the more potent third-generation
ALK TKI lorlatinib, the PFS curve did not reach the 50% line after
36.7 month observation in the Crown study (29).
According to Kaplan-Meier survival analysis,
ALK-rearranged advanced NSCLC exhibited a better OS outcome than
EGFR and WT. Patients without EGFR or ALK driver mutation had
poorer median OS. The superior survival outcome in the ALK group
was observed in the subgroup analysis of patients with stage IV
NSCLC or brain metastasis compared with the EGFR group. From the
indirect comparison of previous clinical trials, we observed
significantly longer PFS and OS in ALK-rearranged NSCLC with the
second- or third generation ALK TKI therapy than in EGFR-mutated
NSCLC with the most potent EGFR TKI of osimertinib (11–13,28,29).
In addition, Camidge et al (30) found that ALK-positive patients have
notably extended PFS following pemetrexed chemotherapy in
comparison with patients without a driver mutation. However,
patients with EGFR mutation do not exhibit the same therapeutic
efficacy to chemotherapy as patients with ALK rearrangement. These
results may explain why ALK-rearranged NSCLC has more diverse and
favorable clinical outcomes than EGFR-mutated NSCLC.
For EGFR and WT groups, survival rates initially
differed notably but converged after 24 months. This may be because
patients with the EGFR mutation received TKI as the primary
therapy, whereas patients without a driver mutation received
chemotherapy. The initial difference in survival rates might be
caused by a highly effective EGFR TKI. Secondly, the EGFR group had
a larger number of EPMSs than the WT group. The larger EPMS burden,
the worse the prognosis (24).
Larger EPMS burden of EGFR group may explain why the survival
curves of the EGFR and WT groups coincided.
Driver mutations with EGFR and ALK, lower smoking
pack-years, younger age, better performance status, no pleural
metastasis and fewer extrapulmonary metastases were key prognostic
factors for survival. Alexander's lung cancer prognostic index
assesses key prognostic factors, including stage, histology,
mutation status, performance status, weight loss, smoking history,
respiratory comorbidity, sex and age (31). In addition, Wu et al found
that patients with malignant pleural effusions (MPEs) at the
initial diagnosis have worse OS than those who develop MPEs after
disease progression (32). The
aforementioned studies corroborate the present findings.
The present retrospective study had limitations.
Firstly, some data about smoking pack-year and performance status
were missing. Secondly, the sample size for ALK rearrangement in a
single medical center was relatively small. In addition, EGFR PCR
and ALK IHC were used to analyze tumor driver genes rather than
next generation sequencing (NGS). Owing to the high cost of
testing, few patients underwent NGS analysis. Therefore, NGS
results were not analyzed. Certain co-mutations, such TP-53, RB-1
and PIK3CA, might have an impact on patient survival (33). Finally, most of patients received
lung cancer treatment with reimbursement of Taiwan National Health
Insurance (NHI). Under Taiwan NHI regulations, patients with EGFR
and ALK gene mutations are not eligible for immune checkpoint
inhibitors as subsequent treatment (34). Osimertinib is only allowed as the
first line therapy for patients with lung cancer with 19-del EGFR
mutation and brain metastasis. To recruit more patients with ALK
mutations, the trial enrollment period was 10 years. Therefore,
most patients did not receive treatment with current standard of
care TKIs.
In conclusion, clinical outcomes are driven by
different driver mutations. EGFR mutation leads to more
extrapulmonary metastases. Driver mutation, lower smoking burden,
younger age, better performance status, no pleural metastasis and
fewer extrapulmonary metastases are key prognostic factors for
patient outcomes. Superior median OS was observed in patients with
ALK rearrangement than with EGFR-mutated NSCLC regardless of brain
metastasis.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the study may be requested
from the corresponding author.
Authors' contributions
CWL, MHH and SHL conceived the study and edited the
manuscript. CWL analyzed data and wrote the manuscript. CWL and SHL
confirm the authenticity of all the raw data. CWL, KYH, CHL and SHL
performed experiments. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Changhua Christian Hospital, Changhua, Taiwan
(approval no. 200323) and all procedures were carried out in
accordance with the 1996 Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALK
|
anaplastic lymphoma kinase
|
|
EGFR
|
epidermal growth factor receptor
|
|
HR
|
hazard ratio
|
|
IHC
|
immunohistochemistry
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
TKI
|
tyrosine kinase inhibitor
|
|
WT
|
wild-type
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ministry of Health and Welfare, . Cancer
Registry Annual Report, 2020 Taiwan. https://www.hpa.gov.tw/File/Attach/16434/File_21196.pdfMay
12–2024
|
|
3
|
Luo YH, Chiu CH, Scott Kuo CH, Chou TY,
Yeh YC, Hsu HS, Yen SH, Wu YH, Yang JC, Liao BC, et al: Lung cancer
in Republic of China. J Thorac Oncol. 16:519–527. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsao AS, Scagliotti GV, Bunn PA Jr,
Carbone DP, Warren GW, Bai C, de Koning HJ, Yousaf-Khan AU,
McWilliams A, Tsao MS, et al: Scientific advances in lung cancer
2015. J Thorac Oncol. 11:613–638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu KH, Ho CC, Hsia TC, Tseng JS, Su KY,
Wu MF, Chiu KL, Yang TY, Chen KC, Ooi H, et al: Identification of
five driver gene mutations in patients with treatment-naïve lung
adenocarcinoma in Taiwan. PLoS One. 10:e01208522015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Graham RP, Treece AL, Lindeman NI, Vasalos
P, Shan M, Jennings LJ and Rimm DL: Worldwide frequency of commonly
detected EGFR mutations. Arch Pathol Lab Med. 142:163–167. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaw AT, Yeap BY, Mino-Kenudson M,
Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S,
McDermott U, et al: Clinical features and outcome of patients with
non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol.
27:4247–4253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schneider JL, Lin JJ and Shaw AT:
ALK-positive lung cancer: A moving target. Nat Cancer. 4:330–343.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin DY, Na II, Kim CH, Park S, Baek H and
Yang SH: EGFR mutation and brain metastasis in pulmonary
adenocarcinomas. J Thorac Oncol. 9:195–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang P, Kulig K, Boland JM,
Erickson-Johnson MR, Oliveira AM, Wampfler J, Jatoi A, Deschamps C,
Marks R, Fortner C, et al: Worse disease-free survival in
never-smokers with ALK+ lung adenocarcinoma. J Thorac Oncol.
7:90–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mok T, Camidge DR, Gadgeel SM, Rosell R,
Dziadziuszko R, Kim DW, Pérol M, Ou SI, Ahn JS, Shaw AT, et al:
Updated overall survival and final progression-free survival data
for patients with treatment-naive advanced ALK-positive
non-small-cell lung cancer in the ALEX study. Ann Oncol.
31:1056–1064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hendriks LE, Kerr KM, Menis J, Mok TS,
Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, et
al: Oncogene-addicted metastatic non-small-cell lung cancer: ESMO
clinical practice guideline for diagnosis, treatment and follow-up.
Ann Oncol. 34:339–357. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riley GJ, Wood DE, Ettinger DS, Aisner DL,
Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, et
al: Non–Small Cell Lung Cancer, Version 4.2024, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw.
22:249–274. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HR, Shim HS, Chung JH, Lee YJ, Hong
YK, Rha SY, Kim SH, Ha SJ, Kim SK, Chung KY, et al: Distinct
clinical features and outcomes in never-smokers with nonsmall cell
lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement.
Cancer. 118:729–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin JJ, Cardarella S, Lydon CA, Dahlberg
SE, Jackman DM, änne PA and Johnson BE: Five-year survival in
EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs.
J Thorac Oncol. 11:556–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
cobas® EGFR Mutation Test v2
for in vitro diagnostic use. https://elabdoc-prod.roche.com/eLD/api/downloads/71bf89fb-e86d-ee11-2091-005056a71a5d?countryIsoCode=XGOctober
7–2024
|
|
20
|
Roche eLabDoc, . VENTANA®
anti-ALK (D5F3) Rabbit Monoclonal Primary Antibody ROW for in vitro
diagnostic use. https://elabdoc-prod.roche.com/eLD/api/downloads/0d3a124b-d03d-ed11-1791-005056a71a5d?countryIsoCode=XGOctober
7–2024
|
|
21
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu F, Nichol A, Toriumi T and De Caluwe
A: Miliary metastases are associated with epidermal growth factor
receptor mutations in non-small cell lung cancer: A
population-based study. Acta Oncol. 56:1175–1180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mendoza DP, Lin JJ, Rooney MM, Chen T,
Sequist LV, Shaw AT and Digumarthy SR: Imaging features and
metastatic patterns of advanced ALK-rearranged non-small cell lung
cancer. AJR Am J Roentgenol. 214:766–774. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gibson AJW, Li H, D'Silva A, Tudor RA,
Elegbede AA, Otsuka SM, Bebb DG and Cheung WY: Impact of number
versus location of metastases on survival in stage IV M1b non-small
cell lung cancer. Med Oncol. 35:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Corvaja C, Passaro A, Attili I, Aliaga PT,
Spitaleri G, Signore ED and de Marinis F: Advancements in
fourth-generation EGFR TKIs in EGFR-mutant NSCLC: Bridging
biological insights and therapeutic development. Cancer Treat Rev.
130:1028242024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ou SI, Nagasaka M, Brazel D, Hou Y and Zhu
VW: Will the clinical development of 4th-generation ‘double mutant
active’ ALK TKIs (TPX-0131 and NVL-655) change the future treatment
paradigm of ALK+ NSCLC? Transl Oncol. 14:1011912021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Camidge DR, Kim HR, Ahn MJ, Yang JC, Han
JY, Lee JS, Hochmair MJ, Li JYC, Chang GC, Lee KH, et al:
Brigatinib versus crizotinib in ALK-positive non-small-cell lung
cancer. N Engl J Med. 379:2027–2039. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Solomon BJ, Bauer TM, Mok TSK, Liu G,
Mazieres J, de Marinis F, Goto Y, Kim DW, Wu YL, Jassem J, et al:
Efficacy and safety of first-line lorlatinib versus crizotinib in
patients with advanced, ALK-positive non-small-cell lung cancer:
Updated analysis of data from the phase 3, randomised, open-label
CROWN study. Lancet Respir Med. 11:354–366. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Camidge DR, Kono SA, Lu X, Okuyama S,
Barón AE, Oton AB, Davies AM, Varella-Garcia M, Franklin W and
Doebele RC: Anaplastic lymphoma kinase gene rearrangements in
non-small cell lung cancer are associated with prolonged
progression-free survival on pemetrexed. J Thorac Oncol. 6:774–780.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alexander M, Wolfe R, Ball D, Conron M,
Stirling RG, Solomon B, MacManus M, Officer A, Karnam S, Burbury K
and Evans SM: Lung cancer prognostic index: A risk score to predict
overall survival after the diagnosis of non-small-cell lung cancer.
Br J Cancer. 117:744–751. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu SG, Yu CJ, Tsai MF, Liao WY, Yang CH,
Jan IS, Yang PC and Shih JY: Survival of lung adenocarcinoma
patients with malignant pleural effusion. Eur Respir J.
41:1409–1418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pezzuto F, Hofman V, Bontoux C, Fortarezza
F, Lunardi F, Calabrese F and Hofman P: The significance of
co-mutations in EGFR-mutated non-small cell lung cancer: Optimizing
the efficacy of targeted therapies? Lung Cancer. 181:1072492023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
National Health Insurance Administration
and Ministry of Health and Welfare, . History of National Health
Insurance Drug Benefit Regulations. https://www.nhi.gov.tw/ch/cp-2192-9951a-2509-1.htmlSeptember
10–2024
|