Introduction
Squamous cell carcinoma (SCC) is commonly seen in
areas covered by squamous epithelium, such as the skin, mouth,
lips, esophagus, cervix and vagina (1–3).
Moreover, some areas, such as the bladder, renal pelvis and
bronchi, which are not covered by squamous epithelium, can form SCC
through squamous epithelial metaplasia (4,5).
Metastatic SCC of the spleen is a rare occurrence, and to the best
of our knowledge, there are no studies reporting gastric SCC with
metastasis to the spleen in humans. Only one case of a 21-year-old
female spotted seal (Phoca largha) with SCC of presumed
pancreatic origin that metastasized to the spleen has previously
been reported (6). The present case
reported a gastric SCC that metastasized to the spleen, and the
link between the cases is that both the pancreas and stomach
surround the spleen anatomically. More commonly observed metastases
to the spleen are from the lung, breast, colorectal organs and
ovary (7,8). The present study reports a case of
splenic metastasis from gastric SCC in a 58-year-old man.
Case report
A 58-year-old man presented to the Emergency
Department of Shaoxing Second Hospital (Shaoxing, China) with left
upper quadrant abdominal pain in December 2023. The patient had
undergone a radical gastrectomy, with the postoperative pathology
showing SCC of the gastric fundus and cardia, with medium-low
differentiation and deep muscle layer invasion in July 2022. A
perigastric lymph node dissection showed no lymph node metastasis.
No invasion of the gastric fundus by esophageal cancer was found
according to the pathology report. The patient received
chemotherapy and radiotherapy after the operation. In December
2022, the patient experienced anemia for >3 months and underwent
a subtotal splenectomy in Zhejiang Tumor Hospital (Hangzhou,
China). The patient had initially developed fatigue, dyspnea,
low-grade fever and abdominal pain since December 2022.
At 2 days prior to the current admission, the
symptoms worsened. On physical examination, the patient was
conscious, with a body temperature of 37.4°C (reference range
36.0–37.0°C), a pulse rate of 103 beats/min (reference range 60–100
beats/min) and a blood pressure of 113/71 mmHg (reference range
90/60-140/90 mmHg). Physical examination revealed splenomegaly and
tenderness over the spleen. Laboratory tests showed the following
results: Leukocytes, 14.1×109/l (reference range
3.5–9.5×109/l); granulocytes, 0.81×109/l
(reference range 0.40–0.75×109/l); hemoglobin, 91 g/l
(reference range 130–175 g/l); and platelets, 461×109/l
(reference range 125–350×109/l). Biochemical examination
showed the following results: Alkaline phosphatase, 267 U/l
(reference range 45–125 U/l); γ-glutamyl transferase, 347 U/l
(reference range 10–60 U/l); albumin, 30.9 g/l (reference range
10–60 g/l); urea, 4.9 mmol/l (reference range 3.1–8.0 mmol/l); and
creatinine, 39 µmol/l (reference range 57–97 µmol/l). The results
of the coagulation spectrum analysis showed a plasma prothrombin
time of 16.2 sec (reference range 10–14 sec) and a D-dimer level of
667 ng/ml (reference range 0–200 ng/ml). The tumor marker
examination showed a carbohydrate antigen 125 level of 98.2 U/ml
(reference range 0–35 U/ml), a SCC antigen (SCCA) level of 2.5
ng/ml (reference range 0.0–1.5 ng/ml) and a ferritin level of
1,080.7 ng/ml (reference range 22.0–273.0 g/ml) (Table I).
 | Table I.Notable laboratory test results at
the admission. |
Table I.
Notable laboratory test results at
the admission.
| Inspection
item | Values | Reference
range |
|---|
| Temperature,
°C | 37.4 | 36.0–37.0 |
| Blood pressure,
mmHg | 113/71 | 90/60-140/90 |
| Heart rate,
beats/min | 103 | 60-100 |
| Respiratory
frequency, breaths/min | 20 | 12-20 |
| WBC count,
×109/l | 14.1 | 3.5–9.5 |
| SCCA, ng/ml | 2.5 | 0.0–1.5 |
| Blood glucose
levels, mmol/l | 3.13 | 3.89–6.11 |
| Granulocytes,
×109/l | 0.81 | 0.40–0.75 |
| Hemoglobin,
g/l | 91 | 130-175 |
| Platelets,
×109/l | 461 | 125-350 |
| Alkaline
phosphatase, U/l | 267 | 45-125 |
| γ-glutamyl
transferase, U/l | 347 | 10-60 |
| Albumin, g/l | 30.9 | 40.0–55.0 |
| Urea, mmol/l | 4.9 | 3.1–8.0 |
| Creatinine,
µmol/l | 39 | 57-97 |
| Ferritin,
ng/ml | 1,080.7 | 22.0–273.0 |
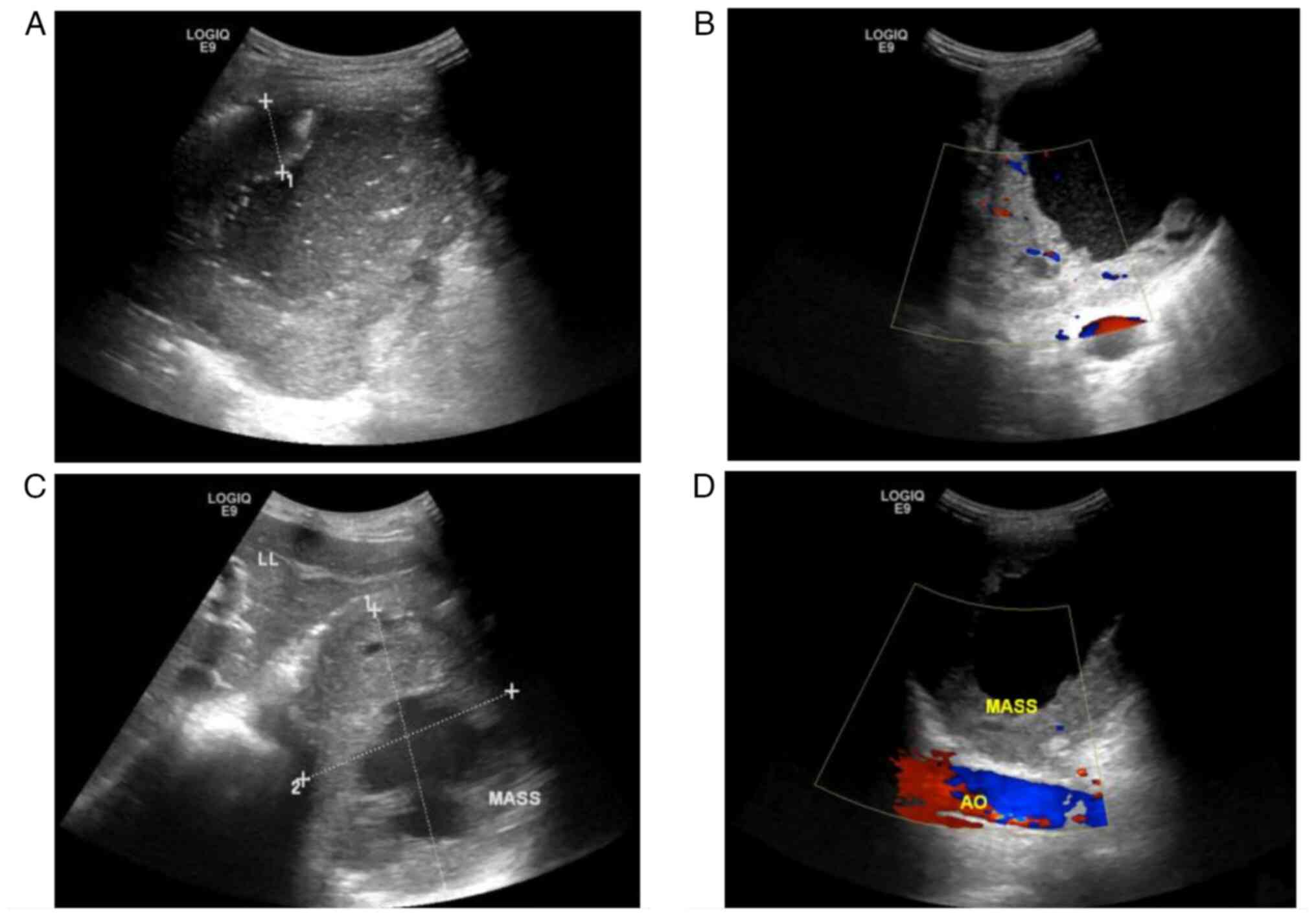
The patient underwent an immediate emergency
ultrasound (US) examination (Fig.
1), which detected a 116×91-mm cystic and solid mixed-echo mass
in the residual spleen under the left diaphragm. The mass was
surrounded by a 22.5-mm thick hypoechoic rough wall (Fig. 1A). Blood flow was detected in the
surrounding wall (Fig. 1B). An
ultrasonic median longitudinal abdominal scan found that the mass
was close to the left lobe of the liver (Fig. 1C), and a xiphoid downward oblique
scan found that it was positioned anterior to the abdominal aorta
(Fig. 1D). The patient was
initially diagnosed with a splenic abscess and treated with
anti-inflammatory therapy (cefotaxime 2.00 g diluted in 100 ml 0.9%
sodium chloride injection, intravenous drip twice a day), but the
abdominal pain was not relieved.
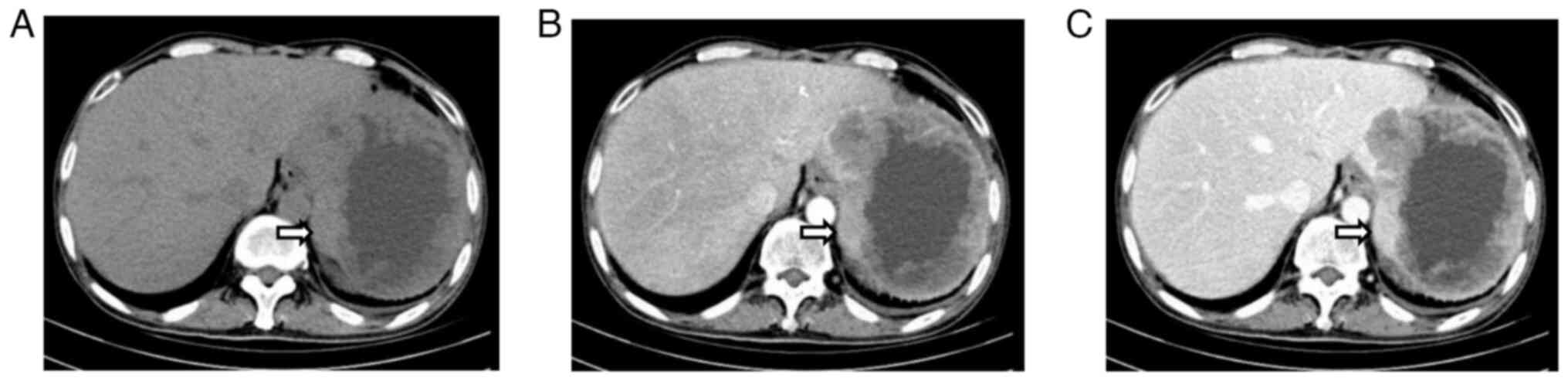
A subsequent computed tomography (CT) scan confirmed
the presence of an abdominal mass (Fig.
2). An abdominal plain CT scan detected an irregular
soft-tissue mass measuring 125.9×101.3 mm with an irregularly
thickened wall in the residual spleen (Fig. 2A). The mass showed uneven
enhancement in the arterial phase (Fig.
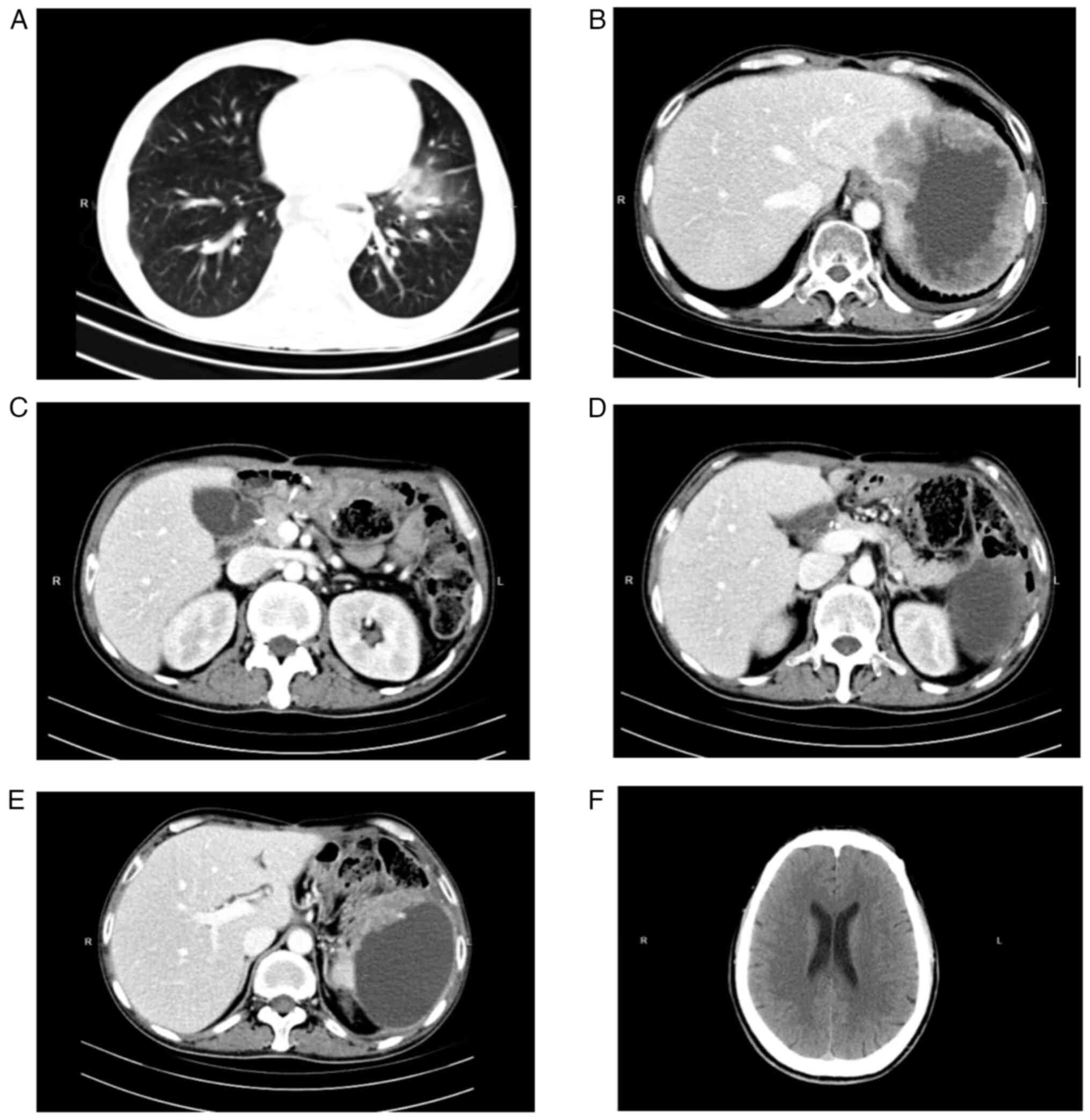
2B) and no obvious regression in the delayed phase (Fig. 2C). Imaging examinations, such as CT
scans (Fig. 3) of the brain, lungs
and abdomen, together with US of the liver, gallbladder and
pancreas were performed. There were no metastases in the lungs
(Fig. 3A), the liver (Fig. 3B), the lymph nodes in the upper
abdomen (Fig. 3C), the pancreas
(Fig. 3D and E) or the brain
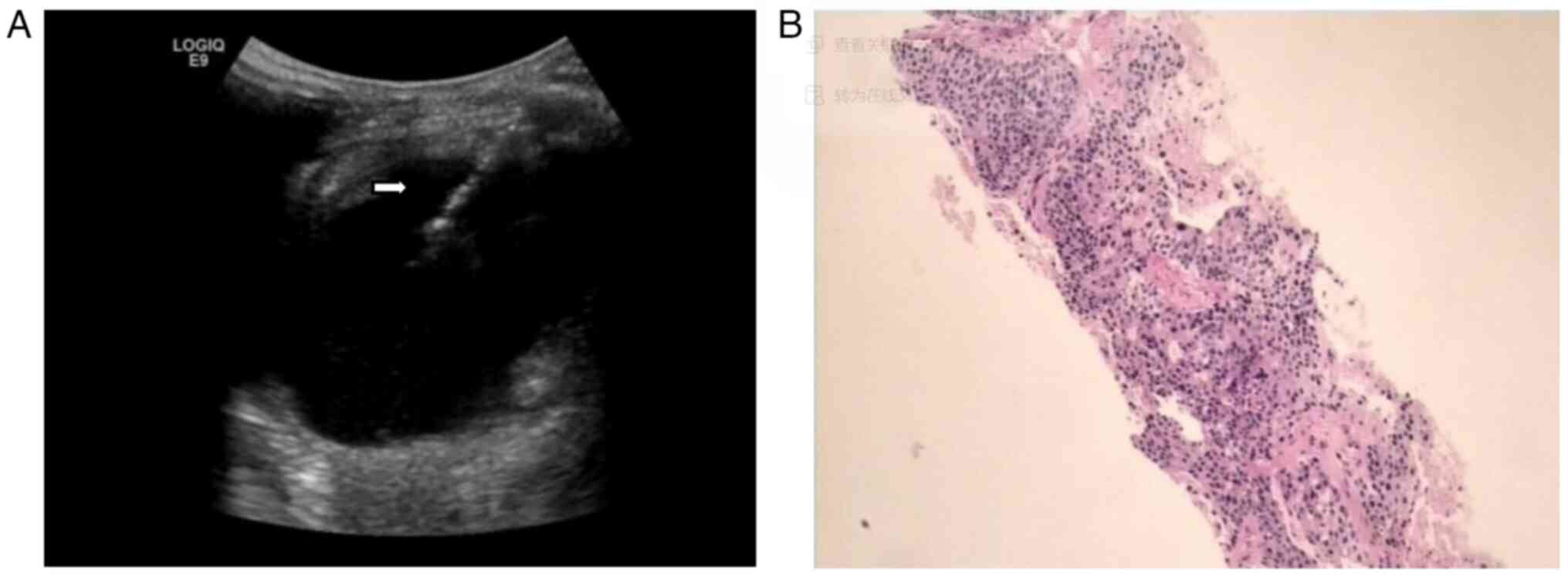
(Fig. 3F). A bedside percutaneous
splenic mass biopsy was next performed under US guidance (Fig. 4).
The patient was placed lying on their right side,
with routine disinfection, ultrasonic point selection and
positioning. Local anesthesia was applied using 2% lidocaine
hydrochloride and an incision was made into the skin
subcutaneously. Two percutaneous splenic mass biopsies were taken
from different regions of the mass (Fig. 4A). The biopsy samples were
immediately fixed with 3 ml 10% neutral buffered formalin fixative
at 25°C for 12 h and sent for pathological examination.
Percutaneous catheterization and drainage with an 8F pigtail needle
was performed in the cystic region of the mass, and dark brown
liquid was drawn out. There was no obvious bleeding during the
operation and the patient's vital signs were stable. A total of 667
ml of dark brown liquid was drained out. The results of the
drainage fluid culture showed hemolytic Staphylococcus. The
pathological diagnosis of the biopsy was provided by the Department
of Pathology, Shaoxing Second Hospital. The pathological
examination was performed on 3-µm sections, which were stained with
and hematoxylin and eosin at 70°C for 30 min and were observed
under a light microscope (magnification, ×50). The result (Fig. 4B) showed that the splenic puncture
tissue came from a SCC metastasis of medium-low differentiation,
composed of sheets of large polymorphic cells exhibiting
intercellular bridges and keratin pearls.
Resection of the residual spleen was recommended;
however, the patient abandoned treatment after the pathological
diagnosis due to a lack of confidence in the procedure and due to
the cost of the required hospital stay post-surgery, and
subsequently died in June 2024.
Discussion
SCC, also known as epidermal carcinoma, is a
malignant tumor that occurs in epidermal cells and areas covered by
squamous epithelium. However, although there is no squamous
epithelium in some regions of the body, such as the bronchus and
the urethra, squamous metaplasia can also occur to form SCC
(9). Most gastric cancers are
adenocarcinoma, and primary SCC is rare. Primary SCC is formed by
squamous metaplasia of the human gastric mucosal epithelium based
on chronic inflammation (10).
To the best of our knowledge, gastric SCC metastasis
to the spleen has not been reported in humans in the literature.
Splenic metastatic SCC should be differentiated from other
space-occupying lesions of the spleen (11). Splenic hemangioma (12) is the most common benign splenic
tumor in the spleen. Pathologically, it is classified into
cavernous hemangioma and capillary hemangioma, and its ultrasonic
manifestations are as a mostly circular or quasicircular
hyperechoic or hypoechoic mass in the spleen. The boundary of a
splenic hemangioma is clear, the shape is regular, and the edge is
not smooth. Some hemangiomas have blood necrosis and cystic
degeneration inside. Color Doppler flow imaging mostly shows no
blood flow signal inside. Splenic lymphoma (13) is the most common malignant tumor in
the spleen. Ultrasonic manifestations consist of homogeneous
enlargement of the spleen, a smooth capsule, multiple hypoechoic
nodules and a few internal ethmoid reticular or solitary
heterogeneous echo nodules. Splenic metastatic SCC should also be
differentiated from splenic abscesses (14,15).
Patients with splenic metastatic SCC usually have a normal body
temperature. In addition, the lesions of splenic metastatic SCC are
usually a regular shape, have clear boundaries and a hard texture,
which is not easily deformed under the pressure of the probe in
imaging. These patients often have history of primary SCC in other
areas and no risk factors, and anti-inflammatory treatment is
ineffective. Furthermore, the tumor marker SCCA is often elevated.
By contrast, patients with splenic abscesses usually have elevated
body temperature and white blood cell count. The lesions of splenic
abscesses mostly have unclear boundaries with a soft texture, which
is easy to deform under the pressure of the probe in imaging. These
patients often have risk factors for abscesses, such as diabetes.
In these patients, anti-inflammatory treatment is effective, no
primary SCC in other areas can be found and the tumor marker SCCA
is often normal. Notably, pathological biopsy is the gold standard
for differential diagnosis. Tumor cells will be detected in splenic
metastatic SCC lesions, whereas they will not be detected in
splenic abscess lesions (Table
II).
 | Table II.Differentiation of splenic metastatic
SCC from splenic abscess. |
Table II.
Differentiation of splenic metastatic
SCC from splenic abscess.
| Factor | Splenic metastatic
SCC | Splenic
abscess |
|---|
| Body
temperature | Mostly normal | Mostly
elevated |
| Morphology of
lesions | Regular shape,
clear boundaries | Unclear boundaries,
rupture to the outside |
| Imaging
characteristics | Hard texture, not
easily deformed | Soft texture, easy
to deform under compression |
| Presence of risk
factors for abscess | None | Mostly (for
example, diabetes) |
| Blood WBC
count | Mostly normal | Mostly
elevated |
| Anti-inflammatory
treatment | Ineffective | Effective |
| Primary SCC in
other areas | History of primary
SCC | Not applicable |
| Pathological
biopsy | Can detect tumor
cells | Tumor cells cannot
be detected |
| Anti-inflammatory
effect of antibiotics | Ineffective | Effective |
| Tumor marker
SCCA | Mostly
elevated | Mostly normal |
The metastasis of tumors comprises of a series of
cascade reactions that include tumor cells detaching from the
primary lesion, invading the extracellular matrix, invading nearby
blood vessels and lymphatic vessels, entering the circulatory
system, and adhering and interacting with platelets and target
endothelial cells to penetrate the vascular system and form new
clones at the target site, effectively evading immune clearance in
the body (16,17). To date, stem cell-like cells, also
known as tumor stem cells, have been isolated from a small number
of solid tumors. Although these cells are few in number, they have
high tumorigenicity and may be the root cause of tumor occurrence,
development and metastasis. The risk factors for tumor metastasis
include high malignancy, delayed treatment, poor physical
condition, genetic factors (such as gene mutations) and tumor
microenvironment factors (such as immune escape, angiogenesis and
extracellular matrix degradation) (18,19).
There are several methods of tumor metastasis, including direct
infiltration, hematogenous metastasis, lymphatic metastasis and
implantation metastasis (20).
Splenic resistance to metastatic seeding and the rarity of splenic
metastases are probably due to its high density of immune system
cells, and high concentration of angiogenesis inhibition factors,
such as tissue inhibitor of matrix metalloproteinases and P53
(21). Kinoshita et al
(22) reported that constant
splenic sinus blood flow decreased the adhesion of cancer cells to
the spleen, and that the presence of a humoral substance in the
spleen destroyed cancer cells and inhibited the splenic metastasis.
Lee et al (23) reported
that most splenic metastases appeared within the parenchyma,
reflecting probable hematogenous spread. In cases with SCC
metastasis to the spleen from the lung, a preferentially higher
blood flow to the spleen via the left lung, in comparison to that
of the right lung, may explain the increased rate of splenic
metastasis from left-sided lung cancer (8). In this case, the cancer cells are most
likely to invade the blood vessel wall and enter the bloodstream,
spreading through the circulatory system vessels, such as the
gastric artery and portal vein system, to reach the splenic
tissues. The spleen is similar to the liver, lungs and bones in
that it has an abundant blood supply and immune cells in the
microenvironment.
The clinical manifestations of splenic metastatic
SCC are often atypical, with abdominal discomfort being the most
common symptom (24–27). In the present case, upon
examination, the patient presented with abdominal discomfort and a
low-grade fever, while the other vital signs were stable. The
patient was also found to have a large abdominal mass. Initial
treatment included anti-inflammatory therapy and other symptomatic
treatments. However, there was no improvement in the patient's
condition. Patients with splenic abscesses are often observed to
have concurrent diabetes, and anti-inflammatory therapy is
effective (27–32). However, in the current case, the
patient presented with normal blood glucose levels and no other
high-risk factors for splenic abscess development. Although
standardized anti-inflammatory therapy was administered, the
dyspnea did not markedly improve. As a result, the patient was
initially diagnosed with metastatic SCC to the spleen. The patient
underwent a pathological biopsy for diagnosis, which is the gold
standard for this disease.
Splenic metastatic SCC, non-Hodgkin's lymphoma,
abscess and other lesions can all lead to splenic masses (8,33). CT
and US are commonly used for the diagnosis of splenic metastatic
SCC (31). The main manifestations
of CT and US are a regular shape, clear boundaries, a hard texture
and a mass that is not easily deformed under probe compression. US
can intuitively show the metastasis of SCC, the spleen, the
diaphragm and other organs of the left upper abdomen, and the
relationship among them. Metastasis of splenic SCC has relatively
characteristic manifestations in US and color Doppler flow imaging,
including a regular shape, smooth boundary, internal ischemia and
necrosis, fine and dense light spots floating in the necrotic
cavity, a thick capsule wall, an irregular inner wall and a few
blood flow signals on color Doppler flow imaging. These
characteristics make it easy to distinguish SCC metastasis from
other splenic space occupying lesions.
SCCA is a glycoprotein that was first identified in
cervical SCC tissue; it is a highly specific tumor marker that
exists in the cytoplasm of SCC cells such as the uterus, cervix,
esophagus, lungs, and head and neck, but exhibits low sensitivity
(34–37). SCCA is involved in both epithelial
differentiation in normal squamous epithelial cells and the growth
of SCC cells (37). The serum
concentration of SCCA in normal subjects is <2 ng/ml. The
detection of SCCA serum concentration level has high specificity
for the diagnosis of SCC and can be used as an auxiliary diagnostic
indicator and prognostic monitoring indicator of SCC, such as oral
and cervical SCC, for efficacy, recurrence and metastasis (38,39).
The patient in the present case exhibited increased SCCA at the
current admission, while it was normal when the radical gastrectomy
for gastric SCC was completed. After the subtotal splenectomy, the
patient had SCC metastasis to the residual spleen, and the cancer
cells in the splenic mass grew rapidly and released SCCA into the
serum, which elevated the serum SCCA level.
Metastatic SCC in the spleen is rare, and few cases
have been reported (7,40,40–42).
In the present study, the patient's overall condition was
relatively poor, with anemia, fever and fatigue for a period of
time. In addition, the spleen is an organ adjacent to the stomach,
which may have helped the quick and easy metastasis of the SCC
cells. Lastly, the spleen is an organ with an abundant blood
supply, which may have helped the SCC cells to survive and grow
(43).
Cases with metastases to the spleen from other
organs should undergo individualized therapy. A splenectomy can be
performed for those individuals with controlled local recurrence
that is associated with enlargement of the splenic mass despite
chemotherapy, as suggested in the present case, or for those with a
mass large enough that a risk of rupture exists (44). Only one previous case (7) reported intra-abdominal hemorrhaging
and was diagnosed with a splenic rupture. Coil embolization to the
splenic artery was performed and adjuvant chemotherapy was
administered after the surgery. The other reported cases were
mainly administered chemotherapy (45–47).
Diagnosis of metastases to the spleen from other organs can be
achieved via a splenectomy or using less invasive methods such as
fine-needle aspiration or transcutaneous biopsy, as in the present
case, with a low complication rate (<2%) and a high rate of
success (37,39). Pathological confirmation of the
lesions is achieved through histological analysis (46,47).
In conclusion, metastasis of residual splenic SCC is
clinically rare and easily confused with a splenic abscess. The
final diagnosis requires histopathological confirmation. The
present case report highlights the role of interventional radiology
in managing splenic occupations. Ultrasonography has positive
advantages in the localization and qualitative judgment of splenic
space-occupying lesions. Specific manifestations of US can be used
to distinguish splenic SCC metastasis from other diseases. In
addition, in splenic space-occupying diseases, especially those
with increased SCCA levels, the possibility of SCC metastasis
should be considered, which has clinical relevance to the
diagnosis, treatment and prognosis of the patients. The clinical
diagnosis and treatment process in the present case highlighted the
need for doctors to consider the possibility of metastatic splenic
SCC in patients diagnosed with splenic abscess when
anti-inflammatory therapy is ineffective, and when there are no
causative factors for the splenic abscess, such as diabetes or a
high blood glucose level.
Acknowledgements
Not applicable.
Funding
The study was funded by the Training Project of New Talents in
Medicine of Zhejiang Province (grant no. Zhejiang Health and Family
Planning Commission 2017-102).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LW contributed to the conception of the study, as
well as to the literature search for related studies. SZ designed
the study, and was involved in the writing of the manuscript. Both
authors read and approved the final version of the manuscript. LW
and SZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written consent for the
publication of the present report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cabral SC, Gouveia E, Nunes H and Pereira
PM: Use of preoperative immunotherapy in locally advanced
unresectable cutaneous squamous cell carcinoma: A case report. Case
Rep Oncol. 17:1109–1114. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iguchi R and Inoue K: Distal preservation
and retrograde resection of the anterior vaginal wall in female
robot-assisted radical cystectomy. Asian J Endosc Surg.
18:e133992025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piersiala K, Hjalmarsson E, Lagebro V,
Farrajota Neves da Silva P, Bark R, Elliot A, Marklund L, Margolin
G, Georén SK and Cardell LO: Prognostic value of T regulatory cells
and immune checkpoints expression in tumor-draining lymph nodes for
oral squamous cell carcinoma. Front Immunol. 15:14554262024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grizzi F, Chiriva-Internati M, Miranda E,
Zaharie R, Hajjar NA, Zaharie F, Del Arco CD, Fernández-Aceñero MJ,
Bresalier RS and Moiş E: Sperm protein antigen 17 and Sperm
flagellar 1 cancer testis antigens are expressed in a rare case of
ciliated foregut cyst of the common hepatic duct. Pathol Res Pract.
247:1545462023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samardali M, Shanti I, Samardaly J and
Alhusari L: An interesting finding of xanthogranulomatous
pyelonephritis revealed concomitant incidental squamous cell
carcinoma (SCC) of the renal pelvis. Cureus.
16:e633832024.PubMed/NCBI
|
|
6
|
Tsuka T, Kozu T, Sunden Y, Morita T,
Okamoto Y, Yamashita M, Osaki T, Amaha T, Ito N, Murahata Y and
Imagawa T: Detection of squamous cell carcinoma of presumed
pancreatic origin and its metastasis in a spotted seal (Phoca
largha) using ultrasonography and computed tomography. J Vet
Med Sci. 84:373–377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka K, Iwata T, Yoshida S, Nishii K,
Matsui Y, Sugiyama T, Itami M and Iizasa T: A surgical case of
synchronous solitary splenic metastasis from lung squamous cell
carcinoma: Report of a case and review of the literature. Gen
Thorac Cardiovasc Surg. 68:866–870. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dias AR, Pinto RA, Ravanini JN, Lupinacci
RM, Cecconello I and Ribeiro U Jr: Isolated splenic metastasis from
lung squamous cell carcinoma. World J Surg Oncol. 10:242012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valentine EH: Squamous metaplasia of the
bronchus; a study of metaplastic changes occurring in the
epithelium of the major bronchi in cancerous and noncancerous
cases. Cancer. 10:272–279. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DE Miranda Neto AA, Marques SB, Baba ER,
Yamazaki K, Ribeiro IB and DE Moura EGH: Extensive squamous
metaplasia of the stomach. Arq Gastroenterol. 57:335–336. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou X, Chai C, Li S and Li Y: Metastatic
squamous cell carcinoma of the spleen: A case report. Chin J Gen
Surg. 17:852002.(In Chinese).
|
|
12
|
Vancauwenberghe T, Snoeckx A,
Vanbeckevoort D, Dymarkowski S and Vanhoenacker FM: Imaging of the
spleen: What the clinician needs to know. Singapore Med J.
56:133–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Findeisen H, Görg C, Winter H, Trenker C,
Dietrich CF, Alhyari A, Eilsberger F and Safai Zadeh E: B-mode
ultrasound and contrast-enhanced ultrasound for the detection of
splenic involvement in Hodgkin lymphoma: A retrospective analysis
of 112 patients. Ultraschall Med. 45:484–492. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Staudt MD, Langdon KD, Hammond RR and
Lownie SP: Incisional seeding of metastatic squamous cell carcinoma
following carotid endarterectomy: An unusual case of an unknown
primary cancer presenting as a presumed neck abscess. Oper
Neurosurg (Hagerstown). 17:202–207. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Govindaraj S, Prakash C, Ananthamurthy A
and Govindaraj S: Unique diagnostic challenge in surgery: Hepatic
abscess versus malignancy. BMJ Case Rep. 15:e2504892022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Zhang J, Guo X, Huang J, Lou Z,
Zhao X, Lin Q, Li X, You J and Luo L: Enhancing tumor immunotherapy
via photodynamic therapy with a cascade reaction of reactive oxygen
species and sustaining nutrient supply. J Control Release.
S0168-3659(23)00687-9. 2023.(Epub ahead of print). View Article : Google Scholar
|
|
17
|
Wang X, Yang Y, Wang P, Li Q, Gao W, Sun
Y, Tian G, Zhang G and Xiao J: Oxygen self-supplying
nanoradiosensitizer activates cGAS-STING pathway to enhance
radioimmunotherapy of triple negative breast cancer. J Control
Release. 376:794–805. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozdemir Y, Cag M, Colak E, Coskun N,
Basgoz N, Sarici H, Kaan D, Dogan M, Deniz K, Inanc M and Ozkul Y:
The effect of gene mutations on metastasis and overall survival in
metastatic and nonmetastatic colon cancers. Asian Pac J Cancer
Prev. 22:3839–3846. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu SY, Fu T, Jiang YZ and Shao ZM: Natural
killer cells in cancer biology and therapy. Mol Cancer. 19:1202020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Gu Q, Sun P, Yang L, Zhang X, Lu B
and Ni Q: NSG2: A promising prognostic marker shaping the immune
landscape of breast cancer. Front Immunol. 15:14874472024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Compérat E, Bardier-Dupas A, Camparo P,
Capron F and Charlotte F: Splenic metastases: Clinicopathologic
presentation, differential diagnosis, and pathogenesis. Arch Pathol
Lab Med. 131:965–969. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kinoshita A, Nakano M, Fukuda M, Kasai T,
Suyama N, Inoue K, Nakata T, Shigematsu K, Oka M and Hara K:
Splenic metastasis from lung cancer. Neth J Med. 47:219–223. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SS, Morgenstern L, Phillips EH, Hiatt
JR and Margulies DR: Splenectomy for splenic metastases: A changing
clinical spectrum. Am Surg. 66:837–840. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agostinis P, Cappello D, Riccardi N,
Michelutti T, Orsaria M, Zerbato V and Di Bella S: A 25-year-old
woman with long-lasting abdominal pain and spleen abscess. Clin
Infect Dis. 77:795–798. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Walsh M, Wasko N, Simms AJ and Hodges J:
Splenic abscess caused by Cutibacterium acnes in a patient with
multiple tooth extractions. BMJ Case Rep. 16:e2504862023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aktas A, Kayaalp C, Gundogan E, Gunes O
and Pıskın T: Percutaneous drainage of a splenic abscess via
laparoscopic trocar in a kidney transplant patient. Exp Clin
Transplant. 20:613–615. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Almasaud AD and Sulaiman IF: The
successful resolution of a large splenic abscess with six years of
follow-up and without recurrence. Cureus. 16:e530422024.PubMed/NCBI
|
|
28
|
Sritharan S, Lau PS, Manan K and Mohan A:
Case report: Aseptic splenic abscesses in childhood-onset systemic
lupus erythematosus. Front Pediatr. 11:12145512023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SB, Kim JH, Park SJ, Park CI and Kim
CW: Complications and recovery patterns after blunt splenic injury:
Recommended duration and follow-up methods. Ulus Travma Acil
Cerrahi Derg. 29:297–303. 2023.PubMed/NCBI
|
|
30
|
Muralitharan J, Nagarajan V and
Ravichandran U: Mumps and splenic abscess: Is there a link? Cureus.
14:e331952022.PubMed/NCBI
|
|
31
|
Hadi IAN, Boleng PP and Mengga HB:
Surgical management of splenic abscess complicated by pleural
effusion in rural setting: A case report from rural Indonesia. Int
J Surg Case Rep. 89:1065792021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Masaki C, Matsushita K, Inoue T, Shima H,
Chikakiyo M, Yamada M, Shirono R, Tashiro M, Tada H, Takamatsu N,
et al: Splenic abscess diagnosed following relapsing sterile
peritonitis in a peritoneal dialysis patient: A case report with
literature review. Semin Dial. 34:245–251. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Z, Cai Z, Tao B and Jin Q: Full-length
spleen tyrosine kinase inhibits the invasion and metastasis of
human laryngeal squamous cell carcinoma. Int J Clin Exp Pathol.
8:15786–15793. 2015.PubMed/NCBI
|
|
34
|
Khan ZA, Aisha M, Farkouh CS, Tango T,
Bereka L, Ul Ain H, Belay NF, Farkouh M and Ali Khan Q: The
diagnostic dilemma of splenic non-Hodgkin's lymphoma and splenic
abscess: A narrative review. Cureus. 14:e319442022.PubMed/NCBI
|
|
35
|
Hong R, Luo L, Xu X, Huang K, Zhao H,
Huang L, Wang Y and Li F: The treatment response evaluation through
the combination of contrast-enhanced ultrasound and squamous cell
carcinoma antigen in cervical cancer. Quant Imaging Med Surg.
14:7587–7599. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu M, Xie X, Cai L, Liu D and Sun P:
Preoperative scoring system for the prediction of risk of lymph
node metastasis in cervical cancer. Sci Rep. 14:238602024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zeng Z, Wang W, Yan J, Liu D, Zhang F and
Hu K: Weekly image guidance in patients with cervical cancer
treated with intensity-modulated radiation therapy: Results of a
large cohort study. Cancer Med. 13:e702692024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim YT, Yoon BS, Kim JW, Kim SH, Kwon JY
and Kim JH: Pretreatment levels of serum squamous cell carcinoma
antigen and urine polyamines in women with squamous cell carcinoma
of the cervix. Int J Gynaecol Obstet. 91:47–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qiu J, Zhang Z, Liu J, Zhao Y, Li Y, Tang
Z, Li L, Tian Y and Tian H: Nomograms to predict tumor regression
grade (TRG) and ypTNM staging in patients with locally advanced
esophageal cancer receiving neoadjuvant therapy. World J Surg
Oncol. 22:1982024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Haque MM, Kadir MI, Badruddoza SM, Alom MA
and Kamal MM: Metastatic squamous cell carcinoma of spleen: A case
report. Mymensingh Med J. 22:410–412. 2013.PubMed/NCBI
|
|
41
|
Carvalho L, Azevedo I, Salgado L, Ferreira
ES, Henrique R, de Carvalho RG and Vieira E: Squamous cell
carcinoma of the cervix metastatic to the spleen-case report.
Gynecol Oncol. 67:107–110. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pang LC: Solitary recurrent metastasis of
squamous cell carcinoma of the uterine cervix in the spleen: Case
report. South Med J. 97:301–304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Krasnov A, Afanasyev S, Hansen MHS, Bou M,
Sveen L and Dessen JE: Smoltification of atlantic salmon (Salmo
salar L.) is associated with enhanced traffic and renewal of B cell
repertoire. Genes (Basel). 15:12202024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chloros D, Bitzikas G, Kakoura M,
Chatzikostas G, Makridis C and Tsitouridis I: Solitary splenic
metastasis of squamous lung cancer: A case report. Cases J.
2:90912009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mitsimponas N, Mitsogianni M, Crespo F,
Hartmann KA, Diederich S, Klosterhalfen B and Giagounidis A:
Isolated splenic metastasis from non-small-cell lung cancer: A case
report and review of the literature. Case Rep Oncol. 10:638–643.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guzman Rojas P, Parikh J, Vishnubhotla P
and Oharriz JJ: Primary gastric squamous cell carcinoma. Cureus.
10:e23892018.PubMed/NCBI
|
|
47
|
Meng Y, Zhang J, Wang H, Zhang Y, Sun R,
Zhang Z, Gao F, Huang C and Zhang S: Poorer prognosis in patients
with advanced gastric squamous cell carcinoma compared with
adenocarcinoma of the stomach: Case report. Medicine (Baltimore).
96:e92242017. View Article : Google Scholar : PubMed/NCBI
|