Introduction
Worldwide, lung cancer has the highest mortality
rate of all types of cancer; ~1.8 million people die from lung
cancer every year (1). Non-small
cell lung cancer (NSCLC) is the most prevalent type of lung cancer,
accounts for approximately 85% of all lung cancers (2). Surgery is an essential treatment for
patients with early stage NSCLC, and a lobectomy with systematic
lymph node dissection (SLND) is regarded as the standard surgical
procedure for patients with NSCLC (3,4).
Nonetheless, the use of lymph node dissection in
early stage NSCLC remains contentious (5–11).
Some studies have suggested that lobe-SLND (LSLND) or lymph node
sampling (LNS) yields survival outcomes comparable to those of SLND
in patients with early stage NSCLC (12–16).
In addition, excessive lymph node dissection and sampling can
increase potential postoperative complications such as bleeding,
chylothorax and nerve damage (17,18).
Furthermore, it has been reported that normal lymph nodes serve an
important role in antitumor immunity, and that lymph node
dissection may alter endogenous antitumor mechanisms, accelerating
tumor growth (17). Therefore,
excessive lymph node dissection may not only be unhelpful, but also
potentially harmful to patients. The right upper paratracheal lymph
nodes (2R lymph nodes) are significant in the development of
right-sided lung cancer. However, in LSLND of lower lobe cancer and
LNS, the dissection of 2R lymph nodes may be unnecessary.
Dissection of the 2R lymph node for right-sided NSCLC is
challenging due to the anatomical constraints imposed by the left
innominate vein. Therefore, some surgeons may choose not to dissect
the 2R lymph nodes during surgical procedures to treat right lung
cancer. Consequently, the present study aimed to investigate the
significance of 2R lymph node resection in patients with early
stage right lung cancer.
Materials and methods
Patient details
Between January 1999 and October 2009, data was
gathered for patients with NSCLC who underwent surgery at the Sun
Yat-Sen University Cancer Center (Guangzhou, China). The inclusion
criteria used were as follows: i) Patients with stage IB NSCLC
located in the right lung; ii) patients treated with a lobectomy;
and iii) no prior history of other malignancies. The exclusion
criteria used were as follows: i) Patients treated with neoadjuvant
chemotherapy; ii) patients who died during the first month after
surgery; and iii) patient records with no detailed follow-up data.
All pathological specimens were confirmed for pathological results
through H&E and immunohistochemical staining. For H&E
staining, the specimen was fixed in 10% neutral buffered formalin
at 37°C for 12–18 h. The section thickness was 4 µm. This was
followed by staining with hematoxylin-eosin for 3–8 min at 37°C.
Finally, the slides were examined under a light microscope and
images (magnification, ×200) were acquired. The immunohistochemical
staining process was as follows. Initial fixation was performed
with 10% neutral buffered formalin solution at 37°C for 12–18 h,
followed by paraffin embedding with a section thickness of 4 µm.
The blocking reagent was 3% hydrogen peroxide for 10 min at 37°C.
The primary antibody was working solution (TTF-1, MAB-0677;
NapsinA, MAB-0704; p40, RMA-0815; CK5/6, MAB-0744, Fuzhou Maixin
Biotechnology Development), undiluted, at 37°C for 30 min, and the
secondary antibody was the working solution (DAKO K8002, Agilent
Technologies), undiluted, at 37°C for 30 min. The conjugate is
biotin/streptavidin, which is labeled with horseradish peroxidase
(HRP). The final slides were examined under a light microscope
(magnification, ×200). To clarify the pathological type,
immunohistochemical staining indicators are usually thyroid
transcription factor-1 (TTF-1), NapsinA, p40 and cytokeratin
(CK)5/6. Patients who were positive for TTF-1 and NapsinA were
diagnosed with adenocarcinoma, and those who were positive for p40
and CK5/6 were diagnosed with squamous cell carcinoma. If the
cytological morphology suggested the possibility of SCLC,
synaptophysin and CD56 staining were added. If these stains were
positive, the case was diagnosed as SCLC and was not included in
this study. All tumors and lymph node samples were evaluated by two
experienced senior pathologists who were blinded to the clinical
outcomes of the patients. T and N stages were adjusted according to
tumor size and lymph node information in the database, and the
Tumor-Node-Metastasis stage was determined according to the 8th
edition of the International Association for the Study of Lung
Cancer staging system (19). A
total of 339 patients were included in the study cohort.
Patient follow-up
The follow-up information was obtained by contact
with patient's relatives by telephone or collected from the
hospital records. Routine examinations, such as blood tests, chest
computed tomography scan images and ultrasound images of the
abdomen and neck were conducted every 3 months for 2 years, every 6
months for the subsequent 3–5 years and annually thereafter. Brain
MRI scans were performed annually and a bone scan was performed
based on the patient's symptoms. Overall survival (OS) was
determined from the surgery date to the date of death and
recurrence-free survival (RFS) was determined from the surgery date
to the recurrence date. All patients were monitored until January
2013.
Statistical analysis
The χ2 test was applied for evaluating
categorical variables between two groups. The Kaplan-Meier method
and log-rank test were used to estimate OS and RFS. Cox regression
analysis was performed both for univariate and multivariate
analyses. Variables with a P-value <0.1 in the univariate
analysis were included in the multivariate analysis. P<0.05 was
considered to indicate a statistically significant difference.
Hazard ratios and 95% confidence intervals were calculated to
quantify the association between covariates and survival outcomes.
Statistical analyses were conducted using R software (version
4.2.0; R Foundation). The ‘matchit’ package was used to perform
propensity score matching (PSM). In PSM analysis, all variables
included in the study were included for calculating propensity
score, with a caliper width set to 0.3. The survival curves and
forest plots were produced utilizing the ‘survival’ and ‘survminer’
packages. The X-tile software (version 3.6.1; Yale University) was
utilized to ascertain the appropriate cut-off values for resected
lymph node counts, which were identified as <12 and ≥12.
Results
Patient characteristics
The baseline characteristics of all 339 patients in
the present study are summarized in Table I. The patients were separated into
two groups according to whether their upper paratracheal lymph
nodes were resected (Yes, n=187 cases/No, n=152 cases). Male
patients accounted for most patients in the present study (70.2%).
The mean age is 59.5, with a range of 18 to 85 and a total of 34.2%
of patients were aged >65 years. Patients with a smoking history
and a family history of malignant tumors accounted for 57.5 and
15.9% of all cases, respectively. The pathological grade of most of
the cases was I + II (61.7%) and the pathological type was mainly
adenocarcinoma (64.6%). The proportion of cases with tumors located
in the upper, middle and lower lobes were 53.7, 13.0 and 33.3%,
respectively. Patients with visceral pleural invasion and bronchial
invasion constituted 61.7 and 25.4% of the total patients,
respectively. A total of 31.0% of cases involved the dissection of
≥6 lymph nodes, whereas 61.7% of cases involved the dissection of
≥12 lymph nodes. Chemotherapy was used to treat 15.0% of the
patients.
 | Table I.Clinicopathological characteristics
between original and matched data set. |
Table I.
Clinicopathological characteristics
between original and matched data set.
|
| Original data
set | Matched data set |
|---|
|
|
|
|
|---|
|
|
| Upper paratracheal
lymph node resection |
|
| Upper paratracheal
lymph node resection |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Variables | Total (n=339) | No (n=152) | Yes (n=187) | P-value | Total (n=202) | No (n=101) | Yes (n=101) | P-value |
|---|
| Sex, n (%) | 0.506 |
| | | 0.446 |
|
|
|
|
Female | 101 (29.8) | 42 (27.6) | 59 (31.6) |
| 62 (30.7) | 28 (27.7) | 34 (33.7) |
|
| Male | 238 (70.2) | 110 (72.4) | 128 (68.4) |
| 140 (69.3) | 73 (72.3) | 67 (66.3) |
|
| Age, n (%) | 0.567 |
| | | 0.659 |
|
|
|
| >65
years | 116 (34.2) | 55 (36.2) | 61 (32.6) |
| 72 (35.6) | 34 (33.7) | 38 (37.6) |
|
| ≤65
years | 223 (65.8) | 97 (63.8) | 126 (67.4) |
| 130 (64.4) | 67 (66.3) | 63 (62.4) |
|
| Smoking, n (%) | 0.498 |
| | | 0.203 |
|
|
|
| No | 144 (42.5) | 61 (40.1) | 83 (44.4) |
| 90 (44.6) | 40 (39.6) | 50 (49.5) |
|
|
Yes | 195 (57.5) | 91 (59.9) | 104 (55.6) |
| 112 (55.4) | 61 (60.4) | 51 (50.5) |
|
| Family history of
malignant tumor, n (%) | 0.932 |
| | | >0.999 |
|
|
|
| No | 285 (84.1) | 127 (83.6) | 158 (84.5) |
| 173 (85.6) | 86 (85.1) | 87 (86.1) |
|
|
Yes | 54 (15.9) | 25 (16.4) | 29 (15.5) |
| 29 (14.4) | 15 (14.9) | 14 (13.9) |
|
| Grade, n (%) | 0.127 |
| | | >0.999 |
|
|
|
| I +
II | 209 (61.7) | 101 (66.4) | 108 (57.8) |
| 124 (61.4) | 62 (61.4) | 62 (61.4) |
|
| III +
IV | 130 (38.3) | 51 (33.6) | 79 (42.2) |
| 78 (38.6) | 39 (38.6) | 39 (38.6) |
|
| Histology, n
(%) | 0.735 |
| | | 0.592 |
|
|
|
|
Adenocarcinoma | 219 (64.6) | 102 (67.1) | 117 (62.6) |
| 140 (69.3) | 67 (66.3) | 73 (72.3) |
|
|
Squamous cell carcinoma | 110 (32.4) | 46 (30.3) | 64 (34.2) |
| 55 (27.2) | 31 (30.7) | 24 (23.8) |
|
|
Others | 10 (2.9) | 4 (2.6) | 6 (3.2) |
| 7 (3.5) | 3 (3.0) | 4 (4.0) |
|
| Site, n (%) | 0.002 |
| | | 0.753 |
|
|
|
| Right
lower lobe | 113 (33.3) | 52 (34.2) | 61 (32.6) |
| 65 (32.2) | 35 (34.7) | 30 (29.7) |
|
| Right
middle lobe | 44 (13.0) | 30 (19.7) | 14 (7.5) |
| 23 (11.4) | 11 (10.9) | 12 (11.9) |
|
| Right
upper lobe | 182 (53.7) | 70 (46.1) | 112 (59.9) |
| 114 (56.4) | 55 (54.5) | 59 (58.4) |
|
| Visceral pleura
invasion, n (%) | >0.999 |
| | | >0.999 |
|
|
|
| No | 130 (38.3) | 58 (38.2) | 72 (38.5) |
| 71 (35.1) | 35 (34.7) | 36 (35.6) |
|
|
Yes | 209 (61.7) | 94 (61.8) | 115 (61.5) |
| 131 (64.9) | 66 (65.3) | 65 (64.4) |
|
| Bronchial invasion,
n (%) | 0.605 |
| | | >0.999 |
|
|
|
| No | 253 (74.6) | 116 (76.3) | 137 (73.3) |
| 159 (78.7) | 80 (79.2) | 79 (78.2) |
|
|
Yes | 86 (25.4) | 36 (23.7) | 50 (26.7) |
| 43 (21.3) | 21 (20.8) | 22 (21.8) |
|
| Resected lymph node
stations, n (%) | <0.001 |
| | | 0.148 |
|
|
|
|
<6 | 234 (69.0) | 129 (84.9) | 105 (56.1) |
| 150 (74.3) | 80 (79.2) | 70 (69.3) |
|
| ≥6 | 105 (31.0) | 23 (15.1) | 82 (43.9) |
| 52 (25.7) | 21 (20.8) | 31 (30.7) |
|
| Resected lymph node
numbers, n (%) | <0.001 |
| | | 0.472 |
|
|
|
|
<12 | 130 (38.3) | 93 (61.2) | 37 (19.8) |
| 80 (39.6) | 43 (42.6) | 37 (36.6) |
|
|
≥12 | 209 (61.7) | 59 (38.8) | 150 (80.2) |
| 122 (60.4) | 58 (57.4) | 64 (63.4) |
|
| Chemotherapy, n
(%) | 0.847 |
| | | 0.847 |
|
|
|
| No | 288 (85.0) | 128 (84.2) | 160 (85.6) |
| 170 (84.2) | 84 (83.2) | 86 (85.1) |
|
|
Yes | 51 (15.0) | 24 (15.8) | 27 (14.4) | | 32 (15.8) | 17 (16.8) | 15 (14.9) |
|
Prognostic factors
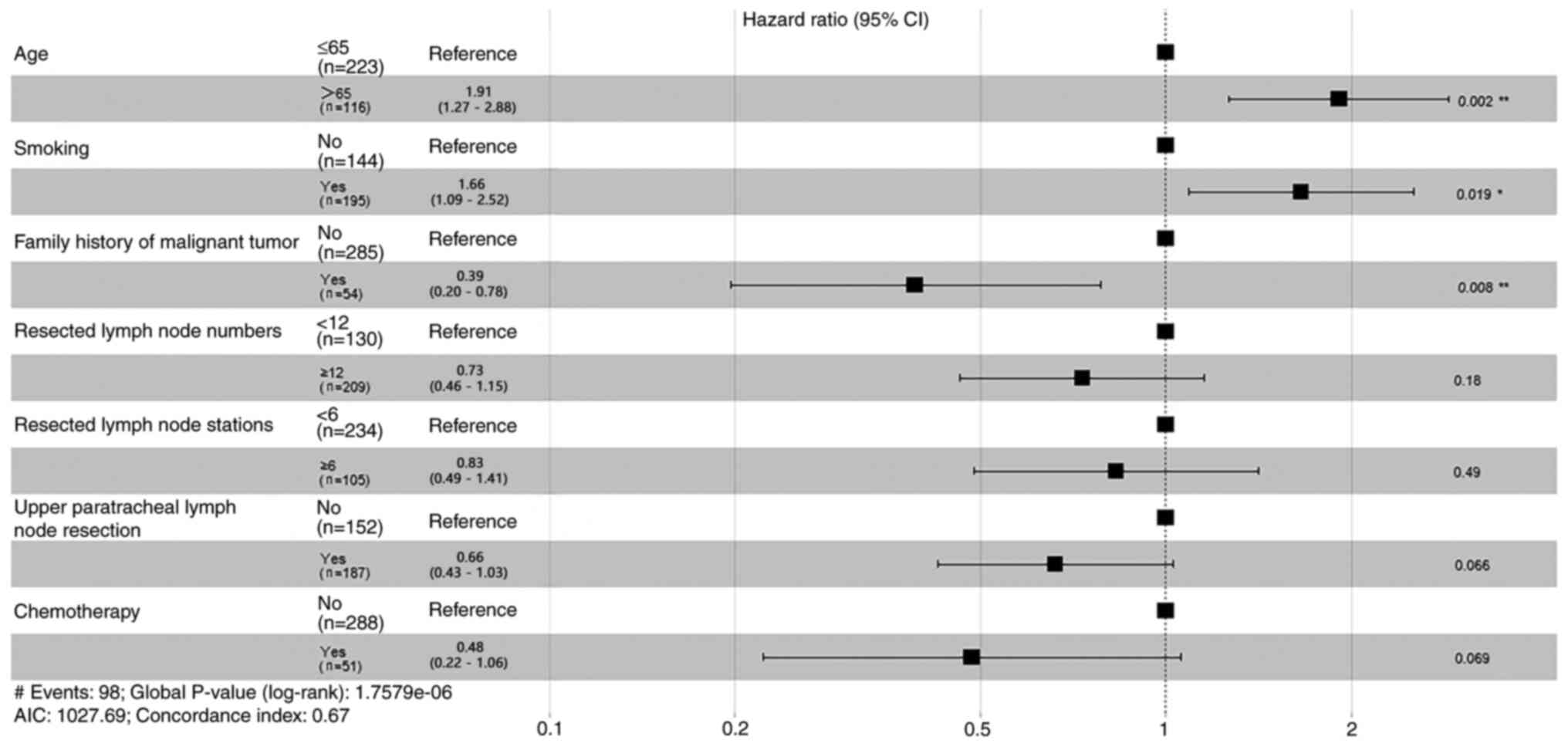
Prior to PSM, Cox proportional hazards regression
models were applied to explore prognostic factors for OS and RFS.
The results of univariate analysis (Table II) and the results of multivariate
analysis were summarized (Figs. 1
and 2). Patient age, smoking
status, family history of malignant tumor, resected lymph node
stations, resected lymph node numbers, upper paratracheal lymph
node resection and chemotherapy treatment were all significant
prognostic indicators for OS (Table
II). These variables were included in the multivariate
analysis; age, smoking and family history of malignant tumor were
statistically significant factors (Fig.
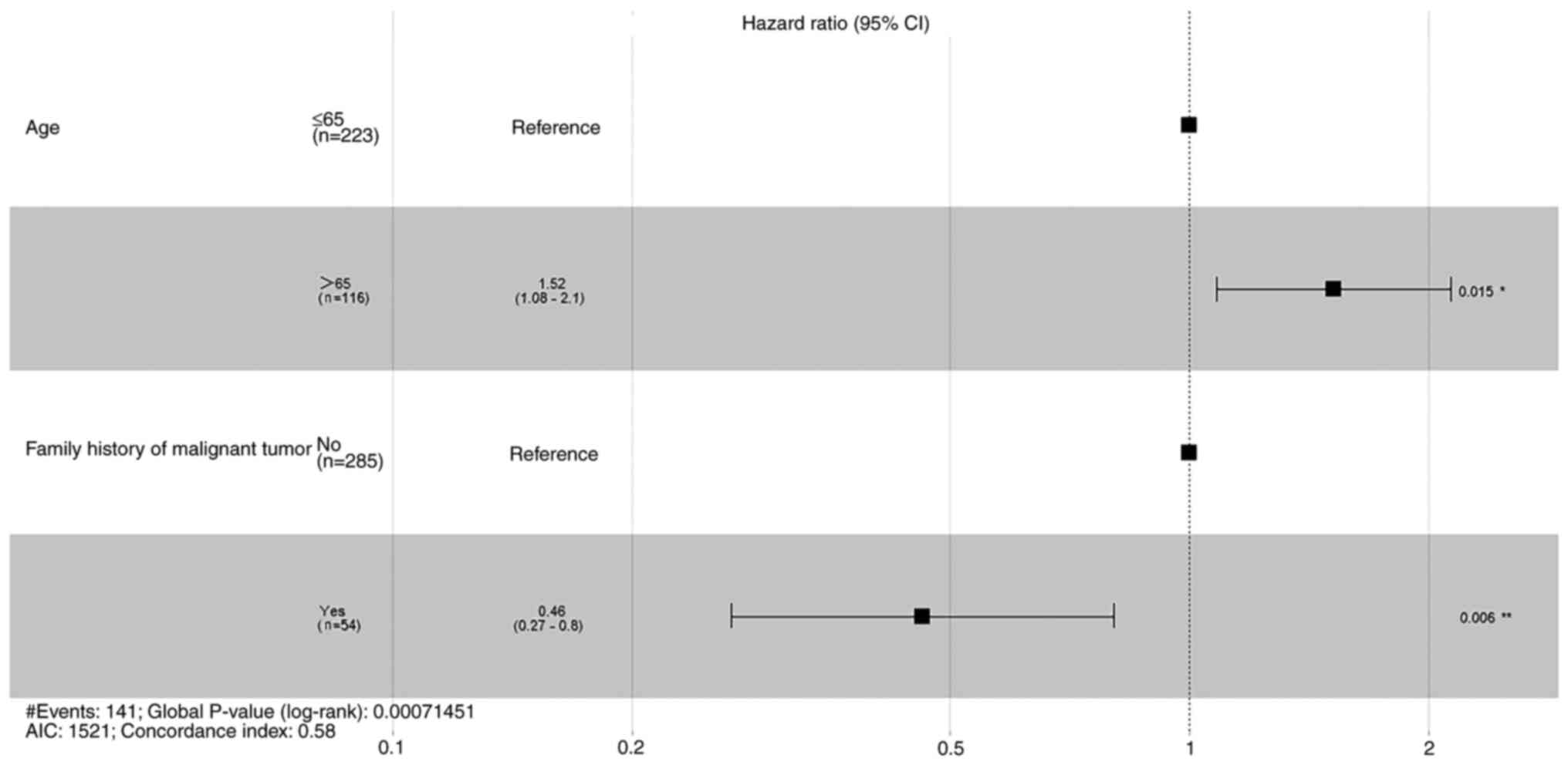
1). Univariate analysis demonstrated that patient age and
family history of malignant tumors were statistically significant
prognostic factors for RFS (Table
II). Multivariate analysis also demonstrated that these factors
were statistically significant. Therefore, patient age and family
history of malignant tumors were both significant independent
prognostic factors for RFS.
 | Table II.Univariate analysis of overall
survival and recurrence free survival before propensity score
matching. |
Table II.
Univariate analysis of overall
survival and recurrence free survival before propensity score
matching.
|
|
| Overall
survival | Recurrence-free
survival |
|---|
|
|
|
|
|
|---|
| Variables | Total, n (%) | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
|
| 0.226 |
| 0.379 |
|
Female | 101 (29.8) | Reference |
| Reference |
|
|
Male | 238 (70.2) | 1.33
(0.84–2.13) |
| 1.18
(0.81–1.72) |
|
| Age, years |
|
| 0.001 |
| 0.018 |
| 65 | 116 (34.2) | Reference |
| Reference |
|
|
≤65 | 223 (65.8) | 0.52
(0.34–0.77) |
| 0.67
(0.48–0.94) |
|
| Smoking |
|
| 0.024 |
| 0.476 |
| No | 144 (42.5) | Reference |
| Reference |
|
|
Yes | 195 (57.5) | 1.62
(1.06–2.46) |
| 1.13
(0.81–1.58) |
|
| Family history of
malignant tumor |
|
| 0.014 |
| 0.006 |
| No | 285 (84.1) | Reference |
| Reference |
|
|
Yes | 54 (15.9) | 0.43
(0.22–0.86) |
| 0.47
(0.27–0.82) |
|
| Grade |
|
| 0.750 |
| 0.866 |
| I +
II | 209 (61.7) | Reference |
| Reference |
|
| III +
IV | 130 (38.3) | 1.07
(0.71–1.61) |
| 1.03
(0.73–1.45) |
|
| Histology |
|
| 0.455 |
| 0.306 |
|
Adenocarcinoma | 219 (64.6) | Reference |
| Reference |
|
|
Others | 10 (2.9) | 0.36
(0.05–2.60) |
| 0.46
(0.11–1.88) |
|
|
Squamous cell carcinoma | 110 (32.4) | 0.85
(0.55–1.30) |
| 0.81
(0.56–1.16) |
|
| Site |
|
| 0.850 |
| 0.395 |
| Right
lower lobe | 113 (33.3) | Reference |
| Reference |
|
| Right
middle lobe | 44 (13.0) | 1.19
(0.63–2.22) |
| 1.17
(0.67–2.05) |
|
| Right
upper lobe | 182 (53.7) | 1.01
(0.65–1.57) |
| 1.29
(0.89–1.87) |
|
| Visceral pleura
invasion |
|
| 0.401 |
| 0.807 |
| No | 130 (38.3) | Reference |
| Reference |
|
|
Yes | 209 (61.7) | 0.84
(0.56–1.26) |
| 1.04
(0.74–1.47) |
|
| Bronchial
invasion |
|
| 0.355 |
| 0.480 |
| No | 253 (74.6) | Reference |
| Reference |
|
|
Yes | 86 (25.4) | 0.79
(0.48–1.30) |
| 0.87
(0.58–1.29) |
|
| Resected lymph node
stations |
|
| 0.034 |
| 0.401 |
|
<6 | 234 (69.0) | Reference |
| Reference |
|
| ≥6 | 105 (31.0) | 0.60
(0.37–0.97) |
| 0.85
(0.59–1.23) |
|
| Resected lymph node
numbers |
|
| 0.007 |
| 0.152 |
|
>12 | 130 (38.3) | Reference |
| Reference |
|
|
≥12 | 209 (61.7) | 0.58
(0.39–0.87) |
| 0.78
(0.56–1.09) |
|
| Upper paratracheal
lymph node resection |
|
| 0.007 |
| 0.271 |
| No | 152 (44.8) | Reference |
| Reference |
|
|
Yes | 187 (55.2) | 0.58
(0.39–0.87) |
| 0.83
(0.60–1.16) |
|
| Chemotherapy |
|
| 0.018 |
| 0.197 |
| No | 288 (85.0) | Reference |
| Reference |
|
|
Yes | 51 (15.0) | 0.41
(0.19–0.88) |
| 0.72
(0.43–1.19) |
|
Survival analysis
Comparisons of the two groups of patients showed
statistically significant differences between tumor site, resected
lymph node stations and the number of resected lymph nodes
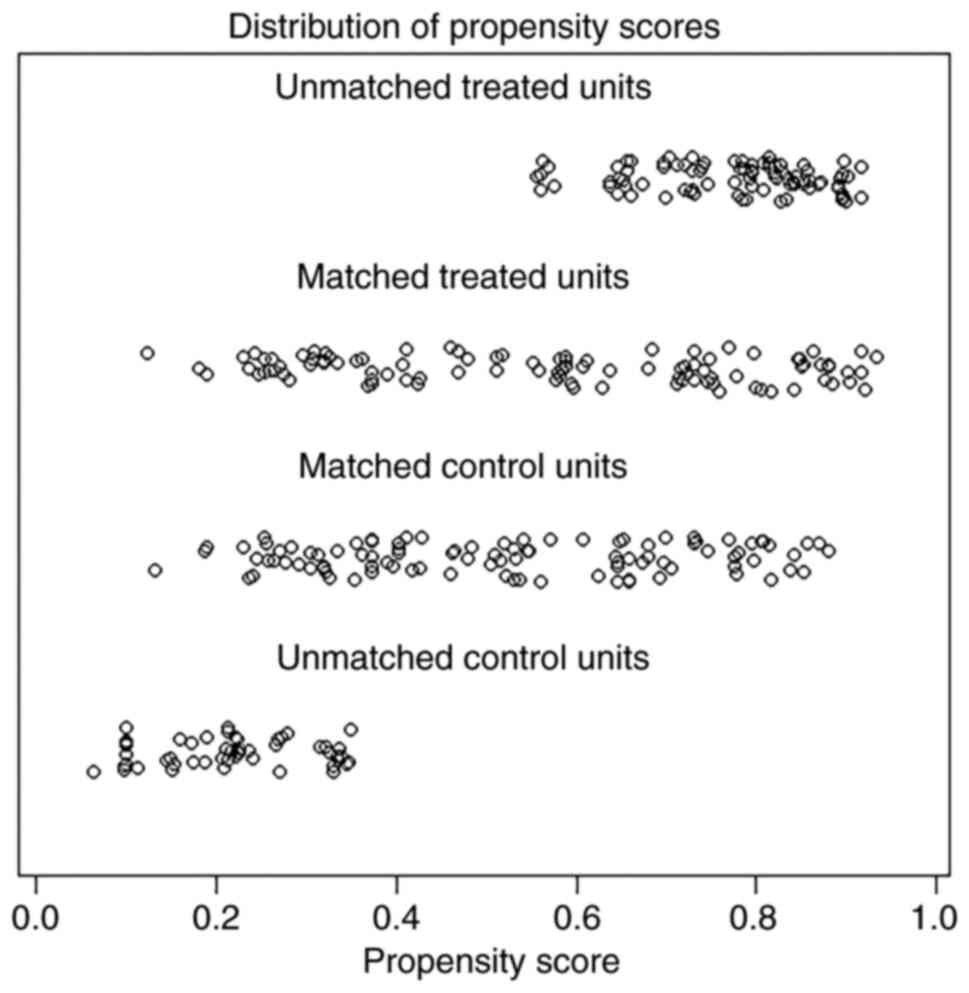
(Table I). A 1-to-1 PSM was
performed to minimize potential bias when comparing the impact of
upper paratracheal lymph node resection on survival. The
distribution of propensity scores were assessed and a perfect match
was obtained (Fig. 3).
Additionally, there were no statistically significant differences
between the two groups of patients in each variable tested
following PSM (Table I).
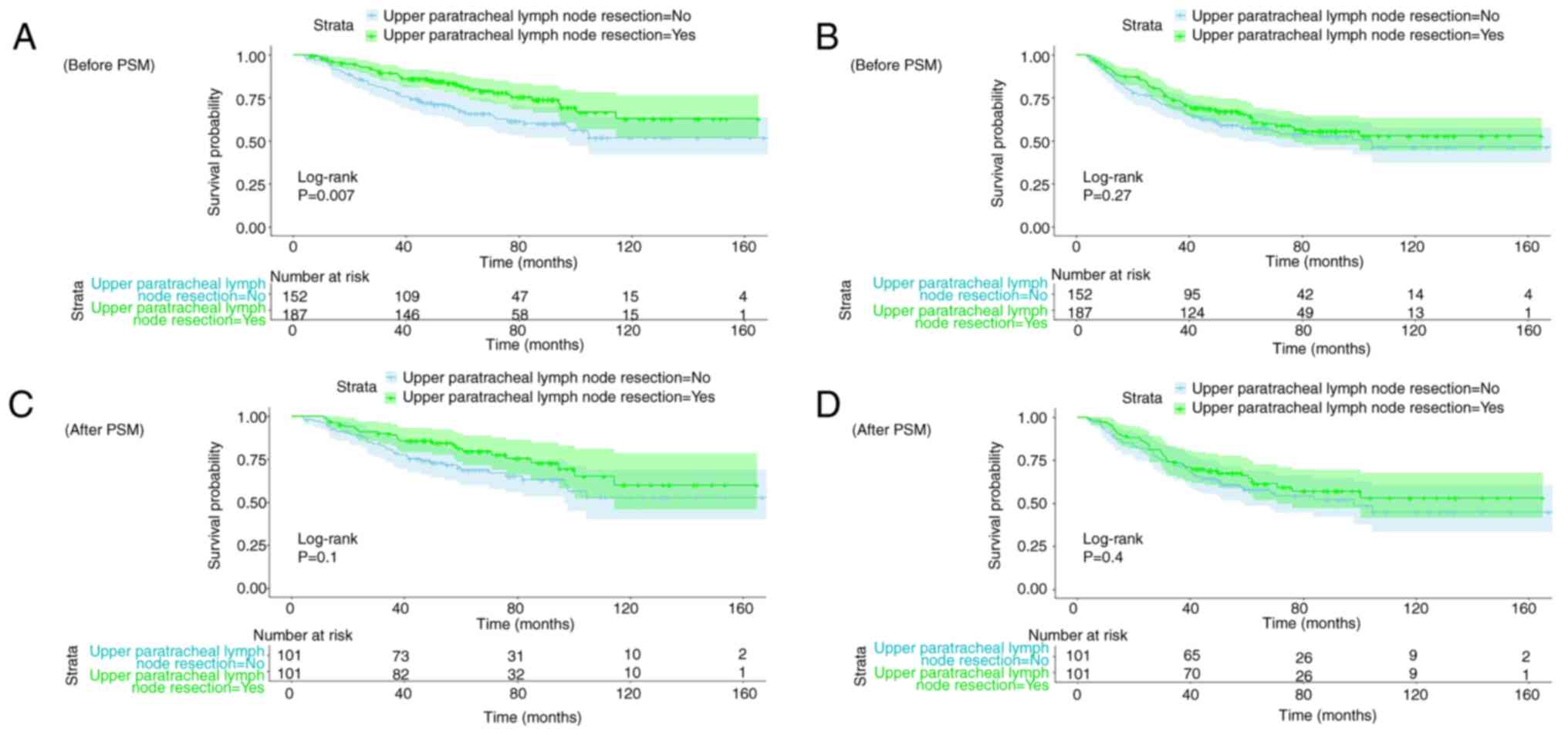
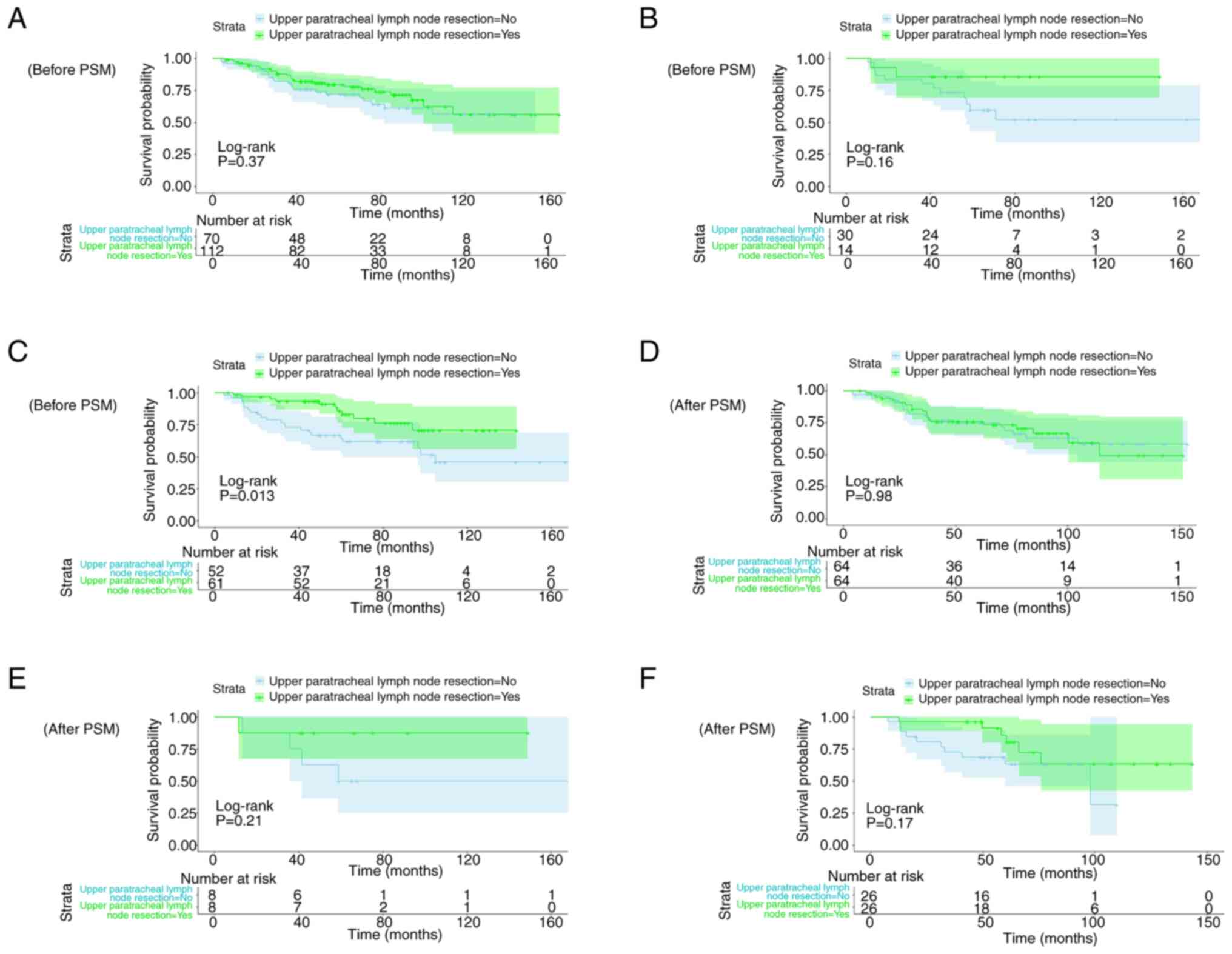
Kaplan-Meier curves of OS (Fig. 4A and
C) and RFS (Fig. 4B and D)
before and after PSM were constructed. Before PSM, upper
paratracheal lymph node resection had a statistically significant
association with OS but not RFS. However, after PSM, the log-rank
test demonstrated that upper paratracheal lymph node resection was
not significantly associated with either OS or RFS.
As the upper paratracheal lymph nodes were
significantly associated with OS in univariate analysis and before
PSM, to further clarify the impact of upper paratracheal lymph node
resection on OS in different lobes, patients were further divided
into three groups (upper, middle and lower lobes). Next,
Kaplan-Meier curves were generated and log-rank tests were
conducted before and after PSM. The impact of upper paratracheal
lymph node resection in different lobes on OS before and after PSM
was assessed (Fig. 5). Before PSM,
for right lower lobe tumors, upper paratracheal lymph node
resection was significantly associated with OS, while after PSM,
there was no significant association between upper paratracheal
lymph node resection and OS, regardless of the tumor lobe
location.
Discussion
The metastasis of tumor cells is an important factor
affecting patient prognosis, and lymph nodes serve a key role in
lung cancer metastasis; therefore, lymph node dissection is
important for the success of lung cancer surgery (20). However, lymph node dissection may
cause potential surgery-related complications, such as lymphatic
fistula, recurrent laryngeal nerve injury and increased blood loss
(21,22). Therefore, the extent of lymph node
dissection for lung cancer treatment, particularly in early stage
NSCLC, has previously been a contentious issue. Although SLND is
currently considered the standard lymph node dissection in lung
cancer treatment, studies have reported differing results. A
prospective clinical trial that included 1,023 patients with early
stage NSCLC showed no significant difference in RFS and OS between
patients who underwent SLND and LNS (13,23).
In patients with early stage NSCLC, several studies reported no
significant difference in survival and recurrence rate between
patients who underwent LSLND and SLND (16,24,25).
The 2R lymph nodes are located above the left
innominate vein, adjacent to the manubrium and the brachiocephalic
artery. Due to its complex anatomical position, it can be difficult
and risky to dissect. Some surgeons may choose not to resect upper
paratracheal lymph nodes when performing right-sided lung cancer
surgery. A number of studies on the lymph node metastasis of NSCLC
suggest that right upper lobe cancer typically metastasizes to
lymph nodes 4R, 10 and 11, that right middle lobe cancer typically
metastasizes to lymph nodes 4R, 7, 10 and 11, and that right lower
lobe cancer typically metastasizes to lymph nodes 7, 10 and 11
(26–28). Therefore, the 2R lymph node is not a
common metastasis zone for right-sided lung cancer. It is
recommended that the upper paratracheal lymph nodes should be
dissected for all right-sided lung cancers in SLND (3,4). The
National Comprehensive Cancer Network guidelines recommend that
station 2, 4, 7, 8 and 9 lymph nodes should be dissected for all
right-sided lung cancers (29).
However, the upper paratracheal lymph nodes should only be
dissected for tumors in the upper and middle lobes in LSLND
(5,7,12,14).
The LNS has no particular requirements for 2R lymph node dissection
(30–32). Due to the proximity of 2R lymph
nodes to the superior vena cava, innominate vein and
brachiocephalic artery, dissecting 2R lymph nodes increases the
risk of large vessel bleeding and increases surgical time due to
the complexity of the operation. It could be suggested that the
necessity of upper paratracheal lymph node dissection for early
stage right lung cancer is currently still controversial; to the
best of our knowledge, there are no reports on the effect of 2R
lymph node dissection on survival outcomes for patients with
right-sided lung cancer, highlighting the importance of the present
study.
The present study included 339 patients with stage
IB right-sided lung cancer. A Cox proportional hazards model was
used to investigate prognostic factors before PSM. In univariate
analysis, upper paratracheal lymph node resection was associated
with OS. However, following multivariate analysis, upper
paratracheal lymph node resection was not an independent prognostic
factor for OS and RFS. To reduce bias, the original data were
divided into two groups according to whether the upper paratracheal
lymph nodes were resected and matched with a 1:1 propensity score.
The Kaplan-Meier method was used for survival analysis. Following
PSM, there was no significant difference in OS and RFS between the
two groups of patients. However, before PSM, the survival curve of
OS demonstrated a significant difference between the two groups of
patients. To clarify the effect of upper paratracheal lymph node
resection on OS in different lobes, survival curves analyzing OS in
different lobes before and after PSM were constructed. There was a
significant difference between the two groups for OS in the right
lower lobe cancer before PSM, but not after PSM. For the upper and
middle lobes of the right lung, no significant difference was
demonstrated between groups before and after PSM. The results of
the present study contradict a number of previous studies that
showed that 2R lymph node dissection was required for right upper
lobe cancer in both SLND and LSLND (5,7,10,12).
A number of previous studies reported that 2R lymph nodes are more
likely to metastasize in right upper lobe cancer (26,28).
The contradictory results obtained in the present study may be
related to the small sample size. In the future, larger sample size
studies on lymph node metastasis in lung cancer are required to
validate the results of the present study.
The present study had a number of limitations.
First, the present study was a single-center retrospective study
and the sample size was relatively small, although PSM was used to
balance variables that may have influenced the results. In
addition, although no sensitivity analysis of PSM was performed in
this study, consistent conclusions were drawn through two different
statistical methods, PSM and Cox regression, which also proves the
robustness of the findings. Sensitivity analyses will be performed
in future research to further validate the outcomes. Second, due to
the inclusion of early lung cancer cases in the present study, a
longer follow-up time is required to obtain OS data, as the present
study cohort was followed from 1999–2009. However, using earlier
data may affect research conclusions due to certain factors, such
as new treatment methods, not being included. Third, since the
present study did not collect information on the surgeon, it could
not be included in the present study. In addition, information on
perioperative management was not included in the study variables
due to the difficulty in quantification. In conclusion, a
multicenter prospective clinical trial with a larger sample size
may validate the findings of the present study in the future. As
research progresses, there could be more accurate lymph node
dissection guidelines for patients with early stage lung cancer in
the future, so that these patients could experience less surgical
trauma and achieve increased survival rates.
Overall, for patients with stage IB NSCLC, upper
paratracheal lymph node resection did not demonstrate a
statistically significant association with OS or RFS. Upper
paratracheal lymph node resection may therefore be unnecessary for
early stage NSCLC, which could potentially reduce unnecessary
surgical trauma and decrease lymph node-related complications.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Beijing Municipal
Administration of Hospitals Incubating Program (grant no.
PX2024057).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FW and XY wrote the manuscript. FW, YH and XY
participated in the design of the study and were involved in data
collection. LZ and SL participated in the design and oversight of
the study. YH and XY participated in the design of the study and
were involved in data collection. FW and XY were involved in
statistical analysis. FW, XY and LZ confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethics Committee of Sun Yat-Sen University Cancer Center and
Beijing Chest Hospital Institutional Review Board (Beijing, China;
approval no. B2018-011). The patients provided written informed
consent to participate in this study.
Patient consent for publication
The patients/participants provided written informed
consent for the publication of any data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Leyn P, Lardinois D, Van Schil P,
Rami-Porta R, Passlick B, Zielinski M, Waller D, Lerut T and Weder
W; ESTS: European trends in preoperative and intraoperative nodal
staging: ESTS guidelines. J Thorac Oncol. 2:357–361. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Howington JA, Blum MG, Chang AC, Balekian
AA and Murthy SC: Treatment of stage I and II non-small cell lung
cancer: Diagnosis and management of lung cancer, 3rd ed: American
college of chest physicians evidence-based clinical practice
guidelines. Chest. 143 (5 Suppl):e278S–e313S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abughararah TZ, Jeong YH, Alabbood F,
Chong Y, Yun JK, Lee GD, Choi S, Kim HR, Kim YH, Kim DK and Park
SI: Lobe-specific lymph node dissection in stage IA non-small-cell
lung cancer: A retrospective cohort study. Eur J Cardiothorac Surg.
59:783–790. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Darling GE: Lymph node assessment in early
stage non-small cell lung cancer lymph node dissection or sampling?
Gen Thorac Cardiovasc Surg. 68:716–724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deng HY, Zhou J, Wang RL, Jiang R, Zhu DX,
Tang XJ and Zhou Q: Lobe-specific lymph node dissection for
clinical early-stage (cIA) peripheral non-small cell lung cancer
patients: What and how? Ann Surg Oncol. 27:472–480. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dezube AR, Mazzola E, Bravo-Iñiguez CE, De
León LE, Rochefort MM, Bueno R, Wiener DC and Jaklitsch MT; Brigham
Large Database Lab, : Analysis of lymph node sampling minimums in
early stage non-small-cell lung cancer. Semin Thorac Cardiovasc
Surg. 33:834–845. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ray MA, Smeltzer MP, Faris NR and
Osarogiagbon RU: Survival after mediastinal node dissection,
systematic sampling, or neither for early stage NSCLC. J Thorac
Oncol. 15:1670–1681. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Qi Z, Cheng D, Hao X, Pu Q and Liu
L: Lobe-specific node dissection can be a suitable alternative to
systematic lymph node dissection in highly selective early-stage
non-small-cell lung cancer patients: A meta-analysis. Ann Thorac
Cardiovasc Surg. 27:143–150. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao D, Zhang R, Yang L, Huang Z, Lin Y,
Wen Y, Zhang X, Wang G, Guo G, Yu X, et al: The independent
prognostic effect of lymph node dissection on patients with stage
IA NSCLC with different T stages. Front Surg. 8:7980462021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adachi H, Sakamaki K, Nishii T, Yamamoto
T, Nagashima T, Ishikawa Y, Ando K, Yamanaka K, Watanabe K,
Kumakiri Y, et al: Lobe-specific lymph node dissection as a
standard procedure in surgery for non-small cell lung cancer: A
propensity score matching study. J Thorac Oncol. 12:85–93. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Darling GE, Allen MS, Decker PA, Ballman
K, Malthaner RA, Inculet RI, Jones DR, McKenna RJ, Landreneau RJ,
Rusch VW and Putnam JB Jr: Randomized trial of mediastinal lymph
node sampling versus complete lymphadenectomy during pulmonary
resection in the patient with N0 or N1 (less than hilar) non-small
cell carcinoma: Results of the American college of surgery oncology
group Z0030 trial. J Thorac Cardiovasc Surg. 141:662–670. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hishida T, Miyaoka E, Yokoi K, Tsuboi M,
Asamura H, Kiura K, Takahashi K, Dosaka-Akita H, Kobayashi H, Date
H, et al: Lobe-specific nodal dissection for clinical stage I and
II NSCLC: Japanese multi-institutional retrospective study using a
propensity score analysis. J Thorac Oncol. 11:1529–1537. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hughes MJ, Chowdhry MF, Woolley SM and
Walker WS: In patients undergoing lung resection for non-small cell
lung cancer, is lymph node dissection or sampling superior?
Interact Cardiovasc Thorac Surg. 13:311–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okada M, Sakamoto T, Yuki T, Mimura T,
Miyoshi K and Tsubota N: Selective mediastinal lymphadenectomy for
clinico-surgical stage I non-small cell lung cancer. Ann Thorac
Surg. 81:1028–1032. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Deng C, Zheng Q, Qian B, Ma J,
Zhang C, Jin Y, Shen X, Zang Y, Guo Y, et al: Selective mediastinal
lymph node dissection strategy for clinical T1N0 invasive lung
cancer: A prospective, multicenter, clinical trial. J Thorac Oncol.
18:931–939. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang C, Zhang Y, Fu F, Deng P and Chen H:
A shift in paradigm: Selective lymph node dissection for minimizing
oversurgery in early stage lung cancer. J Thorac Oncol. 19:25–35.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Detterbeck FC, Chansky K, Groome P,
Bolejack V, Crowley J, Shemanski L, Kennedy C, Krasnik M, Peake M,
Rami-Porta R, et al: The IASLC lung cancer staging project:
Methodology and validation used in the development of proposals for
revision of the stage classification of NSCLC in the forthcoming
(eighth) edition of the TNM classification of lung cancer. J Thorac
Oncol. 11:1433–1446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whitson BA, Groth SS and Maddaus MA:
Surgical assessment and intraoperative management of mediastinal
lymph nodes in non-small cell lung cancer. Ann Thorac Surg.
84:1059–1065. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bollen EC, van Duin CJ, Theunissen PH, vt
Hof-Grootenboer BE and Blijham GH: Mediastinal lymph node
dissection in resected lung cancer: Morbidity and accuracy of
staging. Ann Thorac Surg. 55:961–966. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen-Tu Y, Mao F, Pan Y, Wang W, Zhang L,
Zhang H, Cheng B, Guo H and Wang Z: Lymph node dissection and
survival in patients with early stage nonsmall cell lung cancer: A
10-year cohort study. Medicine (Baltimore). 96:e83562017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng D, Zhou Z, Wang Y, Wang L, Lv W and
Hu J: Lymphadenectomy for clinical early-stage non-small-cell lung
cancer: A systematic review and meta-analysis. Eur J Cardiothorac
Surg. 50:597–604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asamura H, Nakayama H, Kondo H, Tsuchiya R
and Naruke T: Lobe-specific extent of systematic lymph node
dissection for non-small cell lung carcinomas according to a
retrospective study of metastasis and prognosis. J Thorac
Cardiovasc Surg. 117:1102–1111. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adachi H, Maehara T, Nakayama H and Masuda
M: Mediastinal lymph node dissection in surgical treatment for
early stage non-small-cell lung cancer: Lobe-specific or
systematic? J Thorac Dis. 9:2728–2731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martini N, Flehinger BJ, Zaman MB and
Beattie EJ Jr: Results of resection in non-oat cell carcinoma of
the lung with mediastinal lymph node metastases. Ann Surg.
198:386–397. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ichinose Y, Kato H, Koike T, Tsuchiya R,
Fujisawa T, Shimizu N, Watanabe Y, Mitsudomi T, Yoshimura M and
Tsuboi M; Japanese Clinical Oncology Group, : Completely resected
stage IIIA non-small cell lung cancer: The significance of primary
tumor location and N2 station. J Thorac Cardiovasc Surg.
122:803–808. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kotoulas CS, Foroulis CN, Kostikas K,
Konstantinou M, Kalkandi P, Dimadi M, Bouros D and Lioulias A:
Involvement of lymphatic metastatic spread in non-small cell lung
cancer accordingly to the primary cancer location. Lung Cancer.
44:183–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 2.2021. J Natl Compr Canc Netw. 19:254–266. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaseda S, Hangai N, Yamamoto S and Kitano
M: Lobectomy with extended lymph node dissection by video-assisted
thoracic surgery for lung cancer. Surg Endosc. 11:703–706. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gajra A, Newman N, Gamble GP, Kohman LJ
and Graziano SL: Effect of number of lymph nodes sampled on outcome
in patients with stage I non-small-cell lung cancer. J Clin Oncol.
21:1029–1034. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lardinois D, De Leyn P, Van Schil P, Porta
RR, Waller D, Passlick B, Zielinski M, Lerut T and Weder W: ESTS
guidelines for intraoperative lymph node staging in non-small cell
lung cancer. Eur J Cardiothorac Surg. 30:787–792. 2006. View Article : Google Scholar : PubMed/NCBI
|