Introduction
Pancreatic cancer, characterized by its low survival
rate and late diagnosis, poses a significant challenge to oncology.
Despite the advancements in treatment, the global incidence of
pancreatic cancer continues to increase, with an annual incidence
rate of approximately 5 per 100,000 (1). However, the five-year survival rate
remains only 10%, underscoring the urgent need for innovative
diagnostic and prognostic tools to improve patient outcomes
(1,2). A notable area of research has been the
exploration of the interplay between diabetes mellitus and
pancreatic cancer. Studies have documented an increased risk of
pancreatic cancer in individuals with a long-standing history of
diabetes, suggesting a potential bidirectional relationship between
the two conditions (3,4). In certain cases, diabetes may precede
cancer development by several years, acting as an early risk or
predisposing factor. Conversely, new-onset diabetes or rapidly
worsening hyperglycemia can also occur as a paraneoplastic
phenomenon resulting from the presence of pancreatic tumors
(5,6).
The metabolic relationship between pancreatic cancer
and diabetes is complex. Pancreatic cancer can impair insulin
secretion owing to islet cell dysfunction or destroy
insulin-producing β cells, leading to hyperglycemia. Furthermore,
tumors may secrete inflammatory cytokines and tumor-derived factors
that interfere with insulin action, exacerbating insulin resistance
and increasing blood glucose levels (7). This hyperglycemic state often develops
independent of prior diabetes and can serve as an early clinical
sign of malignancy in a number of patients (8).
Preoperative glycemic control is particularly
important, as hyperglycemia is associated with poor surgical
outcomes, including increased infection rates, delayed wound
healing and longer hospital stays (9). While blood glucose levels in patients
with preexisting diabetes are often monitored during treatment, the
immediate preoperative blood glucose status provides a more
accurate reflection of metabolic homeostasis at the time of surgery
(10,11). Importantly, glycemic variability
among patients with pancreatic cancer is influenced not only by
pre-existing diabetes but also by tumor-induced metabolic
disruptions, which may complicate clinical management (9). Despite the recognized importance of
perioperative glycemic control, particularly in reducing surgical
complications and improving patient outcomes, the specific impact
of preoperative blood glucose levels on the survival of patients
with pancreatic cancer remains insufficiently explored. This gap
presents a critical avenue for research with the potential to
inform targeted interventions aimed at optimizing preoperative
glycemic control (7,9).
The present study investigated the prognostic
significance of preoperative blood glucose levels in patients
undergoing pancreatic cancer surgery. By delineating the
relationship between preoperative glycemic status and postoperative
outcomes, the present study aimed to highlight a potentially
modifiable factor in the management of pancreatic cancer.
Patients and methods
Study design and patient
selection
The retrospective cohort included 225 patients with
histologically confirmed pancreatic cancer who were treated at The
Fourth Hospital of Hebei Medical University (Shijiazhuang, China)
between January 2015 and December 2020. Ethical approval was
obtained from the Institutional Review Board of the Fourth Hospital
of Hebei Medical University (approval no. 2023KS182), and the
requirement for informed consent was waived due to the
retrospective nature of the present study. Patients were eligible
if they were aged ≥18 years and had complete preoperative clinical
data, including fasting blood glucose levels. The exclusion
criteria were as follows: i) Patients with other severe primary
diseases such as respiratory, cardiovascular, cerebrovascular,
liver, kidney or hematopoietic diseases; ii) those who died during
the perioperative period; and iii) those with incomplete clinical
data. The present study was conducted in accordance with the
Declaration of Helsinki (revised in 2013).
Diagnostic criteria
Pancreatic cancer was diagnosed based on the Chinese
Guidelines for Diagnosis and Treatment of Pancreatic Cancer (2020
Edition) (12) using enhanced
computed tomography and/or magnetic resonance imaging. The
pathological diagnosis followed the 5th Edition of the World Health
Organization Classification of Tumors of the Digestive System
(13), which includes ductal
adenocarcinoma and its variants.
Grouping and treatments
The preoperative blood glucose level was defined as
the fasting blood glucose level measured from venous blood samples
collected in the morning before food intake. Patients were
categorized into two groups based on blood glucose levels: Normal
blood glucose (≤6.11 mmol/l) and high blood glucose (>6.11
mmol/l). Additionally, the patients were grouped based on diabetic
status into those with and without diabetes. Treatment regimens,
including surgical resection and adjuvant chemotherapy, followed
the current clinical guidelines. All patients underwent
perioperative management according to the Chinese Guidelines for
Perioperative Management of Pancreatic Cancer (12).
Data collection and outcomes
Demographic characteristics, clinical history, tumor
characteristics, treatment details and laboratory results were
collected. The primary outcome was overall survival (OS), which was
defined as the time from diagnosis to death from pancreatic cancer.
Secondary outcomes included postoperative complications requiring
clinical intervention such as infection, bleeding (arterial or
gastrointestinal), wound disruption and pancreatic fistula, all of
which were documented and analyzed.
Follow-up
Patients were followed up regularly through
outpatient visits, telephone interviews and medical record reviews.
The follow-up period was conducted until December 2023. The
survival status was documented during each follow-up period.
Statistical analysis
Data analysis was performed using SPSS software
(version 26.0; IBM Corp.). Continuous variables are presented as
the mean ± standard deviation or median with interquartile range,
and compared using unpaired Student's t-test or Mann-Whitney U
test, as appropriate. Categorical variables were expressed as
frequencies and percentages, and compared using χ2 or
Fisher's exact tests. Survival analysis was conducted using the
Kaplan-Meier method, and differences were assessed using the
log-rank test. Factors associated with OS were identified using
univariate and multivariate Cox proportional hazard regression
models. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
A total of 225 patients were included in the present
study. The median age was 62 years (range 31–83 years), with a
higher prevalence in men (66.7%) than in women (33.3%). 153 in the
normal blood glucose group and 72 in the high blood glucose group
(Table I).
 | Table I.Patient demographics and baseline
characteristics based on blood glucose levels. |
Table I.
Patient demographics and baseline
characteristics based on blood glucose levels.
|
| Blood glucose |
|
|---|
|
|
|
|
|---|
| Characteristic | Normal (n=153) | Hyperglycemia
(n=72) | P-value |
|---|
| Median age, years
(range) | 61 (31–83) | 64 (35–81) | 0.066 |
| Sex, n (%) |
|
| 0.064 |
|
Female | 71 (46.4) | 24 (33.3) |
|
|
Male | 82 (53.6) | 48 (66.7) |
|
| Smoking history, n
(%) |
|
| 0.531 |
| No | 98 (64.1) | 43 (59.7) |
|
|
Yes | 55 (35.9) | 29 (40.3) |
|
| Weight loss, n
(%) |
|
| 0.459 |
| No | 103 (67.3) | 52 (72.2) |
|
|
Yes | 50 (32.7) | 20 (27.8) |
|
| Median albumin
level (range), g/l | 44.0
(35.8–48.6) | 43.6
(28.0–48.6) | 0.804 |
| Median total
bilirubin (range), mmol/l | 14 (5–281) | 15 (5–281) | 0.087 |
| CA19-9, n (%) |
|
| 0.093 |
| <37
U/ml | 36 (23.5) | 10 (13.9) |
|
|
37-1,000 U/ml | 92 (60.1) | 43 (59.7) |
|
|
>1,000 U/ml | 25 (16.3) | 19 (26.4) |
|
| Median
neutrophil-to-lymphocyte ratio (range) | 2.89
(0.70–18.07) | 2.93
(0.94–98.09) | 0.237 |
| Median
platelet-to-lymphocyte ratio (range) | 223 (150–299) | 211 (151–298) | 0.044 |
| Median tumor size
(range), cm | 3.50
(0.80–10.51) | 3.69
(1.80–11.00) | 0.283 |
| Location, n
(%) |
|
| 0.469 |
| Tail of
pancreas | 63 (41.2) | 26 (36.1) |
|
| Head of
pancreas | 90 (58.8) | 46 (63.9) |
|
| Lymph node
involvement, n (%) |
|
| 0.058 |
| No | 86 (56.2) | 50 (69.4) |
|
|
Yes | 67 (43.8) | 22 (30.6) |
|
| Lymphovascular
invasion, n (%) |
|
| 0.625 |
| No | 110 (71.9) | 54 (75.0) |
|
|
Yes | 43 (28.1) | 18 (25.0) |
|
| Perineural
invasion, n (%) |
|
| 0.912 |
| No | 50 (32.7) | 23 (31.9) |
|
|
Yes | 103 (67.3) | 49 (68.1) |
|
| Grade, n (%) |
|
| 0.740 |
| I | 19 (12.4) | 7 (9.7) |
|
| II | 88 (57.5) | 45 (62.5) |
|
|
III | 46 (30.1) | 20 (27.8) |
|
| TNM stage, n
(%) |
|
| 0.564 |
| I | 60 (39.2) | 32 (44.4) |
|
| II | 79 (51.6) | 36 (50.0) |
|
|
III | 14 (9.2) | 4 (5.6) |
|
| Adjuvant
chemotherapy, n (%) |
|
| 0.750 |
| No | 50 (32.7) | 22 (30.6) |
|
|
Yes | 103 (67.3) | 50 (69.4) |
|
| Postoperative
complications, n (%) |
|
| <0.001 |
| No | 138 (90.2) | 51 (70.8) |
|
|
Yes | 15 (9.8) | 21 (29.2) |
|
No significant differences were observed between the
normal and high blood glucose groups in terms of age, sex
distribution, smoking history, weight loss, albumin levels, total
bilirubin, CA19-9 levels, neutrophil-to-lymphocyte ratio (NLR),
tumor size, tumor location, lymph node involvement, lymphovascular
invasion, perineural invasion, tumor grade, TNM stage or adjuvant
chemotherapy. However, the platelet-to-lymphocyte ratio (PLR) and
postoperative complications were significantly different between
the normal and high blood glucose groups.
A total of 192 patients had no history of diabetes,
whereas 33 patients had a history of diabetes. When grouped by
diabetes history, no significant differences were observed in terms
of age, sex, smoking history, weight loss, albumin levels, total
bilirubin, CA19-9 levels, NLR, PLR, tumor size, location, lymph
node involvement, lymphovascular invasion, perineural invasion,
tumor grade, TNM stage, adjuvant chemotherapy and postoperative
complications between the normal and high blood glucose groups
(Table II).
 | Table II.Patient demographics and baseline
characteristics based on diabetes history. |
Table II.
Patient demographics and baseline
characteristics based on diabetes history.
|
| Diabetes
history |
|
|---|
|
|
|
|
|---|
| Characteristic | No (n=192) | Yes (n=33) | P-value |
|---|
| Median age (range),
years | 62 (55–67) | 64 (59–70) | 0.175 |
| Sex, n (%) |
|
| 0.461 |
|
Female | 83 (43.2) | 12 (36.4) |
|
|
Male | 109 (56.8) | 21 (63.6) |
|
| Smoking history, n
(%) |
|
| 0.296 |
| No | 123 (64.1) | 18 (54.5) |
|
|
Yes | 69 (35.9) | 15 (45.5) |
|
| Weight loss, n
(%) |
|
| 0.606 |
| No | 131 (68.2) | 24 (72.7) |
|
|
Yes | 61 (31.8) | 9 (27.3) |
|
| Median albumin
(range), g/l | 43.7
(40.7–45.6) | 44.1
(41.3–45.2) | 0.721 |
| Median total
bilirubin (range), mmol/l | 14 (10–68) | 14 (10–21) | 0.573 |
| CA19-9, n (%) |
|
| 0.418 |
| <37
U/ml | 41 (21.4) | 5 (15.2) |
|
|
37-1,000 U/ml | 116 (60.4) | 19 (57.6) |
|
|
>1,000 U/ml | 35 (18.2) | 9 (27.3) |
|
| Median
neutrophil-to-lymphocyte ratio (range) | 2.93
(2.19–3.88) | 2.89
(2.31–4.00) | 0.554 |
| Median
platelet-to-lymphocyte ratio (range) | 221 (191–261) | 211 (174–263) | 0.158 |
| Median tumor size
(range), cm | 3.50
(3.00–5.00) | 3.50
(3.00–5.52) | 0.864 |
| Location, n
(%) |
|
| 0.429 |
| Tail of
pancreas | 78 (40.6) | 11 (33.3) |
|
| Head of
pancreas | 114 (59.4) | 22 (66.7) |
|
| Lymph node
involvement, n (%) |
|
| 0.429 |
| No | 114 (59.4) | 22 (66.7) |
|
|
Yes | 78 (40.6) | 11 (33.3) |
|
| Perineural
invasion, n (%) |
|
| 0.776 |
| No | 63 (32.8) | 10 (30.3) |
|
|
Yes | 129 (67.2) | 23 (69.7) |
|
| Lymphovascular
invasion, n (%) |
|
| 0.982 |
| No | 140 (72.9) | 24 (72.7) |
|
|
Yes | 52 (27.1) | 9 (27.3) |
|
| Grade, n (%) |
|
| 0.450 |
| I | 23 (12.0) | 3 (9.1) |
|
| II | 110 (57.3) | 23 (69.7) |
|
|
III | 59 (30.7) | 7 (21.2) |
|
| TNM stage, n
(%) |
|
| 0.806 |
| I | 77 (40.1) | 15 (45.5) |
|
| II | 99 (51.6) | 16 (48.5) |
|
|
III | 16 (8.3) | 2 (6.0) |
|
| Adjuvant
chemotherapy, n (%) |
|
| 0.821 |
| No | 62 (32.3) | 10 (30.3) |
|
|
Yes | 130 (67.7) | 23 (69.7) |
|
| Postoperative
complications, n (%) |
|
| 0.056 |
| No | 165 (85.9) | 24 (72.7) |
|
|
Yes | 27 (14.1) | 9 (27.3) |
|
The baseline characteristics of diabetic patients
divided into normal and high blood glucose groups were analyzed
(Table SI). No significant
differences were noted between these groups in terms of
demographic, clinical or tumor characteristics, such as age, sex,
CA19-9 levels, tumor size and postoperative complications. This
balanced distribution supports the analysis by minimizing the
potential confounding effects related to baseline
characteristics.
A comparison of the prevalence of hyperglycemia
between the diabetic and non-diabetic groups was conducted, which
demonstrated a significantly higher proportion of hyperglycemia in
patients with diabetes (54.5%) compared with that in patients
without diabetes (28.1%) (P=0.005; Table SII).
Factors associated with OS
Univariate analysis identified high blood glucose
levels as significantly associated with decreased OS [hazard ratio
(HR), 1.96; 95% CI, 1.38–2.77]. In the multivariate Cox regression
model, high blood glucose level was an independent predictor of
poor OS (HR 1.68; 95% CI, 1.15–2.45), along with CA19-9 >1,000
U/ml (HR 1.70; 95% CI, 1.06–2.74). Diabetes history was also
associated with decreased OS in the univariate analysis (HR 1.70;
95% CI, 1.13–2.58), but not in the multivariate analysis (HR 1.35;
95% CI, 0.87–2.10). Similarly, grade III was associated with
decreased OS in univariate analysis (HR 1.92; 95% CI, 1.01–3.66),
but showed no significant impact in multivariate analysis (HR 1.13;
95% CI, 0.77–1.65; Table
III).
 | Table III.Univariate and multivariate analysis
of influencing factors using Cox's regression. |
Table III.
Univariate and multivariate analysis
of influencing factors using Cox's regression.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | No. of
patients | Patient deaths | HR | 95% CI | P-value | No. of
patients | Patient deaths | HR | 95% CI | P-value |
|---|
| Age | 225 | 153 | 1.00 | 0.99–1.02 | 0.736 |
|
|
|
|
|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
Female | 95 | 64 | - | - |
|
|
|
|
|
|
|
Male | 130 | 89 | 1.12 | 0.81–1.55 | 0.506 |
|
|
|
|
|
| Blood glucose |
|
|
|
|
|
|
|
|
|
|
| Normal
blood glucose | 153 | 101 | - | - |
| 153 | 101 | - | - |
|
|
Hyperglycemia | 72 | 52 | 1.96 | 1.38–2.77 | <0.001 | 72 | 52 | 1.68 | 1.15–2.45 | 0.007 |
| Diabetes
history |
|
|
|
|
|
|
|
|
|
|
| No | 192 | 125 | - | - |
| 192 | 125 | - | - |
|
|
Yes | 33 | 28 | 1.70 | 1.13–2.58 | 0.011 | 33 | 28 | 1.35 | 0.87–2.10 | 0.184 |
| Smoking
history |
|
|
|
|
|
|
|
|
|
|
| No | 141 | 96 | - | - |
|
|
|
|
|
|
|
Yes | 84 | 57 | 0.99 | 0.71–1.37 | 0.935 |
|
|
|
|
|
| CA199, U/ml |
|
|
|
|
|
|
|
|
|
|
|
<37 | 46 | 32 | - | - |
| 46 | 32 | - | - |
|
|
37-1,000 | 135 | 77 | 0.78 | 0.51–1.18 | 0.237 | 135 | 77 | 0.73 | 0.48–1.11 | 0.136 |
|
>1,000 | 44 | 44 | 1.91 | 1.21–3.04 | 0.006 | 44 | 44 | 1.70 | 1.06–2.74 | 0.029 |
| Weight loss |
|
|
|
|
|
|
|
|
|
|
| No | 155 | 107 | - | - |
|
|
|
|
|
|
|
Yes | 70 | 46 | 1.01 | 0.71–1.44 | 0.940 |
|
|
|
|
|
| Albumin | 225 | 153 | 1.02 | 0.97–1.07 | 0.496 |
|
|
|
|
|
| Total
bilirubin | 225 | 153 | 1.00 | 1.00–1.00 | 0.981 |
|
|
|
|
|
|
Neutrophil-to-lymphocyte ratio | 225 | 153 | 1.02 | 1.00–1.03 | 0.084 | 225 | 153 | 1.02 | 1.00–1.04 | 0.081 |
|
Platelet-to-lymphocyte ratio | 225 | 153 | 1.00 | 1.00–1.00 | 0.960 | 225 | 153 | 1.00 | 1.00–1.01 | 0.220 |
| Location |
|
|
|
|
|
|
|
|
|
|
| Tail of
pancreas | 89 | 57 | - | - |
|
|
|
|
|
|
| Head of
pancreas | 136 | 96 | 1.13 | 0.81–1.57 | 0.468 |
|
|
|
|
|
| Size | 225 | 153 | 1.07 | 0.98–1.16 | 0.130 |
|
|
|
|
|
| Lymph node
involvement |
|
|
|
|
|
|
|
|
|
|
| No | 136 | 90 | - | - |
|
|
|
|
|
|
|
Yes | 89 | 63 | 1.07 | 0.77–1.48 | 0.693 |
|
|
|
|
|
| Perineural
invasion |
|
|
|
|
|
|
|
|
|
|
| No | 73 | 55 | - | - |
|
|
|
|
|
|
|
Yes | 152 | 98 | 0.95 | 0.68–1.32 | 0.746 |
|
|
|
|
|
| Lymphovascular
invasion |
|
|
|
|
|
|
|
|
|
|
| No | 164 | 109 | - | - |
|
|
|
|
|
|
|
Yes | 61 | 44 | 1.14 | 0.80–1.62 | 0.474 |
|
|
|
|
|
| Grade |
|
|
|
|
|
|
|
|
|
|
| I | 26 | 13 | - | - |
| 26 | 13 | - | - |
|
| II | 133 | 94 | 2.15 | 1.17–3.96 | 0.013 | 133 | 94 | 1.03 | 0.69–1.30 | 0.093 |
|
III | 66 | 46 | 1.92 | 1.01–3.66 | 0.046 | 66 | 46 | 1.13 | 0.77–1.65 | 0.089 |
| Adjuvant
chemotherapy |
|
|
|
|
|
|
|
|
|
|
| No | 72 | 44 | - | - |
|
|
|
|
|
|
|
Yes | 153 | 109 | 1.19 | 0.84–1.70 | 0.325 |
|
|
|
|
|
| Postoperative
complications |
|
|
|
|
|
|
|
|
|
|
| No | 189 | 128 | - | - |
|
|
|
|
|
|
|
Yes | 36 | 25 | 1.04 | 0.67–1.60 | 0.871 |
|
|
|
|
|
Survival analysis
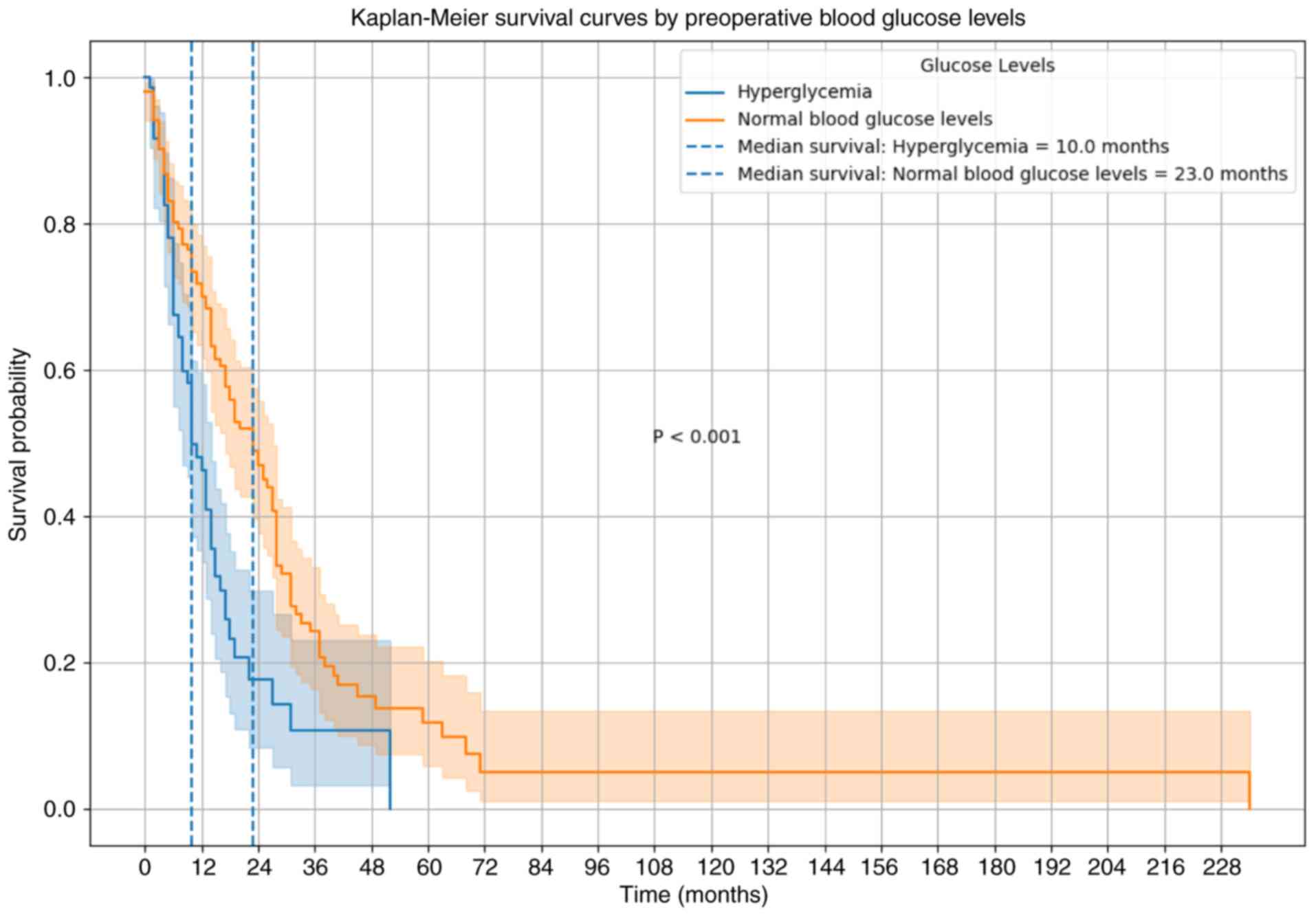
Kaplan-Meier analysis indicated a significantly
shorter median OS time in the high blood glucose group (10.0
months) compared with that in the normal blood glucose group (23.0
months) (P<0.001). Additionally, the 1- and 2-year survival
rates were markedly lower in patients with high blood glucose
levels, at 45 and 20%, respectively, compared with 65 and 35% in
the normal glucose group (Fig.
1).
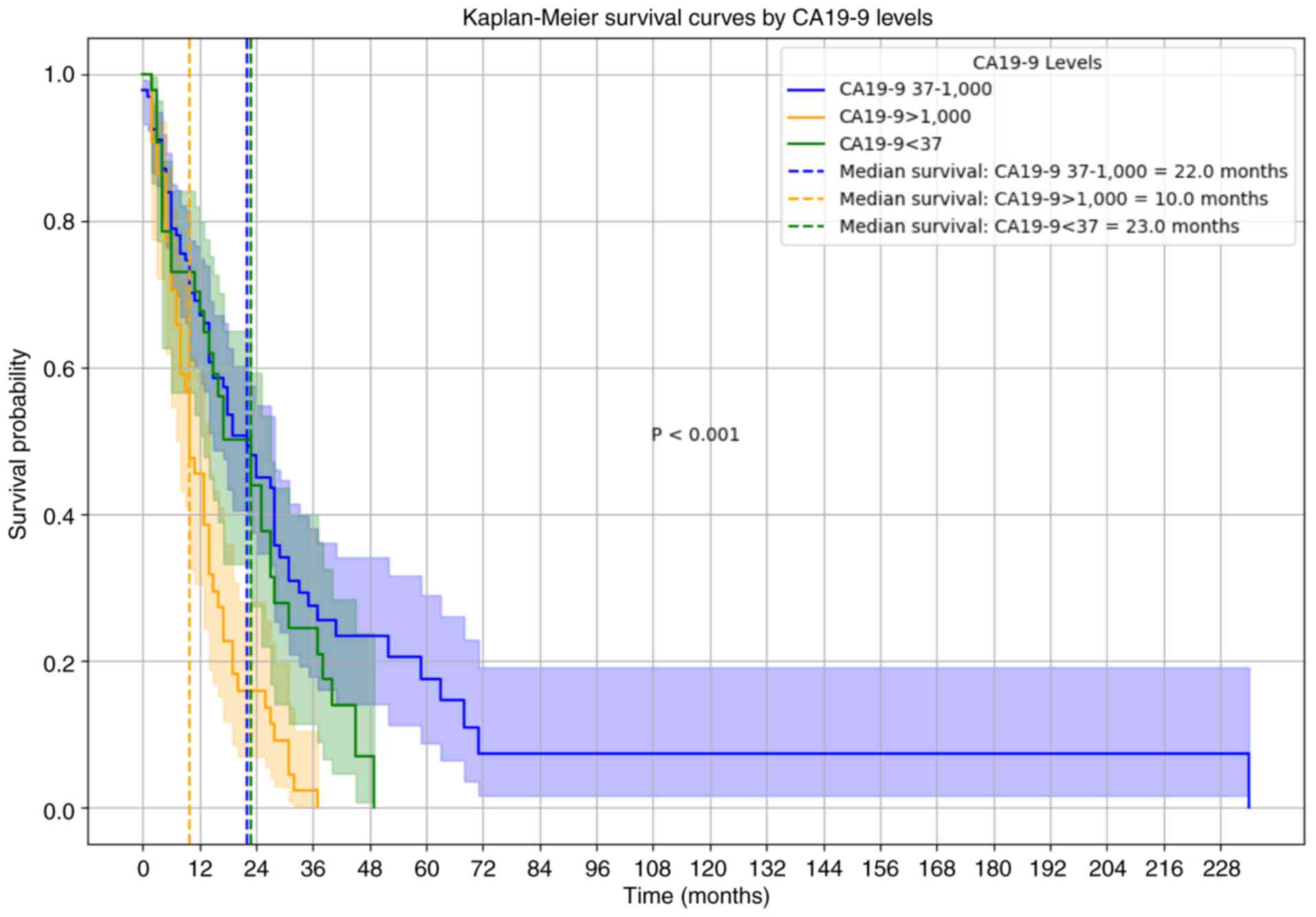
For patients with CA19-9 levels >1,000 U/ml, the
median OS was significantly reduced (10.0 months), compared with
those with CA19-9 levels between 37 and 1,000 U/ml (22.0 months)
and those with CA19-9 levels <37 U/ml (23.0 months) (Fig. 2). Multivariate Cox regression
analysis further validated CA19-9 levels >1,000 U/ml as an
independent predictor of poor OS (HR 1.70; 95% CI, 1.06–2.74),
along with high blood glucose levels (HR 1.68; 95% CI, 1.15–2.45).
Although diabetes history was associated with significantly
decreased OS in univariate analysis (HR 1.70; 95% CI, 1.13–2.58),
this association did not hold in the multivariate model (HR 1.35;
95% CI, 0.87–2.10) (Table III),
suggesting that immediate metabolic status, rather than diabetes
history, serves a critical role in prognosis.
Postoperative complications
Postoperative complications were more common in the
high blood glucose group compared with the normal glucose group
(29.2 vs. 9.8%; (Table I). The most
frequent complications were infection, delayed wound healing and
anastomotic leakage. Postoperative complications were more frequent
in patients with a history of diabetes than in those without
diabetes; however, the difference was not significant (27.3 vs.
14.1%; Table II).
Discussion
Pancreatic cancer is a highly malignant tumor with a
poor prognosis. Despite significant advancements in diagnostic and
therapeutic approaches in recent years, the OS rate remains low
(1). The aggressive nature of
pancreatic cancer, combined with its often asymptomatic early
stages, results in numerous patients being diagnosed at an advanced
stage, which negatively affects treatment outcomes and survival
rates (7). Among the numerous
factors that influence prognosis, preoperative metabolic status and
tumor markers are considered to be key predictive factors (8). Diabetes mellitus, a common metabolic
disorder, has been extensively studied and linked to the incidence
and prognosis of various malignancies, including renal and prostate
cancer (14,15). Additionally, preoperative blood
glucose levels and tumor markers such as CA19-9 are suggested to
serve important roles in cancer prognosis (16). However, the specific effects of
these factors on patients with pancreatic cancer remain
controversial. The present study aimed to systematically evaluate
the effects of a preoperative history of diabetes and preoperative
blood glucose and CA19-9 levels on the prognosis of patients with
pancreatic cancer to provide insights for clinical decision-making
(17).
The present study included clinical data from
>200 patients with pancreatic cancer and performed Kaplan-Meier
survival, Cox's regression, and univariate and multivariate
analyses. The baseline characteristics of the patients with
diabetes were similar between the normal and high blood glucose
groups, which reduced the potential effects of confounding factors
in the outcome analysis. The prevalence of hyperglycemia was
significantly higher in the diabetic group compared with that in
the non-diabetic group, which indicated distinct differences in
blood glucose control between these populations. Patients with
diabetes often face challenges in maintaining normoglycemia due to
factors such as insulin resistance and impaired pancreatic function
(11). This result underscores the
importance of intensified blood glucose management in patients with
diabetes to reduce the risk of hyperglycemia-related
complications.
After adjusting for other potential confounding
factors, preoperative blood glucose level was an independent
prognostic factor in patients with pancreatic cancer. High blood
glucose levels were significantly associated with poor survival
rates. Secondly, CA19-9 levels >1,000 U/ml were an independent
prognostic factor, which indicated that patients with elevated
CA19-9 levels had significantly worse survival outcomes. Finally,
while preoperative diabetes history was associated with worse
survival rates in the univariate analysis, it did not remain
significant in the multivariate analysis, suggesting that its
impact might be mediated through other factors, such as blood
glucose levels and inflammatory status.
The present study demonstrated a significantly
higher incidence of postoperative complications, including
infections, delayed wound healing and anastomotic leaks in the high
blood glucose group compared with that in the normoglycemic group.
This finding aligns with previous research indicating that elevated
blood glucose levels markedly increase the risk of postoperative
complications, particularly those related to infection and delayed
healing (18). Compared with
analyzing individual complications separately, combining
complications into a single composite measure offers a more
comprehensive perspective and reduces statistical variability,
especially given the lower incidence of individual events (19).
Hyperglycemia is considered to increase the
complication rates through several mechanisms. First, elevated
blood glucose levels have been shown to suppress immune function,
particularly by inhibiting neutrophil chemotaxis and phagocytosis,
which lowers the body's defense against pathogens and increases
infection risks, such as wound infections and anastomotic leaks
(20). Additionally, hyperglycemia
delays wound healing by reducing collagen synthesis, impairing
fibroblast activity and restricting blood flow, thereby hindering
tissue access to the oxygen and nutrients that are essential for
healing. This delayed healing effect likely contributes to an
increased risk of wound dehiscence and anastomotic leakage
(18). Moreover, hyperglycemia
amplifies the inflammatory response, promoting the release of
pro-inflammatory cytokines such as TNF-α and IL-6, which exacerbate
tissue damage and elevate the risk of complications. The
accumulation of advanced glycation end products (AGEs) further
exacerbates tissue injury and impedes normal repair processes,
making patients more vulnerable to postoperative complications
(21).
Although combining complications provides a broader
view and increased statistical power, this approach may obscure the
unique effects of hyperglycemia on each type of complication. A
previous study has highlighted the advantages of such combined
analyses, particularly in patients with diabetes or preoperative
hyperglycemia, as the interrelated nature of complications such as
cardiovascular events and infections enhances the statistical
reliability and clinical interpretability of the findings (22). For example, when assessing the
impact of hyperglycemia on infection risk, combining complications
assists in identifying high-risk patients and underscores the
importance of rigorous preoperative glucose management (21). Despite these benefits, future
studies should explore individual complications to evaluate the
distinct effects of hyperglycemia on various postoperative
outcomes, which may aid in creating personalized glucose management
strategies for high-risk patients (20). Overall, these findings support the
need for stringent preoperative glucose control to reduce infection
risk and delay wound healing and anastomotic leaks, thus improving
surgical outcomes.
High preoperative blood glucose levels have been
associated with a poor prognosis in various types of cancer,
including pancreatic cancer. Balzano et al (23) found that elevated blood glucose
levels were significantly associated with an increased incidence
and mortality of pancreatic cancer. Huxley et al (24) also reported that preoperative
hyperglycemia was significantly correlated with reduced OS in
patients with pancreatic cancer. In the present study, preoperative
blood glucose level emerged as an independent prognostic factor,
even after adjusting for other potential confounding factors. This
suggested that glycemic control has a direct impact on patient
outcomes, independent of other metabolic disorders or
comorbidities. Hyperglycemia may promote tumor cell proliferation,
inhibit apoptosis and affect the tumor microenvironment, thereby
influencing the prognosis (25).
These findings highlighted the critical role of preoperative
metabolic control in the management of patients with pancreatic
cancer.
The relationship between preoperative blood glucose
levels and a history of diabetes is complex. Previous studies have
shown that history of diabetes is generally associated with a worse
cancer prognosis (6,14,15).
Meyerhardt et al (26)
conducted a meta-analysis and found that diabetes was linked to
poor outcomes in various types of cancer, including pancreatic
cancer. However, in the present study, although a preoperative
history of diabetes was significant in the univariate analysis, it
did not remain significant in the multivariate analysis. This
discrepancy may be explained by the fact that the impact of a
history of diabetes on prognosis may be mediated by current blood
glucose levels and related metabolic disturbances (26). Chronic hyperglycemia and associated
metabolic abnormalities, such as insulin resistance and chronic
inflammation, may contribute to poor outcomes in patients with
diabetes (27). Therefore, the
immediate preoperative metabolic state, as reflected by blood
glucose levels, may be more critical in predicting outcomes
compared with presence of a history of diabetes.
The non-significance of a history of preoperative
diabetes in the multivariate analysis could be due to several
factors. First, while a history of diabetes indicates long-term
metabolic dysregulation, preoperative blood glucose levels may have
a more direct impact on surgical and oncological outcomes. Kleeff
et al (28) suggested that,
although a history of diabetes is related to prognosis, pre-and
postoperative glycemic controls are more significant factors
affecting outcomes. Second, a history of diabetes may indirectly
influence the prognosis through blood glucose levels and other
metabolic parameters. Patients with diabetes often exhibit insulin
resistance, chronic inflammation and other metabolic issues that
affect their prognosis through various pathways (28). Additionally, a history of diabetes
may be associated with other comorbidities and risk factors that
were accounted for in the multivariate analysis, reducing its
independent significance.
CA19-9 is one of the most commonly used tumor
markers for pancreatic cancer. Several studies have confirmed its
prognostic value. Duffy et al (29) reported that elevated CA19-9 levels
were significantly associated with tumor burden and poor prognosis
in patients with pancreatic cancer. Ballehaninna and Chamberlain
(30) provided a comprehensive
review of CA19-9 as a pancreatic cancer biomarker, highlighting its
potential for risk stratification and prognosis. In the present
study, CA19-9 levels >1,000 U/ml were identified as an
independent prognostic factor, which is consistent with previous
research (29,30). Elevated CA19-9 levels indicate
higher tumor burden and more aggressive disease, thus contributing
to worse survival outcomes (31).
This finding supports the clinical utility of CA19-9 in stratifying
patients with pancreatic cancer and guiding therapeutic decisions
such as more aggressive treatment approaches for patients with high
CA19-9 levels (32).
Hyperglycemia may affect the prognosis of pancreatic
cancer through several mechanisms. Firstly, hyperglycemia promotes
cancer cell proliferation and inhibits apoptosis. Previous research
has shown that high glucose levels can enhance the glycolytic
pathway in cancer cells by providing them with the energy required
for rapid growth and division (33). Second, hyperglycemia can create a
pro-inflammatory environment by increasing the levels of
pro-inflammatory cytokines, such as TNF-α and IL-6, which can
support tumor progression and metastasis (34). Third, chronic hyperglycemia can lead
to insulin resistance, which in turn can result in higher insulin
and insulin-like growth factor levels. These factors promote cell
proliferation and inhibit apoptosis, further contributing to tumor
growth and poor prognosis (26).
Additionally, hyperglycemia alters the composition of the tumor
microenvironment to promote cancer cell proliferation. For example,
the inflammatory environment in hyperglycemia promotes the
accumulation of immunosuppressive cells that suppress antitumor
immune responses (35). High
glucose levels may encourage angiogenesis, provide more nutrients
and oxygen to cancer cells, and accelerate tumor growth and spread
(6). In the present study, no
significant differences were observed in the baseline
characteristics between the normal and high blood glucose groups,
suggesting that blood glucose levels do not influence pancreatic
cancer prognosis through these clinicopathological factors. This
finding further supports the results of Cox's regression analysis,
which indicated that a high blood glucose level is an independent
risk factor affecting patient prognosis.
Inflammation serves an important bridging role in
the relationship between hyperglycemia, diabetes and cancer
prognosis (35). Both hyperglycemia
and diabetes are closely associated with chronic low-grade
inflammation, which is a key factor in cancer progression and
worsening outcomes (35,36). Inflammatory markers, such as
C-reactive protein and fibrinogen, have been shown to be elevated
in diabetes patients and are associated with worse outcomes in
various types of cancer, including pancreatic cancer (37). In patients with diabetes, persistent
hyperglycemia induces oxidative stress and promotes the formation
of AGEs that activate multiple inflammatory pathways, thereby
enhancing the invasiveness and metastatic potential of cancer cells
(6). An inflammatory environment
can promote angiogenesis, tissue remodeling and immune evasion, all
of which are conducive to cancer progression. This highlights the
importance of managing not only blood glucose levels but also the
inflammatory status in patients with diabetes to improve
outcomes.
Although the present study provides valuable
insights into the prognostic factors of pancreatic cancer, it has
numerous limitations. First, the retrospective nature of the
present study may have introduced a selection bias. Second, the
single-center design limits the generalizability of the present
findings. Future multicenter prospective studies are required to
validate these findings. Additionally, further research should
explore the underlying biological mechanisms linking hyperglycemia,
diabetes and pancreatic cancer prognosis, as well as potential
interventions to optimize metabolic control in patients with
pancreatic cancer. Notably, owing to the retrospective nature of
data collection, certain important prognostic factors, such as
venous invasion, arterial invasion and neoadjuvant chemotherapy,
were not consistently available for all included patients. These
factors are known to influence survival in pancreatic cancer
(38,39), and their absence may limit the
robustness of the survival analysis, potentially introducing a bias
in evaluating the impact of preoperative blood glucose levels and
CA19-9 on OS.
In conclusion, the present study emphasizes the
significant impact of the preoperative metabolic status on the
prognosis of patients with pancreatic cancer. High preoperative
blood glucose levels and elevated CA19-9 levels were identified as
independent prognostic factors, whereas the impact of a history of
preoperative diabetes may be mediated through blood glucose levels
and related metabolic disturbances. These findings highlight the
importance of comprehensive preoperative metabolic evaluation and
control to improve patient outcomes. Future research should focus
on elucidating the mechanisms underlying these associations and on
developing strategies to optimize metabolic management in patients
with pancreatic cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Hebei Province (grant no. H2022206335) and the 2023
Hebei Provincial Medical Science Research Project (grant no.
20230756).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SBW, LP and WY conceptualized and designed the
study. SBW and TP performed the data analyses. XZ and SYW collected
the clinical samples. LP interpreted the data. SBW drafted the
manuscript, which was revised by WY and LP. SBW, TP, SYW, XZ, LP
and WY confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki (as revised in 2013). The present study
was approved by the Institutional Research Ethics Committee at the
Fourth Hospital of Hebei Medical University (Shijiazhuang, China;
approval no. 2023KS182) and all patients provided written consent
for participation in the present study.
Patient consent for publication
Written informed consent for publication was
obtained from all individuals involved in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duan X, Wang W, Pan Q and Guo L: Type 2
diabetes mellitus intersects with pancreatic cancer diagnosis and
development. Front Oncol. 11:7300382021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamaguchi A, Tazuma S, Tamaru Y, Kusunoki
R, Kuwai T, Kouno H, Toyota N, Sudo T, Kuraoka K and Kohno H:
Long-standing diabetes mellitus increases concomitant pancreatic
cancer risk in patients with intraductal papillary mucinous
neoplasms. BMC Gastroenterol. 22:5292022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bures J, Kohoutova D, Skrha J, Bunganic B,
Ngo O, Suchanek S, Skrha P and Zavoral M: Diabetes mellitus in
pancreatic cancer: A distinct approach to older subjects with
new-onset diabetes mellitus. Cancers (Basel). 15:36692023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Xu W, Hu X, Yang X and Zhang M:
The prognostic role of glycemia in patients with pancreatic
carcinoma: A systematic review and meta-analysis. Front Oncol.
12:7809092022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lv X, Qiao W, Leng Y, Wu L and Zhou Y:
Impact of diabetes mellitus on clinical outcomes of pancreatic
cancer after surgical resection: A systematic review and
meta-analysis. PLoS One. 12:e01713702017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Q, Feng Z, Miao R, Liu X, Liu C and Liu
Z: Prognosis and survival analysis of patients with pancreatic
cancer: Retrospective experience of a single institution. World J
Surg Oncol. 20:112022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Menini S, Iacobini C, de Latouliere L,
Manni I, Vitale M, Pilozzi E, Pesce C, Cappello P, Novelli F,
Piaggio G and Pugliese G: Diabetes promotes invasive pancreatic
cancer by increasing systemic and tumour carbonyl stress in
KrasG12D/+ mice. J Exp Clin Cancer Res. 39:1522020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong Y, Fan Z, Zhang P, Qian Y, Huang Q,
Deng S, Luo G, Cheng H, Jin K, Ni Q, et al: High pre-operative
fasting blood glucose levels predict a poor prognosis in patients
with pancreatic neuroendocrine tumour. Endocrine. 71:494–501. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raghavan SR, Ballehaninna UK and
Chamberlain RS: The impact of perioperative blood glucose levels on
pancreatic cancer prognosis and surgical outcomes: An
evidence-based review. Pancreas. 42:1210–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andersen DK, Korc M, Petersen GM, Eibl G,
Li D, Rickels MR, Chari ST and Abbruzzese JL: Diabetes,
pancreatogenic diabetes, and pancreatic cancer. Diabetes.
66:1103–1110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pancreatic Cancer Committee of Chinese
Anticancer Association, . Comprehensive guidelines for the
diagnosis and treatment of pancreatic cancer (2020 version).
Zhonghua Wai Ke Za Zhi. 59:81–100. 2021.(In Chinese). PubMed/NCBI
|
|
13
|
Gonzalez RS, Raza A, Propst R, Adeyi O,
Bateman J, Sopha SC, Shaw J and Auerbach A: Recent advances in
digestive tract tumors: Updates from the 5th edition of the World
Health Organization ‘blue book’. Arch Pathol Lab Med. 145:607–626.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan JX, Jiang Q and Yu SJ: Diabetes
mellitus and prostate cancer risk: A mendelian randomization
analysis. World J Diabetes. 14:1839–1848. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Cao Y and Zhu C: Meta-analysis of
the relationship between type 2 diabetes mellitus and renal cancer
risk. Endocr Metab Immune Disord Drug Targets. 24:832–839. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boursi B, Finkelman B, Giantonio BJ,
Haynes K, Rustgi AK, Rhim AD, Mamtani R and Yang YX: A clinical
prediction model to assess risk for pancreatic cancer among
patients with new-onset diabetes. Gastroenterology. 152:840–850.e3.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pannala R, Leibson CL, Rabe KG, Timmons
LJ, Ransom J, de Andrade M, Petersen GM and Chari ST: Temporal
association of changes in fasting blood glucose and body mass index
with diagnosis of pancreatic cancer. Am J Gastroenterol.
104:2318–2325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goodenough CJ, Liang MK, Nguyen MT, Nguyen
DH, Holihan JL, Alawadi ZM, Roth JS, Wray CJ, Ko TC and Kao LS:
Preoperative glycosylated hemoglobin and postoperative glucose
together predict major complications after abdominal surgery. J Am
Coll Surg. 221:854–861.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Hou A, Cao J, Liu Y, Lou J, Li H,
Ma Y, Song Y, Mi W and Liu J: Association of diabetes mellitus with
postoperative complications and mortality after non-cardiac
surgery: A meta-analysis and systematic review. Front Endocrinol
(Lausanne). 13:8412562022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dougherty SM, Schommer J, Salinas JL,
Zilles B, Belding-Schmitt M, Rogers WK, Shibli-Rahhal A and O'Neill
BT: Immediate preoperative hyperglycemia correlates with
complications in non-cardiac surgical cases. J Clin Anesth.
74:1103752021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gu J, Liu J, Hong Y, Feng Y and Huang X:
Nomogram for predicting risk factor of urosepsis in patients with
diabetes after percutaneous nephrolithotomy. BMC Anesthesiol.
22:872022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu S, Xv L, Wu X, Wang F, Wang J, Tang X,
Dong R, Wang B, Lin X and Bi Y: Potential value of preoperative
fasting blood glucose levels in the identification of postoperative
delirium in non-diabetic older patients undergoing total hip
replacement: The perioperative neurocognitive disorder and
biomarker lifestyle study. Front Psychiatry. 13:9410482022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balzano G, Dugnani E, Gandolfi A, Scavini
M, Pasquale V, Aleotti F, Liberati D, Di Terlizzi G, Petrella G,
Reni M, et al: Effect of diabetes on survival after resection of
pancreatic adenocarcinoma. A prospective, observational study. PLoS
One. 11:e01660082016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huxley R, Ansary-Moghaddam A, Berrington
de González A, Barzi F and Woodward M: Type-II diabetes and
pancreatic cancer: A meta-analysis of 36 studies. Br J Cancer.
92:2076–2083. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perazzoli G, Garcia-Valdeavero OM, Peña M,
Prados J, Melguizo C and Jiménez-Luna C: Evaluating
metabolite-based biomarkers for early diagnosis of pancreatic
cancer: A systematic review. Metabolites. 13:8722023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meyerhardt JA, Catalano PJ, Haller DG,
Mayer RJ, Macdonald JS, Benson AB III and Fuchs CS: Impact of
diabetes mellitus on outcomes in patients with colon cancer. J Clin
Oncol. 21:433–440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abel ED, Gloyn AL, Evans-Molina C, Joseph
JJ, Misra S, Pajvani UB, Simcox J, Susztak K and Drucker DJ:
Diabetes mellitus-progress and opportunities in the evolving
epidemic. Cell. 187:3789–3820. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kleeff J, Korc M, Apte M, La Vecchia C,
Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH
and Neoptolemos JP: Pancreatic cancer. Nat Rev Dis Primers.
2:160222016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duffy MJ, Sturgeon C, Lamerz R, Haglund C,
Holubec VL, Klapdor R, Nicolini A, Topolcan O and Heinemann V:
Tumor markers in pancreatic cancer: A European group on tumor
markers (EGTM) status report. Ann Oncol. 21:441–447. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ballehaninna UK and Chamberlain RS: Serum
CA 19-9 as a biomarker for pancreatic cancer-A comprehensive
review. Indian J Surg Oncol. 2:88–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bhandare MS, Gupta V, Chaudhari V, Nandy
K, Ostwal V, Ramaswamy A, Nashikkar C, Engineer R, Krishnatry R and
Shrikhande SV: Differential impact of incrementally elevated CA
19-9 levels on prognosis of resected pancreatic ductal
adenocarcinoma. HPB (Oxford). 26:1237–1247. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guan S, Chen Y, Han Q, Deng G, Wang Y, Shi
Y and Dai G: Preoperative CA19-9 levels predict disease-free
survival and overall survival in pancreatic adenocarcinoma patients
after resection. Transl Cancer Res. 8:811–820. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cummings J, Ward TH, Greystoke A, Ranson M
and Dive C: Biomarker method validation in anticancer drug
development. Br J Pharmacol. 153:646–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Currie CJ, Poole CD and Gale EAM: The
influence of glucose-lowering therapies on cancer risk in type 2
diabetes. Diabetologia. 52:1766–1777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghareghomi S, Arghavani P, Mahdavi M,
Khatibi A, García-Jiménez C and Moosavi-Movahedi AA:
Hyperglycemia-driven signaling bridges between diabetes and cancer.
Biochem Pharmacol. 229:1164502024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lomelí Martínez SM, Cortés Trujillo I,
Martínez Nieto M and Mercado González AE: Periodontal disease: A
silent factor in the development and progression of diabetic
retinopathy. World J Diabetes. 15:1672–1676. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bowers LW, Rossi EL, O'Flanagan CH,
deGraffenried LA and Hursting SD: The role of the insulin/IGF
system in cancer: Lessons learned from clinical trials and the
energy balance-cancer link. Front Endocrinol (Lausanne). 6:772015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang JS, Choi YJ, Byun Y, Han Y, Kim JH,
Lee JM, Sohn HJ, Kim H, Kwon W and Jang JY: Radiological tumour
invasion of splenic artery or vein in patients with pancreatic body
or tail adenocarcinoma and effect on recurrence and survival. Br J
Surg. 109:105–113. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dillon DL, Park JY, Mederos MA, Seo YJ,
King J, Hines J, Donahue T and Girgis MD: Neoadjuvant chemotherapy
is associated with improved disease-free survival in pancreatic
cancer patients undergoing pancreaticoduodenectomy with vascular
resection. J Surg Oncol. 130:72–82. 2024. View Article : Google Scholar : PubMed/NCBI
|