Introduction
Kidney clear cell carcinoma (KIRC) is a malignant
tumor with high morbidity and an increasing mortality rate.
Notably, the incidence rate of KIRC has been increasing by 1–2%
annually, which is due to factors such as unhealthy lifestyle
habits, delays in diagnosis and treatment, and the development of
resistance to treatment (1).
Treatments frequently used for KIRC include surgical treatment and
chemotherapy (2); however, given
that most individuals diagnosed with KIRC are in the advanced
stages of the disease, chemotherapy is vital in the treatment
regimen (3). Doxorubicin is used to
treat a variety of malignancies (4); however, its therapeutic applicability
is limited because it can cause adverse effects and the development
of drug resistance (5).
Consequently, it is critical to enhance the ability of doxorubicin
to target cancer cells and reduce the side effects associated with
its use.
Elevated levels of ErbB3-binding protein 1 (EBP1)
have been reported in numerous types of cancer, in which this
protein is associated with a poor prognosis, treatment resistance
and tumor development (6).
Hypoxia-inducible factor-1α (HIF-1α) also plays a pivotal role as a
transcription factor in the onset of cancer and serves as a target
for the advancement of personalized cancer therapies (7). HIF-1α is crucial in controlling the
expression of a number of downstream target genes, which influences
the progression of various types of cancer (8). The knockdown of EBP1 in
hepatocellular carcinoma cells has been shown to lead to reduced
p38 mitogen-activated protein kinase (p38MAPK) phosphorylation and
the downregulation of HIF-1α expression, thereby affecting the
proliferation and migration ability of the cells (9). In addition, EBP1 has been demonstrated
to promote the occurrence and development of KIRC by regulating the
p38/HIF-1α signaling pathway (10).
p38MAPK is essential in various aspects of cell
proliferation, differentiation, death and embryogenesis, in
addition to tumorigenesis (11). A
novel antitumor drug targeting p38MAPK, named ralimetinib, is
currently being developed and has entered clinical trials for the
treatment of ovarian cancer (12).
However, p38MAPK inhibitors are not effective when administered
alone, and so are often used in combination with other drugs
(13). Doxorubicin penetrates cells
and regulates a variety of target genes to prevent cancer cell
growth and proliferation and to trigger apoptosis (14). The effects of doxorubicin include
the promotion of reactive oxygen species formation (15), DNA modification and topoisomerase II
inhibition (4). Previous research
has also shown that the administration of doxorubicin alters the
phosphorylation level of p38 in gastric cancer cells (16), with a time-dependent inhibitory
effect. Furthermore, the p38MAPK-specific inhibitor SB203580 has
been demonstrated to increase the sensitivity of gastric cancer
cells to doxorubicin (17). It
could be hypothesized that the inhibition of p38MAPK expression may
be associated with increased sensitivity of KIRC to doxorubicin
chemotherapy. In addition, we further hypothesize that the EBP1
protein may regulate the sensitivity of KIRC cells to doxorubicin
via the p38MAPK/HIF-1α pathway.
Therefore, the present study knocked down
EBP1 in two human KIRC cell lies, 786-O and 769-P, with the
aim of investigating the effect of EBP1 on
doxorubicin-induced apoptosis and exploring the underlying
mechanism of action.
Materials and methods
Bioinformatics
The UALCAN (https://ualcan.path.uab.edu/index.html) and GEPIA
(gepia.cancer-pku.cn) databases were used to evaluate the
expression of EBP1 in the tissues of patients with KIRC and
its association with tumor grade, stage and overall survival. In
GEPIA, the ‘match TCGA normal and GTEx data’ setting was used.
Cell culture and processing
Preliminary data were obtained demonstrating that
EBP1 is expressed at high levels in 786-O and 769-P human KIRC cell
lines. Specifically, the mining and analysis of relevant public
databases revealed that the expression level of EBP1 in these two
cell lines was significantly higher compared with that in most
other renal cancer cell lines, a trend consistently validated
across multiple bioinformatics platforms, including the Cancer Cell
Line Encyclopedia database (https://sites.broadinstitute.org/ccle/), The Cancer
Genome Atlas (TCGA; https://www.cancer.gov/tcga; TCGA-KIRC project) and
Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/). The GSE15641
(18) dataset was downloaded from
the GEO.
Therefore, these cell lines were chosen for
analysis. The 786-O and 769-P cell lines were sourced from the Cell
Bank of Type Culture Collection of The Chinese Academy of Sciences.
They were cultured at 37°C in an atmosphere consisting of 5%
CO2 and 95% air, using RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) enriched with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), along with 100 µg/ml
streptomycin and 100 U/ml penicillin. The 786-O and 769-P cells
were seeded into culture dishes, and the medium was supplemented
with doxorubicin (3 µM; cat. no. E2516; Selleck Chemicals) and the
p38MAPK inhibitor SB203580 (10 µM; cat. no. S1076; Selleck
Chemicals). All cells were incubated at 37°C with 5% CO2
in a humidified incubator for 48 h to ensure effective drug
treatment.
Cell Counting Kit-3 (CCK-8) assay
In order to assess the effect of doxorubicin on the
viability of the 786-O and 769-P cell lines, 200 µl complete growth
medium was added to the cells in each well of a 96-well plate, and
the cells were then incubated for 24 or 48 h with 3 µM doxorubicin.
The CCK-8 assay kit from Shanghai Biyuntian Biotechnology Co., Ltd.
China was then used to analyze the cells. In summary, each sample
was treated with 10 µl CCK-8 solution and incubated at 37°C for 2
h. The optical density at 450 nm was then assessed using a
microtiter plate reader, and cell survival curves were plotted.
Colony formation assay
A colony formation assay was performed to assess the
ability of doxorubicin to inhibit 786-O and 769-P cell
proliferation. The cells were inoculated into 6-well plates at a
density of 3×103 cells/well and the plates were
incubated for 1 week. After aspirating the liquid from the 6-well
plate, the cells were fixed with 4% paraformaldehyde for 15 min at
room temperature and washed with PBS. Subsequently, the cells were
stained with Giemsa (Beijing Solarbio Science & Technology Co.,
Ltd.) at room temperature for 20 min, followed by two washes with
PBS. Colonies were defined as containing ≥50 cells and were
quantified using ImageJ bundled with Java8 64-bit (National
Institutes of Health).
Migration assay
The scratch test was performed to determine the
effect of doxorubicin on the migration of 786-O and 769-P cells.
The 769-P and 786-O cells were seeded in 6-well plates at a density
of 1×106 cells/well and grown to 90% confluence.
Subsequently, the cells were cultured overnight in serum-free
medium. In brief, a sanitized grid scale was placed over the
confluent cells in a 6-well plate, and a 1,000-µl pipette tip was
applied from the top to bottom of the grid to create a scratch in
the cells. The cells were washed twice with PBS and then cultured
in serum-free medium containing doxorubicin for 48 h. Using a Nikon
Eclipse Ti-S/L100 inverted phase contrast fluorescence Microscope
(Nikon Corporation), images of cell migration into the wound were
captured at 0, 24 and 48 h at the same lesion site. Wound
measurement was performed using ImageJ, and statistical analysis
was conducted using GraphPad Prism 10.0 (Dotmatics). Each
experiment was repeated at least three times.
TUNEL assay of apoptosis
Apoptosis was assessed using the DeadEnd™
Fluorometric TUNEL System (Promega Corporation) and fluorescence
microscopy. Six-well plates were seeded with 1×105 786-O
or 769-P cells/well, and the cells were cultured at 37°C in an
incubator with 5% CO2. The cells were treated with
doxorubicin for 24 h before analysis using the TUNEL assay kit.
First, the cells were fixed with 4% paraformaldehyde at room
temperature for 15 min, washed three times with PBS, then incubated
with rTdT enzyme incubation buffer at 37°C for 70 min in the dark.
Subsequently, the cells were stained with DAPI solution (1 ml/well)
at room temperature for 15 min. The samples were then immediately
examined under a fluorescence microscope and images were captured
(magnification, ×20). Quantification was performed from ~10 fields
of view using ImageJ, and statistical analysis was conducted using
GraphPad Prism 10.0. Each experiment was repeated at least three
times.
Western blotting
Cells were lysed and then washed twice with ice-cold
PBS (pH 7.4). Protein lysates were extracted using RIPA buffer
(Beijing Solarbio Science & Technology Co., Ltd.), and nuclear
and cytoplasmic extracts were prepared using the Nuclear and
Cytoplasmic Extraction Reagents Kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
total protein concentration of the lysate was determined using a
BCA kit (Thermo Fisher Scientific, Inc.). The protein lysates (30
µg/lane) were then separated by SDS-PAGE on 8–10% gels to ensure
effective protein separation and accurate quantification. The
proteins were subsequently transferred to a PVDF membrane. The
membranes were blocked using 5% skimmed milk (Yuanda Mountain Dairy
Co.) in TBS with 10% Tween-20 (TBS-T) for 2 h at room temperature.
After blocking, the membranes were incubated with the following
primary antibodies in TBS-T overnight at 4°C: EBP1 (cat. no.
sc-393114; Santa Cruz Biotechnology, Inc.), phosphorylated (p-)p38
(cat. no. sc-166182; Santa Cruz Biotechnology, Inc.), p38 (cat. no.
sc-81621; Santa Cruz Biotechnology, Inc.), HIF-1α (cat. no.
sc-53546; Santa Cruz Biotechnology, Inc.), Bcl-2 (cat. no. sc-7382;
Santa Cruz Biotechnology, Inc.), Bax (cat. no. sc-70407; Santa Cruz
Biotechnology, Inc.), cleaved-caspase 3 (cat. no. sc-373730; Santa
Cruz Biotechnology, Inc.), caspase 3 (cat. no. sc-56053; Santa Cruz
Biotechnology, Inc.) and β-actin (cat. no. sc-47778; Santa Cruz
Biotechnology, Inc.). The primary antibodies were diluted 1:1,000
in antibody diluent buffer (Beyotime Institute of Biotechnology).
Subsequently, the membranes were incubated with HRP-conjugated goat
anti-mouse secondary antibodies (1:5,000; cat. no. A0216; Beyotime
Institute of Biotechnology) at 4°C for 2 h. The proteins were
visualized using the ECL Western Blotting Substrate system (Beijing
Solarbio Science & Technology Co., Ltd.). Protein grayscale
values were measured using ImageJ, and statistical analysis was
performed using GraphPad Prism 10.0, with each experiment repeated
≥3 times.
Lentiviral transduction
The 786-O and 769-P cells were transduced with two
different short hairpin RNA (shRNA) lentiviral vectors targeting
EBP1 (sh-EBP1*1 and sh-EBP1*2) and two different shRNA lentiviral
vector negative controls (sh-NC*1 and sh-NC*2; Shanghai GeneChem
Co., Ltd.). The sh-EBP1 target sequence was
5′-ACCAGCATTTCGGTAAATA-3′, and the sh-EBP1 sequences were as
follows: sh-EBP1*1 (PA2G4-RNAi(23071–1)-a),
5′-CCGGCCACCAGCATTTCGGTAAATACTCGAGTATTTACCGAAATGCTGGTGGTTTTTG-3′
and sh-EBP1*2 (PA2G4-RNAi(23071–1)-b),
5′-AATTCAAAAACCACCAGCATTTCGGTAAATACTCGAGTATTTACCGAAATGCTGGTGG-3′.
The control gene insertion sequence was 5′-TTCTCCGAACGTGTCACGT-3′,
and the sequences of the shRNA controls were as follows: sh-NC*1,
5′-CCGGTTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAATTTTTG-3′ and
sh-NC*2,
5′-AATTCAAAAATTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAA-3′. An
EBP1 overexpression lentiviral vector (pHS-AVC-LY124) and
control vector (pHS-AVC-ZQ328) were purchased from SyngenTech Co.,
Ltd. Lentiviral infection was carried out immediately after virus
preparation. The virus titers were as follows: shNC1,
8×108 TU/ml; shNC2, 8×108 TU/ml; sh1
(shEBP1*1), 7×108 TU/ml; sh2 (shEBP1*2),
7×108 TU/ml. The multiplicity of infection (MOI) values
in 786-O cells for shNC1, shNC2, sh1 and sh2 were 5, 10, 10 and 15,
respectively. In 769-P cells, the MOI values for shNC1, shNC2, sh1
and sh2 were 10, 15, 15 and 20, respectively. The lentiviruses were
added to the cells and incubated for 24 h at 37°C for gene
transduction. Prior to adding the virus to the cells, it was mixed
with 2 µg/ml polybrene to enhance infection efficiency. Selection
was performed with 1 g/ml puromycin to obtain successfully
transduced cells. Transduction efficiency was assessed by western
blotting immediately.
Statistical analysis
Images were analyzed using ImageJ software.
Differences between two groups were compared by unpaired Student's
t-test while differences among multiple groups were compared using
one-way or two-way ANOVA with Tukey's post hoc analysis.
Statistical evaluation of the results was performed using GraphPad
Prism 10.0. Experimental results are presented as the mean ± SD.
Each experiment was replicated ≥3 times. P<0.05 was considered
to indicate a statistically significant difference.
Results
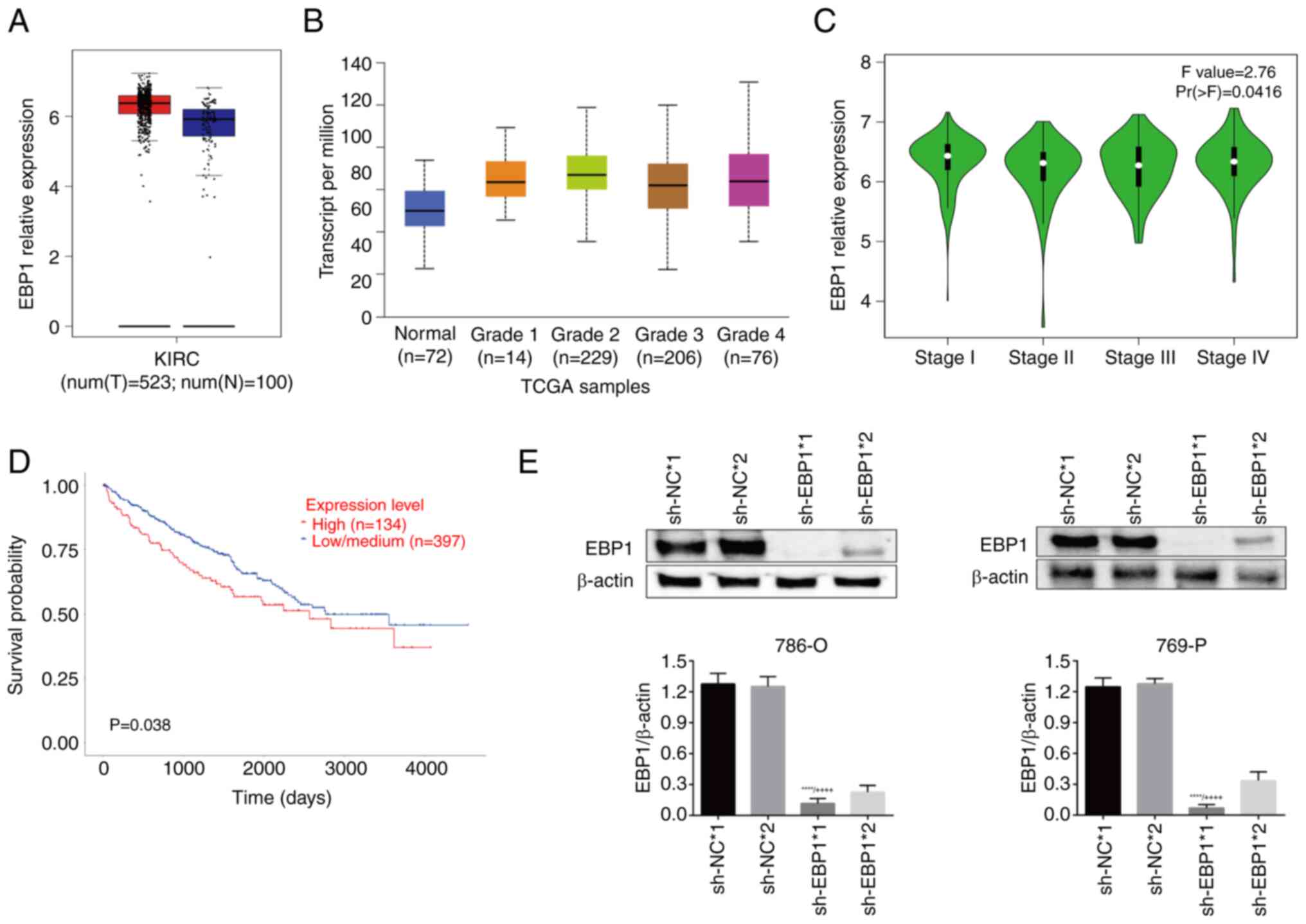
EBP1 is highly expressed in KIRC
The UALCAN and GEPIA databases were used to evaluate
the expression of EBP1 in KIRC. The results showed a marked
upregulation in EBP1 expression in KIRC tissues compared
with normal tissues (Fig. 1A). The
association between KIRC status and EBP1 expression was then
assessed. The results revealed that EBP1 expression was
highly associated with tumor grade (Fig. 1B). Moreover, the analysis of
pathological staging results demonstrated that EBP1
expression was associated with the stage of KIRC (Fig. 1C). In addition, survival curves
(Fig. 1D) showed that the survival
rate of patients with high EBP1 expression was significantly
lower compared with that of patients with low EBP1
expression, indicating a robust association between EBP1
expression and patient survival.
To clarify the regulatory role of EBP1 in
KIRC, EBP1 in 786-O and 769-P cells was stably knocked down,
and the transduction effect was verified using western blotting.
The results revealed that sh-EBP1*1 had a stronger knockdown effect
than sh-EBP*2. Therefore, sh-EBP1*1 and sh-NC*1 were selected for
use in subsequent experiments (Fig.
1E).
Knockdown of EBP1 decreases viability
and proliferation and increases apoptosis in KIRC cells treated
with doxorubicin
CCK-8 assay results showed that 786-O and 769-P
cells in the sh-EBP1 group grew more slowly than those in the sh-NC
group, with a significant difference detected between the groups on
days 4–6; following doxorubicin treatment, the viability of cells
with EBP1 knockdown was significantly lower than that of the
cells transduced with sh-NC (Fig.
2A). The scratch assay demonstrated that 786-O and 769-P cells
in the sh-EBP1 group had a significantly lower capacity to repair
scratches after receiving doxorubicin treatment for 24 and 48 h in
comparison with those in the sh-NC group (Fig. 2B), indicating that the inhibitory
effect of doxorubicin on the migratory ability of KIRC cells was
stronger following the knockdown of EBP1. The results of the
colony formation assay showed that the number of colonies formed by
doxorubicin-treated 786-O and 769-P cells was lower in the sh-EBP1
group than in the sh-NC group (Fig.
2C). This result illustrates that the inhibition of KIRC cell
proliferation by doxorubicin was enhanced following the knockdown
of EBP1. TUNEL staining showed that few 786-O and 769-P
cells underwent apoptosis in the sh-NC group treated with
doxorubicin, and the proportion of apoptotic cells was
significantly higher in the sh-EBP1 group (Fig. 2D). These results indicate that
EBP1 knockdown promotes doxorubicin-induced cell death in
KIRC cells, suggesting that the anticancer effects of doxorubicin
may be potentiated by EBP1 knockdown.
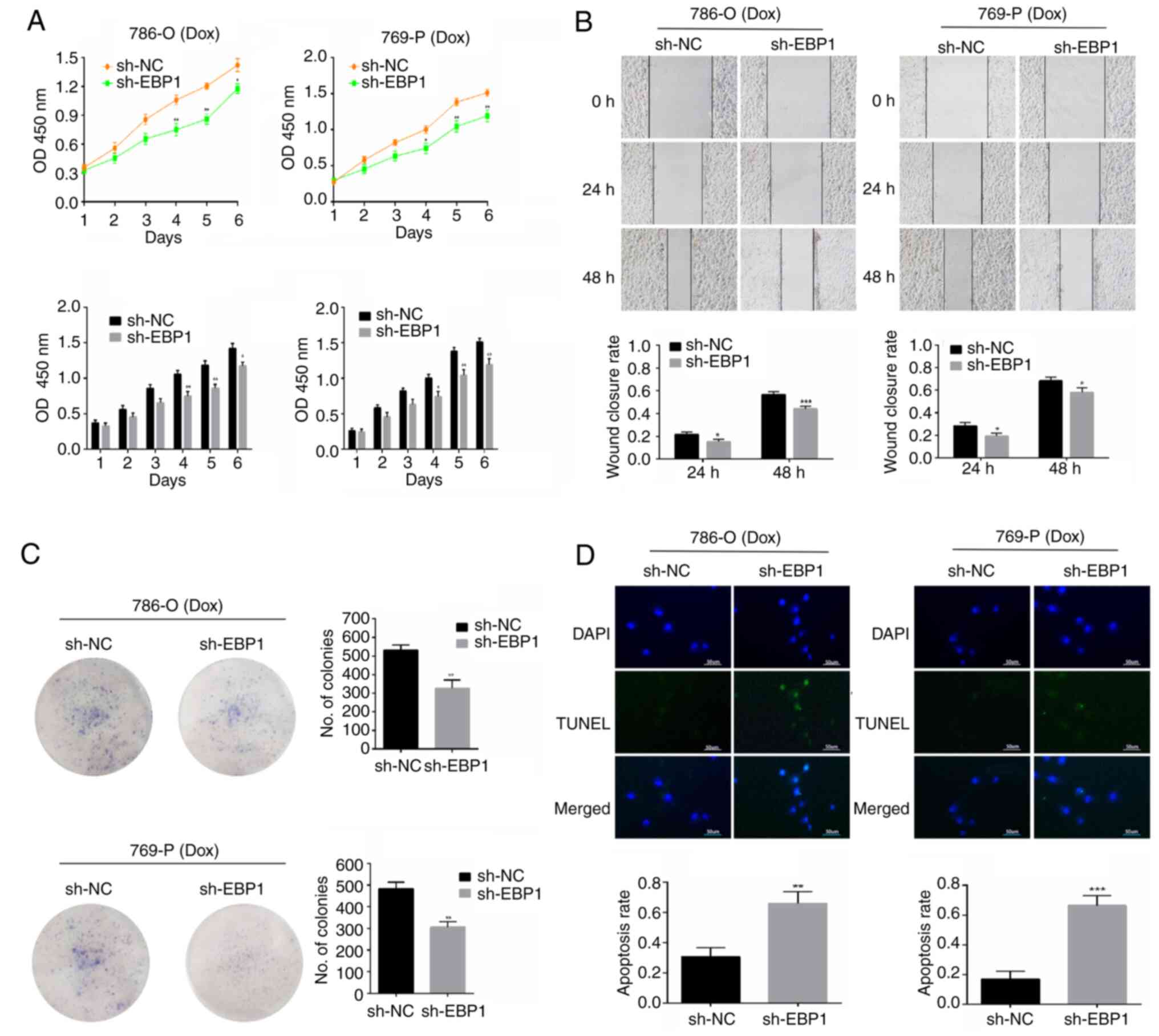 | Figure 2.Knockdown of EBP1 decreases
the viability and proliferation of doxorubicin-treated KIRC cells.
(A) Cell Counting Kit-8 assay, (B) scratch assay (magnification,
×10), (C) colony formation assay and (D) TUNEL assay (scale bar, 50
µm) showing the effect of EBP1 knockdown on the
proliferation, migration and apoptosis of KIRC cells treated with
doxorubicin. Data are presented as the mean ± SD, n=3. *P<0.05,
**P<0.01 and ***P<0.001 vs. sh-NC. EBP1, ErbB3-binding
protein; KIRC, kidney clear cell carcinoma; Dox, doxorubicin; OD450
nm, optical density at 450 nm; sh-NC, short hairpin negative
control; sh-EBP1, short hairpin EBP1. |
Doxorubicin acts by inhibiting p38MAPK
phosphorylation and HIF-1α expression
The 786-O and 769-P cells were cultured in media
containing doxorubicin (3 µM) and the p38MAPK inhibitor SB203580
(10 µM) at 37°C and 5% CO2 for 48 h. When doxorubicin
was administered to cells in the sh-NC group, the p-p38MAPK/p38MAPK
ratio and HIF-1α expression levels significantly decreased
(Fig. 3). In the sh-EBP1 group,
significant reductions in the p-p38MAPK/p38MAPK ratio and HIF-1α
expression levels were also observed after the addition of
doxorubicin compared with those in the sh-NC group. In the
simultaneous presence of doxorubicin and SB203580, the suppression
of the p-p38MAPK/p38MAPK ratio and HIF-1α expression in the sh-NC
group was greater compared with that achieved with doxorubicin
alone; however, a stronger suppression was observed in the sh-EBP1
group. These results suggest that doxorubicin acts by inhibiting
p38MAPK phosphorylation and HIF-1α expression.
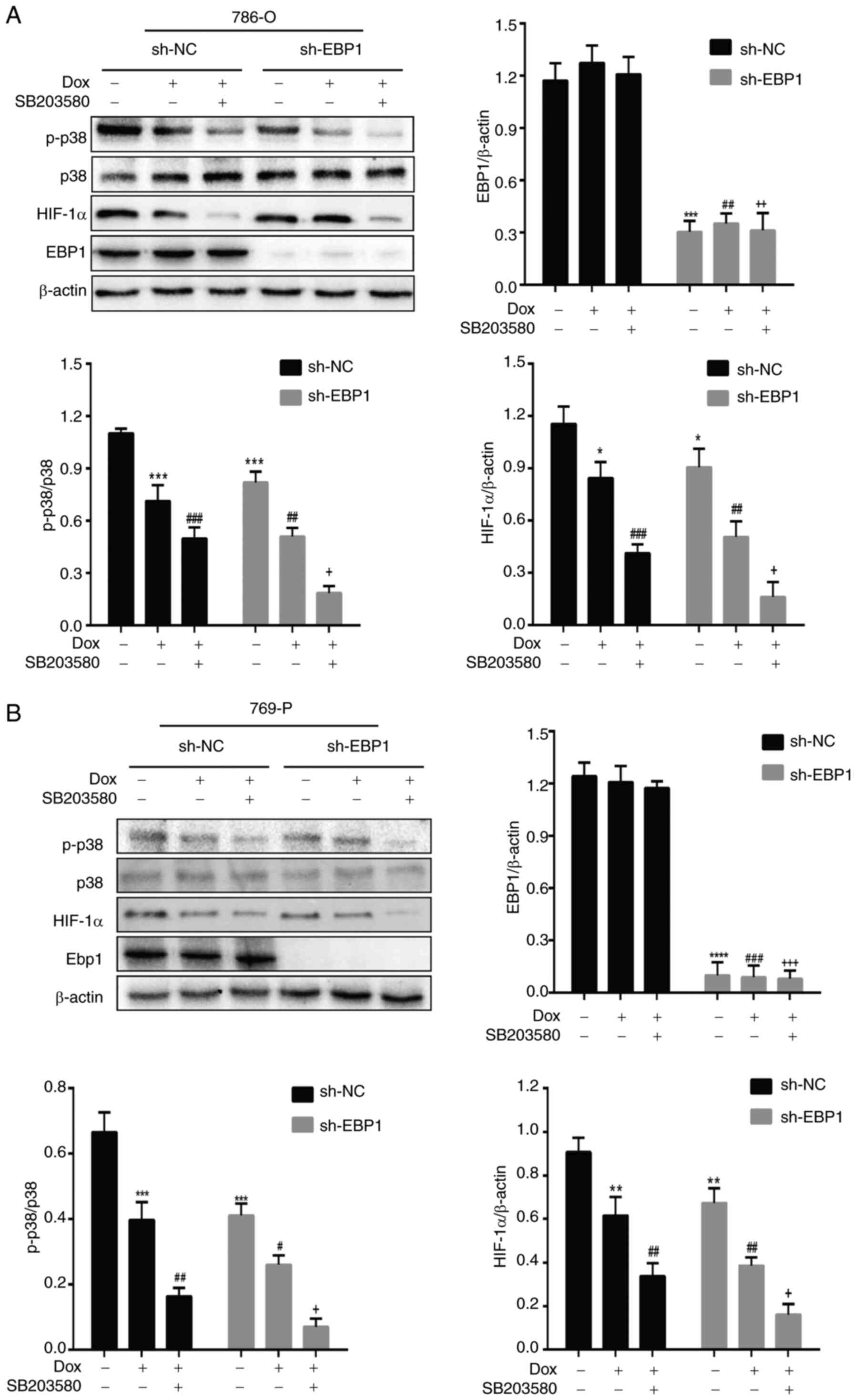 | Figure 3.Doxorubicin acts by inhibiting p38
phosphorylation and HIF-1α expression in kidney clear cell
carcinoma cells. Effect of doxorubicin and SB203580 on p-p38 and
HIF-1α protein levels in (A) 786-O cells and (B) 769-P cells.
Representative western blots and quantitative analyses are shown,
normalized to β-actin. Data are presented as the mean ± SD, n=3.
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs.
sh-NC; #P<0.05, ##P<0.01 and
###P<0.001 vs. sh-NC + Dox; +P<0.05,
++P<0.01 and +++P<0.001 vs. sh-NC + Dox
+ SB203580. p38, p38 mitogen-activated protein kinase; HIF-1α,
hypoxia-inducible factor-1α; EBP1, ErbB3-binding protein; Dox,
doxorubicin; sh-NC, short hairpin negative control; sh-EBP1, short
hairpin EBP1; p-, phosphorylated. |
Combination of doxorubicin and EBP1
knockdown increases KIRC cell apoptosis and reduces cell
viability
The expression of apoptosis-associated proteins Bax
and Bcl-2, and the cleavage of caspase-3 in doxorubicin-treated
KIRC cells were examined using western blotting. When 786-O and
769-P cells were treated with doxorubicin, the proportion of
caspase-3 that was cleaved and the expression of pro-apoptotic
factor Bax increased significantly. Conversely, there was a notable
downregulation in the expression of Bcl-2, an inhibitory apoptotic
component. Furthermore, the Bax/Bcl-2 ratio increased significantly
when SB203580 was combined with doxorubicin (Fig. 4A and B). These results suggest that
the knockdown of EBP1 promotes doxorubicin-induced apoptosis
in KIRC cells by altering the levels of apoptosis-associated
proteins via the p38MAPK pathway.
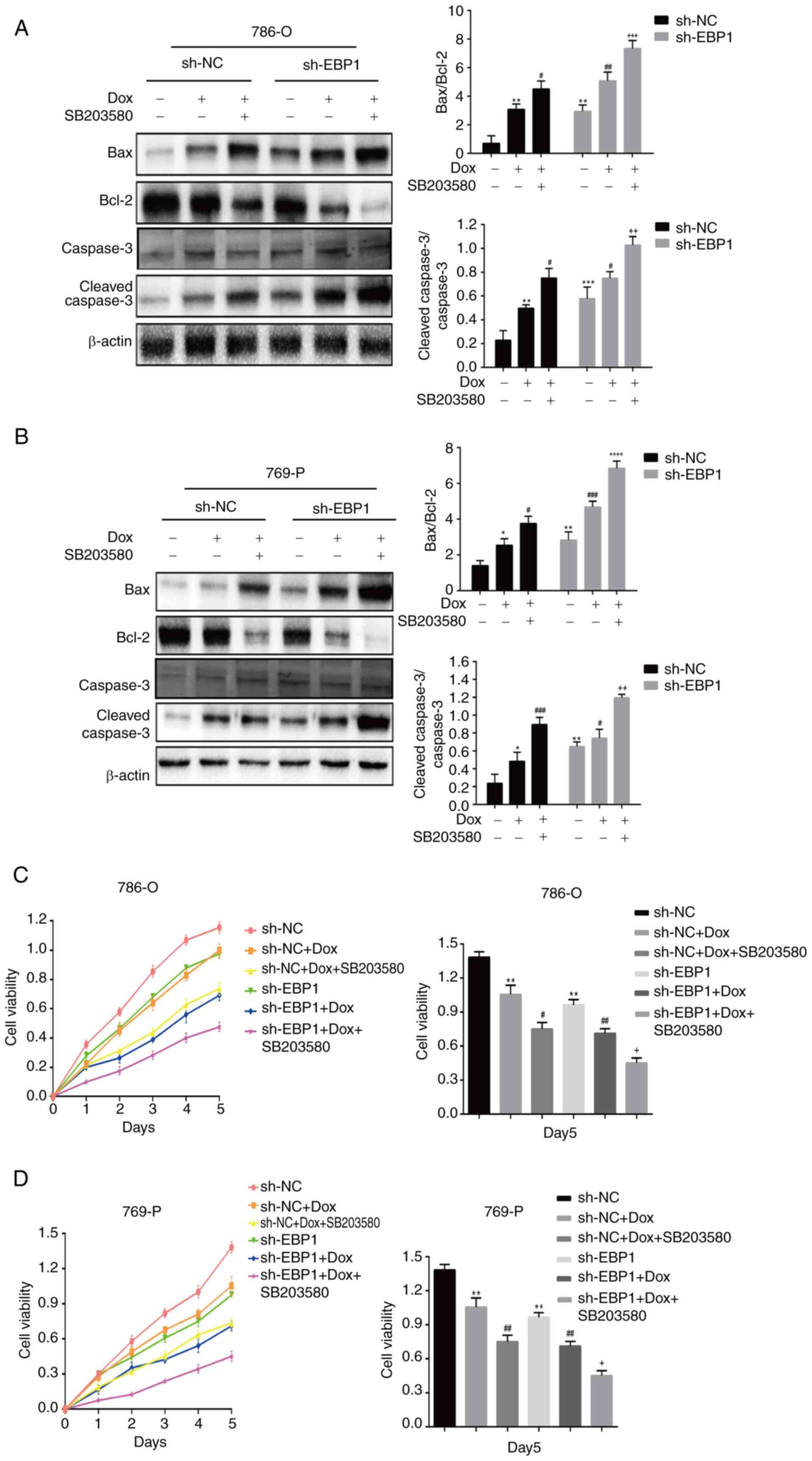 | Figure 4.Concurrent doxorubicin and
EBP1 knockdown together promote apoptosis and reduce the
viability of kidney clear cell carcinoma cells. Effect of
doxorubicin on protein levels associated with apoptosis in (A)
786-O cells and (B) 769-P cells. Representative western blots and
quantitative analyses are shown, normalized to β-actin. Effect of
doxorubicin on (C) 786-O and (D) 769-P cell viability. Changes in
viability over a 5-day period, measured daily, and a quantitative
assessment on day 5 are shown. Data are presented as the mean ± SD,
n=3. *P<0.05, **P<0.01 and ***P<0.001 vs. sh-NC;
#P<0.05, ##P<0.01 and
###P<0.001 vs. sh-NC + Dox; +P<0.05,
++P<0.01, +++P<0.001 and
++++P<0.0001 vs. sh-NC + Dox + SB203580. EBP1,
ErbB3-binding protein; Dox, doxorubicin; sh-NC, short hairpin
negative control; sh-EBP1, short hairpin EBP1. |
The viability of KIRC cells in the sh-NC and sh-EBP1
groups following treatment with doxorubicin and SB203580 was
assessed using the CCK-8 assay. When the sh-EBP1 group was compared
with the sh-NC group in each cell line, it was observed that
doxorubicin treatment reduced cell viability and that this effect
was even more pronounced following the addition of SB203580
(Fig. 4C and D). These findings
indicate that doxorubicin and EBP1 knockdown cooperate via
the p38MAPK pathway to increase the inhibitory effect of
doxorubicin on KIRC cell survival.
Discussion
KIRC is a frequently occurring type of kidney cancer
(1). The treatment of advanced
kidney cancer with radiation and chemotherapy is complicated by
tumor resistance to these treatments (19). Doxorubicin is a broad-spectrum
anticancer medication that has been reported to inhibit the growth
of a variety of cancer cells (4),
and shown to have significant efficacy in the treatment of kidney
cancer (20). However, the toxic
side effects of doxorubicin and the development of drug resistance
have limited its clinical application (5). Therefore, the present study attempted
to identify a means of increasing the susceptibility of cancer
cells to doxorubicin and lowering the dosage of doxorubicin to
lessen its harmful side effects. In preliminary experiments, the
cytotoxicity of doxorubicin to KIRC cell lines was evaluated, and
its half-maximal inhibitory concentration was calculated to be 3 µM
(data not shown). At this concentration, doxorubicin effectively
induced cell death but had a minimal impact on normal cells,
showing suggesting that doxorubicin at this concentration has good
selectivity and efficacy. Therefore, this doxorubicin concentration
was selected for use in the experiments (21). The anticancer effects of doxorubicin
include the induction of apoptosis via the inhibition of RNA and
DNA synthesis (22). Doxorubicin
has also been demonstrated to inhibit cancer progression via the
modulation of several signaling pathways, including the p53 pathway
(23), PI3K/Akt/mTOR pathway
(24), NF-κB pathway (25) and HIF-1α pathway (26). The inhibition of these pathways is
essential to efficiently suppress tumor development and accelerate
the process of apoptosis.
The therapeutic strategy of combining oncogene
knockdown with drug administration has been a topic of in-depth
research in the field of cancer treatment. By specifically
combining the targeting and reducing the expression of oncogenes
with the use of chemotherapy, targeted therapy and other drug
interventions, this strategy can effectively inhibit tumor cell
proliferation and metastasis. In addition, it may help to overcome
the issue of drug resistance that arises when single treatment
methods are used (27,28). Numerous cancers are regulated by
EBP1, which affects cell proliferation and differentiation
(29). Furthermore, EBP1 has
the potential to operate both as an oncostatic and an oncogenic
regulator (30). Studies have shown
that EBP1 protein intensifies the carcinogenic properties of cancer
cells, including those in hepatocellular carcinoma (9) and melanoma (31). In addition, the inhibition of
EBP1 expression has been shown to suppress tumor formation
in colon cancer (32). Although
database analysis reveals that KIRC has significantly upregulated
levels of EBP1 expression, the combined effects of EBP1 and
doxorubicin in KIRC and the underlying mechanism are unclear. EBP1
has been indicated to contribute to KIRC onset and progression via
the regulation of cell cycle-related genes or apoptosis-related
signaling pathways (10).
Therefore, we hypothesized that EBP1 knockdown may enhance
the sensitivity of KIRC to doxorubicin chemotherapy. In the current
study, the combination of EBP1 knockdown and doxorubicin
treatment was observed to have a significant inhibitory effect on
KIRC cells, suggesting that EBP1 promotes the progression of
KIRC, which is consistent with previous research. This suggests
that combination therapy may be critical for the effective
management of KIRC.
EBP1 has previously been demonstrated to promote the
occurrence and development of KIRC by regulating the p38MAPK/HIF-1α
signaling pathway (10). Cellular
inflammatory responses are mainly regulated by mechanisms involving
apoptosis, differentiation and the p38MAPK intracellular signaling
cascade (33). In addition, p38MAPK
and HIF-1α are strongly associated with the medication resistance,
invasiveness and angiogenesis of tumors (34). p38MAPK has been indicated to control
HIF-1α activity, which in turn may control tumor development and
metastasis (35). Notably, research
has shown that p38MAPK inhibitors increase the susceptibility of
cancer cells to doxorubicin (36).
Also, Ye et al (37) have
shown that the activation of p38MAPK is associated with
doxorubicin-induced apoptosis. Therefore, we hypothesized that
doxorubicin might act synergistically with the knockdown of
EBP1 through its effects on the p38MAPK pathway to increase
the sensitivity of KIRC cells to doxorubicin. To demonstrate the
contribution of the p38MAPK/HIF-1α pathway to the effect of
doxorubicin on KIRC cells, a p38MAPK inhibitor was administered to
786-O and 769-P cells. Western blotting results indicated that the
p-p38MAPK/p38MAPK ratio and HIF-1α expression level were reduced
when both doxorubicin and the p38MAPK inhibitor were applied to the
cells, compared with their levels following treatment with
doxorubicin alone. Furthermore, EBP1 protein expression was not
affected by the addition of doxorubicin alone or with the p38MAPK
inhibitor, indicating that EBP1 acts upstream of p38MAPK and
HIF-1α. The effect of EBP1 knockdown on the p38MAPK pathway
was indicated to increase the sensitivity of KIRC cells to
doxorubicin. These findings provide novel insights for the
treatment of KIRC.
Although the present study reveals the potential
role of EBP1 in KIRC cell lines, it has certain limitations, in
particular, the lack of in vivo validation and clinical
data. While cell line experiments can provide valuable molecular
mechanisms and preliminary data, they cannot fully replicate the
complex tumor microenvironment in vivo or the interactions
between the tumor and the host. Therefore, it is necessary to
conduct further validation in mouse or other animal models in
future studies to confirm the biological function and clinical
relevance of EBP1 in KIRC.
In conclusion, the present study indicates that
doxorubicin acts by inhibiting p38MAPK phosphorylation and HIF-1α
expression. EBP1 knockdown and doxorubicin act together via
the p38MAPK pathway, with EBP1 knockdown further enhancing
the inhibitory effect of doxorubicin on KIRC cell viability.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the National
Natural Science Foundation of China (grant no. 82360479), Project
of Education Department of the Jilin province of China (grant no.
JJKH20210589KJ) and The Natural Science Research Foundation of
Jilin Province for Sciences and Technology (grant no.
YDZJ202301ZYTS173).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LM, JH and SC performed the experiments. SC and JH
made significant contributions to the conception or design of the
work. YY and XL prepared the figures and contributed to the
analysis of experimental results. LL and ST wrote the main
manuscript and made substantial contributions to the bioinformatics
analysis. The manuscript was critically reviewed for important
intellectual content. JH and SC confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hishida T, Masai K, Kaseda K, Asakura K
and Asamura H: Debulking surgery for malignant tumors: The current
status, evidence and future perspectives. Jpn J Clin Oncol.
51:1349–1362. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olivares-Urbano MA, Griñán-Lisón C,
Marchal JA and Núñez MI: CSC Radioresistance: A therapeutic
challenge to improve radiotherapy effectiveness in Cancer. Cells.
9:16512020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kciuk M, Gielecińska A, Mujwar S, Kołat D,
Kałuzińska-Kołat Ż, Celik I and Kontek R: Doxorubicin-An Agent with
multiple mechanisms of anticancer activity. Cells. 12:6592023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kong CY, Guo Z, Song P, Zhang X, Yuan YP,
Teng T, Yan L and Tang QZ: Underlying the mechanisms of
Doxorubicin-induced acute cardiotoxicity: Oxidative stress and cell
death. Int J Biol Sci. 18:760–770. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Xing J, Liang Y, Liang H, Liang N,
Li J, Yin G, Li X and Zhang K: The structure and function of
multifunctional protein ErbB3 binding protein 1 (Ebp1) and its role
in diseases. Cell Biol Int. 48:1069–1079. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Xing C, Deng Y, Ye C and Peng H:
HIF-1α signaling: Essential roles in tumorigenesis and implications
in targeted therapies. Genes Dis. 11:234–251. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rashid M, Zadeh LR, Baradaran B, Molavi O,
Ghesmati Z, Sabzichi M and Ramezani F: Up-down regulation of HIF-1α
in cancer progression. Gene. 798:1457962021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bao Y, Suvesh M, Li X, Bai X, Li H, Li X,
Xu D and Liu L: Ebp1 p48 promotes oncogenic properties in
hepatocellular carcinoma through p38 MAPK/HIF1α activation and p53
downregulation. Mol Carcinog. 60:252–264. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng H, Cao S, Tian S, Huo J, Li X, Xu D
and Liu L: EBP1 promotes the malignant biological behaviors of
kidney renal clear cell carcinoma through activation of p38/HIF-1α
signaling pathway. Cancer Cell Int. 24:2612024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vergote I, Heitz F, Buderath P, Powell M,
Sehouli J, Lee CM, Hamilton A, Fiorica J, Moore KN, Teneriello M,
et al: A randomized, double-blind, placebo-controlled phase 1b/2
study of ralimetinib, a p38 MAPK inhibitor, plus gemcitabine and
carboplatin versus gemcitabine and carboplatin for women with
recurrent platinum-sensitive ovarian cancer. Gynecol Oncol.
156:23–31. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grossi V, Peserico A, Tezil T and Simone
C: p38α MAPK pathway: A key factor in colorectal cancer therapy and
chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sohail M, Sun Z, Li Y, Gu X and Xu H:
Research progress in strategies to improve the efficacy and safety
of doxorubicin for cancer chemotherapy. Expert Rev Anticancer Ther.
21:1385–1398. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Girigoswami A, Adhikesavan H, Mudenkattil
S, Devi S and Girigoswami K: Role of cerium oxide nanoparticles and
doxorubicin in improving cancer management: A mini review. Curr
Pharm Des. 29:2640–2654. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao L, Han H, Wang H, Cao L and Feng WH:
IL-10 knockdown with siRNA enhances the efficacy of Doxorubicin
chemotherapy in EBV-positive tumors by inducing lytic cycle via
PI3K/p38 MAPK/NF-kB pathway. Cancer Lett. 462:12–22. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye Y, Ye F, Li X, Yang Q, Zhou J, Xu W,
Aschner M, Lu R and Miao S: 3,3′-diindolylmethane exerts
antiproliferation and apoptosis induction by TRAF2-p38 axis in
gastric cancer. Anticancer Drugs. 32:189–202. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jones J, Otu H, Spentzos D, Kolia S, Inan
M, Beecken WD, Fellbaum C, Gu X, Joseph M, Pantuck AJ, et al: Gene
signatures of progression and metastasis in renal cell cancer. Clin
Cancer Res. 11:5730–5739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Wang Z, Wang H, Zhang J, Wang X,
Xing S and Chen S: IQGAP3 in clear cell renal cell carcinoma
contributes to drug resistance and genome stability. PeerJ.
10:e142012022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu XX, Kakehi Y, Mizutani Y, Nishiyama H,
Kamoto T, Megumi Y, Ito N and Ogawa O: Enhancement of
TRAIL/Apo2L-mediated apoptosis by adriamycin through inducing DR4
and DR5 in renal cell carcinoma cells. Int J Cancer. 104:409–417.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soares LBM, Lima APB, Melo AS, Almeida TC,
de Medeiros Teixeira LF and da Silva GN: Additive effects of
resveratrol and doxorubicin on bladder cancer cells. Anticancer
Drugs. 33:e389–e397. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghelli Luserna Di Rorà A, Ghetti M, Ledda
L, Ferrari A, Bocconcelli M, Padella A, Napolitano R, Fontana MC,
Liverani C, Imbrogno E, et al: Exploring the ATR-CHK1 pathway in
the response of doxorubicin-induced DNA damages in acute
lymphoblastic leukemia cells. Cell Biol Toxicol. 39:795–811. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma Z, Guo D, Wang Q, Liu P, Xiao Y, Wu P,
Wang Y, Chen B, Liu Z and Liu Q: Lgr5-mediated p53 repression
through PDCD5 leads to doxorubicin resistance in hepatocellular
carcinoma. Theranostics. 9:2967–2983. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Babichev Y, Kabaroff L, Datti A, Uehling
D, Isaac M, Al-Awar R, Prakesch M, Sun RX, Boutros PC, Venier R, et
al: PI3K/AKT/mTOR inhibition in combination with doxorubicin is an
effective therapy for leiomyosarcoma. J Transl Med. 14:672016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang Y, Wang Y, Zhang Y, Ye F, Luo D, Li
Y, Jin Y, Han D, Wang Z, Chen B, et al: HSPB1 facilitates
chemoresistance through inhibiting ferroptotic cancer cell death
and regulating NF-κB signaling pathway in breast cancer. Cell Death
Dis. 14:4342023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du G, Lin H, Wang M, Zhang S, Wu X, Lu L,
Ji L and Yu L: Quercetin greatly improved therapeutic index of
doxorubicin against 4T1 breast cancer by its opposing effects on
HIF-1α in tumor and normal cells. Cancer Chemother Pharmacol.
65:277–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin T, Cui XY, Xiu H, Huang C, Sun ZN, Xu
XM, Li LH and Yue L: USP37 downregulation elevates the chemical
sensitivity of human breast cancer cells to adriamycin. Int J Med
Sci. 18:325–334. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan H, Zhao RM, Wang ZJ, Zhao FR and Wang
SL: Knockdown of PRAME enhances adriamycin-induced apoptosis in
chronic myeloid leukemia cells. Eur Rev Med Pharmacol Sci.
19:4827–4834. 2015.PubMed/NCBI
|
|
29
|
Liu Z, Ahn JY, Liu X and Ye K: Ebp1
isoforms distinctively regulate cell survival and differentiation.
Proc Natl Acad Sci USA. 103:10917–10922. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Li Z, Li L, Peng H and Zhang Z:
EBP1 suppresses growth, migration, and invasion of thyroid cancer
cells through upregulating RASAL expression. Tumour Biol.
36:8325–8331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bao Y, Cui J, Yue Y, Cao S, Li X and Liu
L: ERBB3 binding protein 1 promotes the progression of malignant
melanoma through activation of the Wnt/ β-catenin signaling
pathway. Cancer Cell Int. 22:442022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nguyen DQ, Hoang DH, Nguyen TTV, Ho HD,
Huynh V, Shin JH, Ly QT, Thi Nguyen DD, Ghoda L, Marcucci G and
Nguyen LXT: Ebp1 p48 promotes oncogenic activities in human colon
cancer cells through regulation of TIF-90-mediated ribosomal RNA
synthesis. J Cell Physiol. 234:17612–17621. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Coulthard LR, White DE, Jones DL,
McDermott MF and Burchill SA: p38(MAPK): Stress responses from
molecular mechanisms to therapeutics. Trends Mol Med. 15:369–379.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Esposito G, Gigli S, Seguella L, Nobile N,
D'Alessandro A, Pesce M, Capoccia E, Steardo L, Cirillo C, Cuomo R
and Sarnelli G: Rifaximin, a non-absorbable antibiotic, inhibits
the release of pro-angiogenic mediators in colon cancer cells
through a pregnane X receptor-dependent pathway. Int J Oncol.
49:639–645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koodie L, Ramakrishnan S and Roy S:
Morphine suppresses tumor angiogenesis through a HIF-1alpha/p38MAPK
pathway. Am J Pathol. 177:984–997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tan W, Yu HG and Luo HS: Inhibition of the
p38 MAPK pathway sensitizes human gastric cells to doxorubicin
treatment in vitro and in vivo. Mol Med Rep.
10:3275–3281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ye J, Wang Y, Xu Y, Wang Z, Liu L, Wang M,
Ye D, Zhang J, Yang Z, Lin Y, et al: Retraction notice to
‘Interleukin-22 deficiency alleviates doxorubicin-induced oxidative
stress and cardiac injury via the p38 MAPK/macrophage/Fizz3 axis in
mice’ [Redox Biol. 36 (2020) 101636]. Redox Biol. 36:1016362020.
View Article : Google Scholar : PubMed/NCBI
|