Introduction
Malignant melanoma is a highly malignant tumor that
typically arises on the skin surface, with a minority of lesions
occurring in locations such as the intestines, nasal cavity and
uveal tract (1). Both the incidence
and mortality rates of malignant melanoma have shown a rising trend
year by year between 2012 and 2020 (2). Although malignant melanoma accounts
for <1% of all skin malignancies, its mortality rate accounts
for 75% of all associated deaths from skin malignancies (2). The prognosis of patients with
malignant melanoma is closely associated with the stage at
diagnosis, with a 5-year survival rate of >90% for stage I
patients, which is markedly superior to that of stage IV patients
(35%) (2,3). Major risk factors for malignant
melanoma include exposure to ultraviolet radiation, large
congenital nevi and genetic mutations in cyclin-dependent kinase
inhibitor 2A (CDKN2A) and CDK4, among others (4–6). Given
the generally poor prognosis of patients with melanoma, greater
emphasis should be placed on this disease.
Insulin resistance (IR) is a response in which the
body's insulin-mediated regulation of blood glucose diminishes due
to energy surplus, and is closely linked with obesity, type 2
diabetes and fatty liver disease (7–9). In
recent years, an increasing body of research has suggested that the
impact of IR extends beyond endocrinology and cardiovascular
fields, also promoting the progression of malignant tumors,
including breast, colorectal, thyroid and lung cancer (10–13).
However, studies on the association between IR and malignant
melanoma are currently lacking. The hyperglycemic clamp technique
serves as the gold standard for diagnosing IR, but its complexity
and high cost mean that it is difficult to widely adopt in clinical
practice (14). By contrast, the
TyG index has been proven to be a simple and accurate marker of IR,
more suitable for clinical application (15). Therefore, the present study aimed to
explore the association between the TyG index, a marker of IR, and
malignant melanoma.
Patients and methods
Study population
Basic information and hematological test results,
including age, gender, body mass index (BMI), medical history,
fasting triglyceride level and fasting blood glucose level, were
retrospectively collected from patients diagnosed with malignant
melanoma at the First Affiliated Hospital of Nanjing Medical
University (Nanjing, China) between January 2019 and January 2024.
All included patients underwent standard surgical treatment
(consisting of laparoscopic colectomy and lymphadenectomy).
Inclusion criteria comprised histopathological diagnosis of a
malignant melanoma or nevus, age >18 years, no prior treatment,
and sufficient baseline and hematological information. Exclusion
criteria included a history of other tumors, use of lipid-lowering
or blood glucose-lowering medications within a month of diagnosis,
other disorders or conditions affecting lipid or glucose
metabolism, and acute inflammation at the time of diagnosis.
Diabetic patients were also excluded for the following reasons: i)
To reduce the confounding effects of IR: Diabetic patients
typically experience IR and glucose metabolism abnormalities,
leading to significantly elevated IR and TyG index values (16). Including diabetic patients in the
study would introduce additional variability in the TyG index,
which could obscure the true association between the TyG index and
melanoma, thereby reducing the specificity and reliability of the
results. ii) To avoid reverse causality interference: Diabetes may
increase the risk of malignancies through metabolic inflammation
(17). Therefore, diabetes could
play a role in the mechanisms underlying melanoma development.
Without excluding diabetic patients, the results could be
confounded by the potential impact of diabetes itself on melanoma
risk, making it difficult to establish an independent association
between the TyG index and melanoma. iii) To enhance homogeneity of
the study sample: Diabetic patients differ significantly from
non-diabetic individuals in terms of metabolic characteristics,
medication use and other factors (18). By excluding diabetic patients,
sample heterogeneity was reduced, thereby increasing the
consistency of the data. This allowed the changes in the TyG index
among non-diabetic melanoma patients to be more representative.
To minimize potential confounding effects from
undiagnosed diabetes or metabolic syndrome, the following patients
were excluded: i) All patients with a fasting glucose level >7.0
mmol/l; ii) those patients with HbA1c >6%; iii) patients with
marked symptoms such as polydipsia, polyphagia, polyuria and
unexplained weight loss; iv) those with a family history of
diabetes in first-degree relatives; and v) patients with severe
obesity (BMI >30 kg/m2). All relevant examinations
were completed and data collected within 3 days prior to diagnosis.
Initially, 301 patients were enrolled in this study, 10 of whom
were excluded due to pre-existing diabetes, 16 due to recent use of
lipid-lowering medications and 3 due to insufficient information,
resulting in a final inclusion of 272 patients with melanoma.
Additionally, 131 patients with pathological diagnoses of nevi were
included as the control group. The same exclusion criteria were
applied to the control patients.
This study received approval from the Ethics
Committee of the First Affiliated Hospital of Nanjing Medical
University and adheres to the principles outlined in the
Declaration of Helsinki (protocol code, 2024-SR-066; date of
approval, 2024-02-27).
Measurements
In a fasting state, 3–5 ml of blood was drawn from
the median cubital vein into a vacuum tube. The blood sample was
centrifuged at 1,000 × g for 10–15 min at 20°C to separate plasma
from blood cells (centrifuge model no. 5804/5804R; Eppendorf SE).
Glucose concentration was measured using the glucose oxidase method
with photometric detection [PGI-101; Glucose Colorimetric Detection
Kit; Thermo Fisher Scientific, Inc.] according to the
manufacturer's instructions. Triglyceride concentration was
measured using the glycerol oxidase-peroxidase method (TR22421;
Triglyceride Reagent; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Blood was collected from the
individuals enrolled in the study for the purpose of diagnosis and
treatment. The method of calculating the TyG index was: TyG=ln [TG
(mg/dl) × fasting blood glucose (mg/dl)/2].
Statistical analysis
The statistical analysis was conducted using IBM
SPSS (version 27.0; IBM Corp.). Data normality was assessed using
the Shapiro-Wilk test. Categorical variables were described using
numbers and percentages, while continuous variables were described
using means and standard deviations. Continuous variables were
compared using either independent t-tests or Mann-Whitney U tests.
χ2 tests were utilized for comparing categorical
variables. Univariate and multivariate logistic regression analysis
was employed to investigate the association between melanoma risk
and the TyG index. The optimal cutoff value of the TyG index was
determined using a receiver operating characteristic (ROC) curve,
with calculations of area under the curve, sensitivity and
specificity. Patients were stratified into three groups based on
tertiles of the TyG index, and χ2 tests were used to
compare incidence rates among the three groups. P<0.05
(two-sided) was used to indicate a statistically significant
difference.
Results
Baseline characteristics
A total of 403 participants were included in this
study. Among them, there were 272 patients with malignant melanoma
and 131 patients with nevi, defined as the melanoma group and the
control group, respectively. The age range of patients in the
melanoma group was 19 to 76 years (mean ± SD, 59.07±13.70), while
that in the control group was 35 to 78 years (mean ± SD,
57.79±12.09). In the melanoma group, 188 (69.1%) patients had the
acral histological type, 26 (9.6%) the mucosal type and 44 (16.2%)
the cutaneous type, while 14 (5.1%) were of unknown type. Compared
with the control group, the melanoma group exhibited a higher TyG
index (P<0.001). There was also a difference in the drinking
history between the two groups (P=0.037). However, there were no
significant differences between the two groups in terms of age,
gender, BMI, smoking history, presence of hypertension or presence
of hyperlipidemia (P>0.05). Demographic and clinical
characteristics of all participants are presented in Table I.
 | Table I.Baseline clinicopathological
characteristics of patients. |
Table I.
Baseline clinicopathological
characteristics of patients.
| Variables | All patients
(n=403) | Melanoma group
(n=272) | Control group
(n=131) | P-value |
|---|
| Age, years |
|
|
|
|
|
<65 | 259 (64.3) | 180 (66.2) | 79 (60.3) | 0.120 |
|
>65 | 144 (35.7) | 92 (33.8) | 52 (39.7) |
|
| Sex |
|
|
|
|
|
Male | 207 (51.4) | 146 (53.7) | 61 (46.6) | 0.181 |
|
Female | 196 (48.6) | 126 (46.3) | 70 (53.4) |
|
| Mean BMI ± SD | 22.28±2.49 | 22.39±2.56 | 22.17±2.29 | 0.527 |
| Histology |
|
|
|
|
|
Acral | / | 188 (69.) | / | / |
|
Mucosal | / | 26 (9.6) | / | / |
|
Cutaneous | / | 44 (16.2) | / | / |
|
Unknown | / | 14 (5.1) | / | / |
| Clinical stage |
|
|
|
|
| I | / | 46 (16.9) | / | / |
| II | / | 82 (30.1) | / | / |
|
III | / | 112 (41.2) | / | / |
| IV | / | 32 (11.8) | / | / |
| Smoking |
|
|
|
|
|
Yes | 100 (24.8) | 68 (25.0) | 32 (24.4) | 0.9.1 |
| No | 303 (75.2) | 204 (75.0) | 99 (75.6) |
|
| Drinking |
|
|
|
|
|
Yes | 64 (15.9) | 36 (13.2) | 28 (21.4) | 0.037 |
| No | 339 (84.1) | 236 (86.8) | 103 (78.6) |
|
| Hypertension |
|
|
|
|
|
Yes | 132 (32.8) | 98 (36.0) | 34 (26.0) | 0.054 |
| No | 271 (67.2) | 174 (64.0) | 97 (74.0) |
|
| Hyperlipemia |
|
|
|
|
|
Yes | 156 (38.7) | 114 (41.9) | 42 (32.1) | 0.058 |
| No | 247 (61.3) | 158 (58.1) | 89 (67.9) |
|
| Mean fasting blood
glucose ± SD | 4.89±1.11 | 5.05±1.31 | 4.86±0.71 | 0.124 |
| Mean fasting
triglycerides ± SD | 1.46±0.81 | 1.49±0.91 | 1.41±0.65 |
|
| Mean TyG index ±
SD | 8.56±0.59 | 8.80±0.54 | 8.31±0.52 | <0.001 |
Association between malignant melanoma
risk and the TyG index
The optimal cutoff value of the TyG index was 8.87.
The TyG index was categorized into high and low groups according to
the cutoff value. The results of univariate logistic regression
analysis indicated that the melanoma group had a significantly
higher TyG index (OR, 6.45; 95% CI 3.64–11.43; P<0.001; Table II). Variables with P<0.1 were
further included in the multivariate logistic regression analysis.
This analysis demonstrated that the TyG index (OR, 6.33; 95% CI,
3.56–11.27; P<0.001; Table II)
was independently associated with the melanoma incidence.
 | Table II.Univariate and multivariate analysis
of risk factors for melanoma. |
Table II.
Univariate and multivariate analysis
of risk factors for melanoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age (>65/<65
years) | 0.78 | 0.47–1.28 | 0.320 |
|
|
|
| Sex
(female/male) | 0.25 | 0.47–1.22 | 0.752 |
|
|
|
| BMI
(18.5–23.9/>24 kg/m2) | 1.05 | 0.95–1.15 | 0.350 |
|
|
|
| Smoking status
(yes/no) | 1.03 | 0.59–1.80 | 0.914 |
|
|
|
| Drinking status
(yes/no) | 0.56 | 0.29–1.07 | 0.081 | 0.58 | 0.29–1.18 | 0.134 |
| Hypertension
(yes/no) | 1.61 | 0.95–2.72 | 0.076 | 1.47 | 0.83–2.61 | 0.186 |
| Hyperlipemia
(yes/no) | 1.53 | 0.93–2.52 | 0.106 |
|
|
|
| TyG index
(>8.87/<8.87) | 6.45 | 3.64–11.43 | <0.001 | 6.33 | 3.56–11.27 | <0.001 |
Since the most common pathological type in this
study was acral melanoma (69.1%), a logistic regression analysis
was performed for patients with acral melanoma alone. The results
of univariate logistic regression analysis indicated that there
were differences in hypertension (OR, 1.85; 95% CI, 1.05–3.27;
P=0.034; Table III) and TyG index
(OR, 6.09; 95% CI, 3.26–11.37; P<0.001; Table III) between the two groups. The
results of multivariate logistic regression showed that TyG index
was also independently associated with the onset of acral melanoma
(OR, 6.33; 95% CI, 3.34–11.97; P<0.001; Table III).
 | Table III.Univariate and multivariate analysis
of risk factors for acral melanoma. |
Table III.
Univariate and multivariate analysis
of risk factors for acral melanoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age (>65/<65
years) | 0.59 | 0.50–1.49 | 0.861 |
|
|
|
| Sex
(female/male) | 0.67 | 0.39–1.15 | 0.147 |
|
|
|
| BMI
(18.5–23.9/>24 kg/m2) | 1.10 | 0.99–1.23 | 0.089 | 1.14 | 1.01–1.28 | 0.044 |
| Smoking status
(yes/no) | 0.95 | 0.51–1.76 | 0.859 |
|
|
|
| Drinking status
(yes/no) | 0.59 | 0.29–1.21 | 0.151 |
|
|
|
| Hypertension
(yes/no) | 1.85 | 1.05–3.27 | 0.034 | 1.59 | 0.86–2.96 | 0.142 |
| Hyperlipemia
(yes/no) | 1.57 | 0.91–2.72 | 0.108 |
|
|
|
| TyG index
(>8.87/<8.87) | 6.09 | 3.26–11.37 | <0.001 | 6.33 | 3.34–11.97 | <0.001 |
Given the differences in insulin sensitivity between
sexes (19), the TyG index was
analyzed separately for males and females. The results of
univariate logistic regression analysis indicated that the melanoma
group had a significantly higher TyG index in both males (OR, 6.63;
95% CI, 2.85–15.41; P<0.001; Table
IV) and females (OR, 9.09; 95% CI, 3.44–24.06; P<0.001;
Table V). The multivariate logistic
regression results also indicated that the TyG index was an
independent predictor of malignant melanoma in both males (OR,
6.44; 95% CI, 2.75–15.06; P<0.001; Table IV) and females (OR, 10.04; 95% CI,
3.71–27.17; P<0.001; Table V).
Notably, in females, the univariate logistic regression results
indicated that there was no difference in hypertension (OR, 2.08;
95% CI, 0.98–4.42; P=0.058; Table
V) between the two groups, but the multivariate logistic
regression results showed that hypertension was an independent
predictor of malignant melanoma (OR, 2.53; 95% CI, 1.10–5.83;
P=0.029; Table V).
 | Table IV.Univariate and multivariate analysis
of risk factors for melanoma in male patients. |
Table IV.
Univariate and multivariate analysis
of risk factors for melanoma in male patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age (>65/<65
years) | 0.69 | 0.35–1.37 | 0.285 |
|
|
|
| BMI
(18.5–23.9/>24 kg/m2) | 1.09 | 0.95–1.26 | 0.209 |
|
|
|
| Smoking status
(yes/no) | 0.70 | 0.35–1.41 | 0.321 |
|
|
|
| Drinking status
(yes/no) | 0.51 | 0.24–1.06 | 0.070 | 0.55 | 0.25–1.23 | 0.144 |
| Hypertension
(yes/no) | 1.25 | 0.59–2.59 | 0.559 |
|
|
|
| Hyperlipemia
(yes/no) | 1.44 | 0.72–2.88 | 0.300 |
|
|
|
| TyG index
(>8.87/<8.87) | 6.63 | 2.85–15.41 | <0.001 | 6.44 | 2.75–15.06 | <0.001 |
 | Table V.Univariate and multivariate analysis
of risk factors for melanoma in female patients. |
Table V.
Univariate and multivariate analysis
of risk factors for melanoma in female patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age (>65/<65
years) | 0.82 | 0.39–1.71 | 0.593 |
|
|
|
| BMI
(18.5–23.9/>24 kg/m2) | 1.01 | 0.88–1.16 | 0.936 |
|
|
|
| Smoking status
(yes/no) | 1.78 | 0.59–5.31 | 0.303 |
|
|
|
| Drinking status
(yes/no)a | / | / | / | / | / | / |
| Hypertension
(yes/no) | 2.08 | 0.98–4.42 | 0.058 | 2.53 | 1.10–5.83 | 0.029 |
| Hyperlipemia
(yes/no) | 1.54 | 0.74–3.22 | 0.247 |
|
|
|
| TyG index
(>8.87/<8.87) | 9.09 | 3.44–24.06 | <0.001 | 10.04 | 3.71–27.17 | <0.001 |
Incidence of malignant melanoma
compared across the tertiles of the TyG index
Dividing the data into tertiles (from low to high
based on the variable) can help identify the association between
the levels of the variable (TyG index) and disease risk. This
method allows the observer to assess whether there is a gradient
change in the incidence rate across the low (tertile 1), medium
(tertile 2) and high groups (tertile 3). Additionally, tertile
grouping is a commonly used statistical method that evenly
distributes sample sizes, preventing an imbalance in group
distribution that could affect the robustness of the statistical
results. This method has also been applied in a study exploring the
risk factors for non-small cell lung cancer (NSCLC), where the
incidence of NSCLC was observed to consistently increase along the
tertiles of the TyG index (10). To
further investigate the incidence rates among populations with
different levels of the TyG index, all patients in the present
study were divided into three groups based on tertiles of the TyG
index (tertile 1, minimum to 8.29; tertile 2, 8.29 to 8.83; tertile
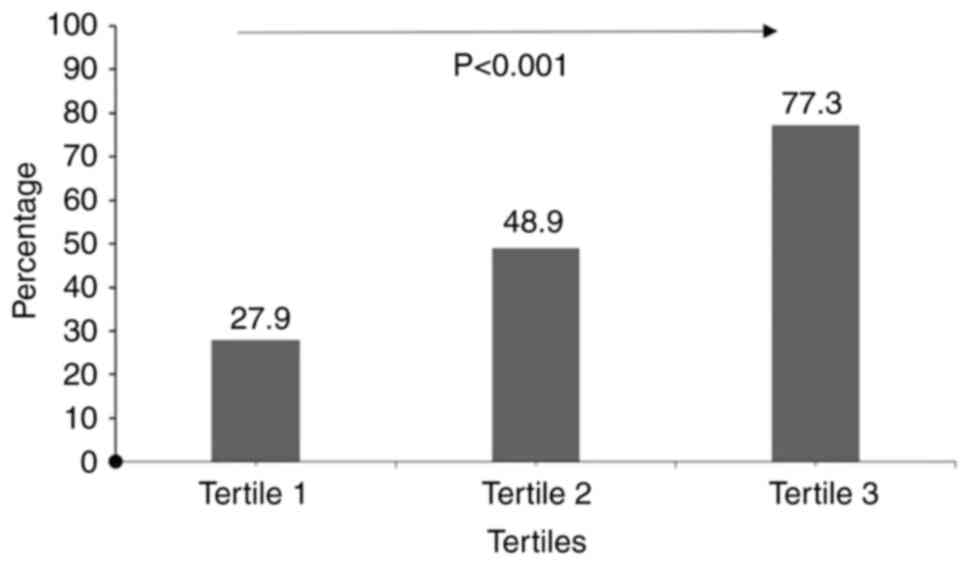
3, 8.83 to maximum). Fig. 1
indicates a rising trend in the incidence rates across the three
groups, with statistically significant differences observed (27.9
vs. 48.9 vs. 77.3%; P<0.001).
Value of the TyG index for predicting
the incident of malignant melanoma
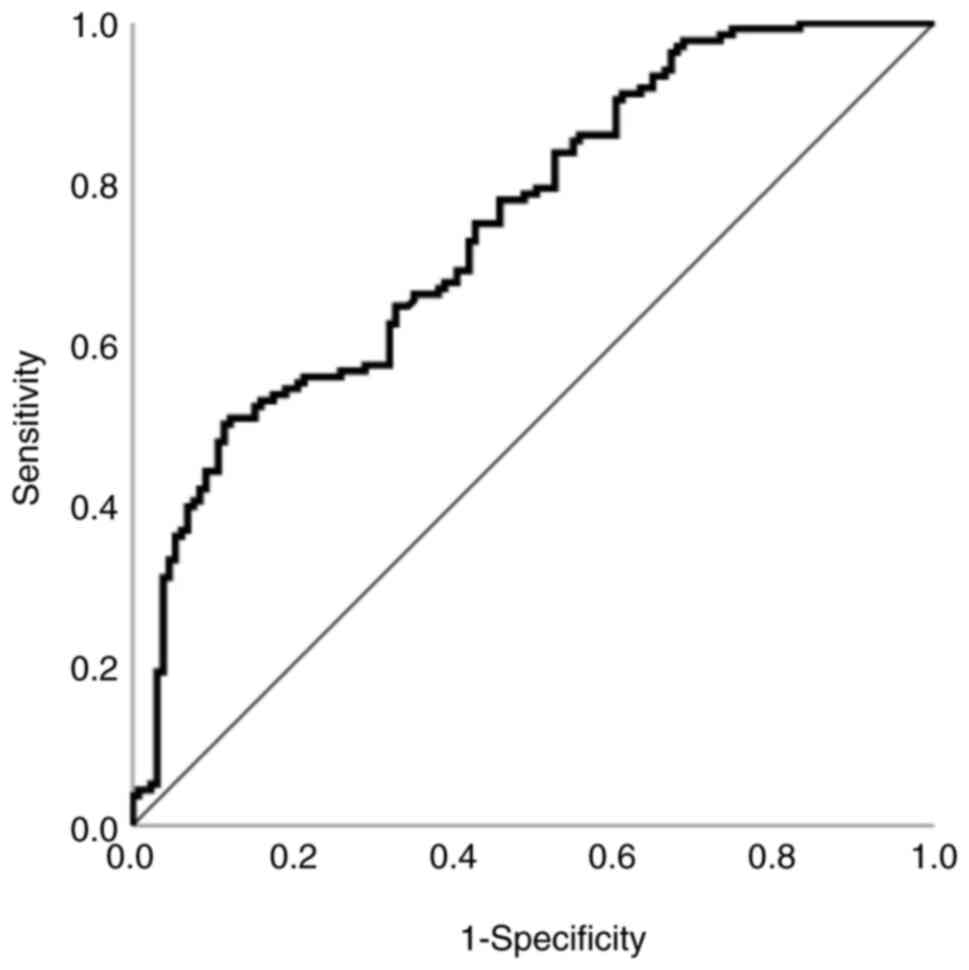
An ROC curve was generated for the TyG index (area
under the curve, 0.748; standard error, 0.03; P<0.001; 95% CI,
0.69–0.806; Fig. 2), with the
optimal cut-off point for the TyG index determined to be 8.87
(sensitivity, 50%; specificity, 88.5%). This implies that the TyG
index is a clinically acceptable predictive indicator for the risk
of malignant melanoma.
Discussion
Malignant melanoma stands as one of the most
malignant tumors, and despite advancements in immunotherapeutic and
targeted approaches that have somewhat ameliorated the prognosis,
the 5-year survival rate for late-stage malignant melanoma patients
remains poor (35.1%) (2).
Established high-risk factors for malignant melanoma include
exposure to ultraviolet radiation, large congenital nevi and
certain genetic mutations (4–6).
However, despite measures taken to address these high-risk factors,
the incidence of malignant melanoma continues to rise annually
(2). This prompts the exploration
of new high-risk factors for malignant melanoma occurrence. The
present study suggested that the TyG index, indicative of IR, is an
independent risk factor for malignant melanoma occurrence. IR is a
potential mechanism underlying elevated TyG index values, and TyG
index may serve as a surrogate marker for IR (15). Although the
hyperinsulinemic-euglycemic clamp technique is considered the gold
standard for diagnosing IR, its high cost and complexity limit its
routine application (14).
Therefore, the present study utilized the TyG index as a substitute
for IR and elaborated on the underlying mechanisms. The study was
retrospective in nature, and fasting insulin levels could not be
obtained, limiting the ability to provide direct evidence of IR.
These aspects will carefully be considered in future research. To
the best of our knowledge, this is the first study to investigate
the association between the TyG index and malignant melanoma
incidence.
Independent t-tests, Mann-Whitney U tests and
χ2 tests were used to analyze baseline variables. Other
than TyG index, there was also a significant difference between the
two groups in terms of drinking history (P=0.037). Multiple studies
have long established that alcohol consumption is a high-risk
factor for the development of malignant tumors (20). Recent studies have also shown a
close association between alcohol consumption and MM. Alcohol
intake may increase the severity of sunburn, which is a well-known
high-risk factor for MM, and this could be a potential mechanism by
which alcohol promotes MM (21). A
Mendelian randomization analysis in 2023 suggested that alcohol
consumption had a positive effect on the development of skin MM
(OR, 2.23; 95% CI, 1.11–4.47; P=0.02) (22). A meta-analysis also indicated that
alcohol consumption might be associated with an increased risk of
melanoma, but residual confounding factors and bias could not be
ruled out (23). This is contrary
to the present findings, where an initial rank-sum test revealed
that the control groups had a higher drinking rate (P=0.037).
However, subsequent univariate and multivariate logistic regression
analyses did not show an association between alcohol consumption
and MM (P=0.081 and P=0.134, respectively). Therefore, the
association between alcohol consumption and MM requires further
exploration through large-scale prospective studies. However, it is
important to note that this variable was not the primary focus of
the present study, and its significance does not alter the central
conclusions regarding the association between the TyG index and
melanoma risk.
IR arises from diminished responsiveness to insulin
in the body. Initial research predominantly focused on the
association of IR with cerebrovascular and cardiovascular diseases,
diabetes and other endocrine disorders (such as Cushing's syndrome
and hyperuricemia) (7,9,24).
However, subsequent studies have revealed significant implications
of IR in the onset and progression of tumors. A comparative study
by Panigoro et al (11)
involving 212 patients with breast cancer and 212 healthy
individuals demonstrated a non-linear correlation between the TyG
index and breast cancer risk, with individuals having a TyG index
>8.87 facing a three-fold increase in breast cancer risk. This
finding aligns with results from a large-scale study indicating a
correlation between IR and breast cancer incidence in
postmenopausal women (25).
Furthermore, research by Li et al (13) on patients with colorectal cancer
suggested an association between elevated TyG index and an
increased risk of colorectal cancer. Similarly, Yan et al
(10) demonstrated that a higher
TyG index elevated the risk of NSCLC. The value of the TyG index is
also reflected in renal cell carcinoma. A retrospective study by
Qin et al (26) showed that
individuals with a higher TyG index had a higher incidence of renal
cell carcinoma, and that patients with renal cell carcinoma had a
poor prognosis. This is consistent with the present study showing
the predictive value of IR (TyG index) for cancer. To the best of
our knowledge, this is the first study to explore the association
between TyG index and melanoma incidence. These studies corroborate
our conclusion that IR, or a higher TyG index, represents a
significant risk factor for malignant melanoma incidence.
IR leads to hyperinsulinemia, which is one of the
mechanisms through which IR promotes tumor onset and progression
(27). Insulin binding to the
insulin receptor (INSR) activates the PI3K/Akt/mTOR pathway,
thereby exerting its effects on promoting angiogenesis, cell
proliferation and differentiation (28). Additionally, INSR-A has been shown
to be upregulated in various tumors (such as breast and lung
cancer) (29). Therefore, the
interaction between elevated insulin levels and upregulated INSR-A
plays a crucial role in promoting tumor onset and development. For
instance, in an animal model study by Zhang et al (30), a decrease in insulin levels
significantly reduced the incidence of pancreatic cancer precursor
lesions. Similarly, a 16-year observational study found that
individuals with higher blood insulin concentrations had a
significantly higher risk of pancreatic cancer compared with those
with lower blood insulin concentrations (31). By contrast, the indirect reduction
of blood insulin levels by metformin demonstrated anticancer
effects (32). The anticancer
properties of metformin stem from its ability to activate adenosine
5′-monophosphate-activated protein kinase, thereby inhibiting mTOR
signaling and protein synthesis, ultimately suppressing cell
proliferation (33). Several
studies have indicated that metformin can inhibit the proliferation
of various tumors, including lung and breast cancer (34,35).
This highlights the importance of not only recognizing the role of
IR in promoting tumor growth, but also considering the improvement
of IR as a potential target for cancer therapy.
In addition, under the state of IR, there is a
decrease in insulin-like growth factor (IGF) binding protein
production, leading to an increase in circulating IGF-1, which is
another mechanism through which IR promotes tumor onset and
development (36). On the one hand,
IGF-1 binds to the IGF-1 receptor (IGF-1R) and exerts its effects
on promoting cell proliferation and inhibiting apoptosis through
the PI3K/Akt/mTOR pathway (37).
Use of a hybrid mouse model in the study by Wang et al
(38) suggested that elevated IGF-1
levels led to increased incidence and invasiveness of prostate
cancer. Additionally, Yakar et al (39) observed that implantation of colon
cancer tissue into IGF-1 gene-deleted (LID) mice and normal mice
showed significantly higher tumor incidence in the control mice
compared with that in the LID mice, with lower circulating IGF-1
levels in the LID mice. However, after administration of IGF-1 to
the LID mice, there was no difference in tumor growth between the
two groups (39). IGF-1 can also
bind to INSR-1 and, through the aforementioned mechanism, promote
tumor onset (40).
IR can also inhibit PTEN function. As a negative
regulator of the PI3K/Akt signaling pathway, PTEN plays a critical
role in tumor suppression. PTEN induces oxidative phosphorylation
and reduces glycolysis, thereby decreasing energy metabolism in
cancer cells, which may be a key mechanism underlying its
tumor-suppressive effects (41).
Inactivation of PTEN leads to increased PIP3 recruitment and
excessive activation of Akt and downstream signaling cascades,
promoting cell survival and tumorigenesis (42). Enhanced Akt activity may increase
immune evasion by downregulating the expression of programmed death
ligand 1 on the tumor cell surface, thereby promoting cancer
development and progression (43).
Additionally, excessive activation of the PI3K/Akt pathway can
further impair insulin sensitivity and contribute to the
development of IR, establishing a vicious cycle of IR and PTEN
inactivation that ultimately leads to tumorigenesis (44). In hepatic malignancies, IR and
hyperinsulinemia lead to upregulation of the IGF axis (including
IGF-1, IGF-2, IGF-1R, IGF-2R, and IGF binding proteins IGFBP1-6 and
INRS-1), subsequently promoting activation and phosphorylation of
the PI3K/PTEN/Akt signaling pathway, inducing cell proliferation
and inhibiting apoptosis, which together drive tumorigenesis
(45). Given that PTEN mutations
are a significant susceptibility factor for malignant melanoma,
greater attention should be paid to the risks associated with IR
(46).
The tumor-promoting effects of IR are also related
to inflammation. IR causes adipocyte dysfunction, increasing the
infiltration of M1-type pro-inflammatory macrophages in adipose
tissue, thereby enhancing the secretion of pro-inflammatory
cytokines from adipocytes, including tumor necrosis factor-α
(TNF-α), interleukin-6 (IL-6) and IL-1β (47–49).
These cytokines further inhibit insulin signaling, exacerbating IR.
IL-6, as an inflammatory cytokine, can induce cancer cell
proliferation through STAT signaling and simultaneously block host
antitumor immune responses (50).
Elevated levels of IL-6 have been observed in overweight and obese
women with IR and early stage breast cancer (51). Additionally, circulating IL-6 levels
are higher in men with prostate cancer compared with those in men
with benign conditions (52).
TNF-α, a key pro-inflammatory factor, is involved in maintaining
immune system homeostasis, inflammation and host defense. TNF-α
promotes and amplifies IR by inhibiting INSR signaling, and plays a
crucial role in the formation and progression of various tumors
(53). For instance, serum TNF-α
levels are significantly associated with tumor stage in patients
with prostate cancer (54). TNF-α
also promotes tumor progression and metastasis in breast cancer by
facilitating epithelial-mesenchymal transition (55). Furthermore, elevated circulating
TNF-α levels can activate the NF-κB pathway, exerting
anti-apoptotic effects that promote tumorigenesis in overweight
patients with IR (56). As melanoma
is an immunogenic tumor, it is not unexpected that this phenomenon
is also observed in malignant melanoma (57). A study by Molinelli et al
(58) indicated that TNF-α
expression was significantly elevated in the peritumoral tissue of
melanoma samples compared with that in controls, suggesting that
the upregulation of TNF-α may contribute to the growth and local
invasiveness of cutaneous melanoma. Therefore, the interaction
between IR and inflammation should be evaluated as a risk factor in
tumor diagnostics.
The present study also had several limitations:
Firstly, it was a retrospective study with a limited number of
participants, and due to excessive missing data and as it was not a
primary focus of this study, high-density lipoprotein (HDL) was
removed. Secondly, an association was not found between the TyG
index and BMI, which may be due to the limited sample size.
Thirdly, there was no direct evidence of IR or hyperinsulinemia due
to the limitations of a retrospective study. Finally, the dynamic
changes in the TyG index were not observed. Furthermore, we also
acknowledge the potential influence of insulin deficiency on the
TyG index (15). However, the
present study was retrospective, and diabetic patients were
excluded to minimize the confounding effect. Fasting insulin is not
routinely measured due to its limited clinical applicability and
cost in patients without specific diseases (59), so we did not have access to fasting
insulin data in the analysis. In future prospective studies,
greater emphasis will be placed on assessing fasting insulin levels
to better elucidate the underlying mechanisms of TyG index
variations. Therefore, further basic research and large-scale
observational studies are needed to further substantiate the
association between IR and the incidence of malignant melanoma.
In conclusion, the present study provides the first
evidence that the TyG index, representing IR, is a significant risk
factor for the incidence of malignant melanoma. This finding has
significant clinical implications, including the following: i)
Early risk assessment: The TyG index can help identify individuals
at higher risk for melanoma, enabling early detection and more
frequent skin monitoring. ii) Comprehensive risk evaluation: The
TyG index provides a tool for assessing the risk of melanoma. iii)
Guiding interventions: High TyG index can prompt lifestyle and
therapeutic interventions to improve insulin sensitivity,
potentially reducing melanoma risk. iv) Prognostic value: The index
may serve as a marker for monitoring metabolic health and treatment
outcomes in patients with melanoma. Overall, the TyG index offers a
simple, cost-effective approach to melanoma risk assessment and
metabolic health management.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JS, ZH and CS contributed to the study conception
and design. JS was responsible for the methodology, and ZH
performed the formal analysis and investigation JS and ZH prepared
the original draft, and CS and JS reviewed and edited the
manuscript. Resources and study supervision were provided by CS.
All authors have read and approved the manuscript. All authors have
read and approved the manuscript. JS and CS confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Written informed consent was not sought from the
patients, as this study was retrospective in nature and involved
only the analysis of existing medical records. No direct patient
contact or intervention occurred, and all patient data were
anonymized to ensure confidentiality. Therefore, only verbal
informed consent was obtained from the patients for participation
in this study. Additionally, the study protocol, including the
waiver of written informed consent, was reviewed and approved by
the Ethics Committee of the First Affiliated Hospital of Nanjing
Medical University (protocol code, 2024-SR-066; date of approval,
2024-02-27).
Patient consent for publication
Verbal informed consent was obtained from all
subjects involved for publication of the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raimondi S, Suppa M and Gandini S:
Melanoma epidemiology and sun exposure. Acta Derm Venereol.
100:adv001362020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waseh S and Lee JB: Advances in melanoma:
Epidemiology, diagnosis, and prognosis. Front Med (Lausanne).
10:12684792023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eisemann N, Schumann L, Baltus H, Labohm
L, Kraywinkel K and Katalinic A: Longer survival from melanoma in
Germany. Dtsch Arztebl Int. 121:45–51. 2024.PubMed/NCBI
|
|
4
|
Toussi A, Mans N, Welborn J and Kiuru M:
Germline mutations predisposing to melanoma. J Cutan Pathol.
47:606–616. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holly EA, Kelly JW, Shpall SN and Chiu SH:
Number of melanocytic nevi as a major risk factor for malignant
melanoma. J Am Acad Dermatol. 17:459–468. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fears TR, Scotto J and Schneiderman MA:
Mathematical models of age and ultraviolet effects on the incidence
of skin cancer among whites in the United States. Am J Epidemiol.
105:420–427. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S, Chen Y, Liu X, Li M, Wu B, Li Y,
Liang Y, Shao X, Holthöfer H and Zou H: Insulin resistance and
metabolic syndrome in normal-weight individuals. Endocrine.
46:496–504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fracanzani AL, Valenti L, Bugianesi E,
Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D,
Marchesini G and Fargion S: Risk of severe liver disease in
nonalcoholic fatty liver disease with normal aminotransferase
levels: A role for insulin resistance and diabetes. Hepatology.
48:792–798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Czech MP: Mechanisms of insulin resistance
related to white, beige, and brown adipocytes. Mol Metab. 34:27–42.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan X, Gao Y, Tong J, Tian M, Dai J and
Zhuang Y: Association between triglyceride glucose index and
non-small cell lung cancer risk in Chinese population. Front Oncol.
11:5853882021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Panigoro SS, Sutandyo N, Witjaksono F,
Siregar NC, Ramli R, Hariani R, Pangarsa EA, Prajoko YW, Puruhita
N, Hamdani W, et al: The association between triglyceride-glucose
index as a marker of insulin resistance and the risk of breast
cancer. Front Endocrinol (Lausanne). 12:7452362021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alkurt EG, Şahin F, Tutan B, Canal K and
Turhan VB: The relationship between papillary thyroid cancer and
triglyceride/glucose index, which is an indicator of insulin
resistance. Eur Rev Med Pharmacol Sci. 26:6114–6120.
2022.PubMed/NCBI
|
|
13
|
Li W, Liu T, Qian L, Wang Y, Ma X, Cao L,
Zhang Q and Qu J: Insulin resistance and inflammation mediate the
association of abdominal obesity with colorectal cancer risk. Front
Endocrinol (Lausanne). 13:9831602022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DeFronzo RA, Tobin JD and Andres R:
Glucose clamp technique: A method for quantifying insulin secretion
and resistance. Am J Physiol. 237:E214–E223. 1979.PubMed/NCBI
|
|
15
|
Sánchez-García A, Rodríguez-Gutiérrez R,
Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A,
Solis RC, Álvarez-Villalobos NA and González-González JG:
Diagnostic accuracy of the triglyceride and glucose index for
insulin resistance: A systematic review. Int J Endocrinol.
2020:46785262020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rohm TV, Meier DT, Olefsky JM and Donath
MY: Inflammation in obesity, diabetes, and related disorders.
Immunity. 55:31–55. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim DS and Scherer PE: Obesity, diabetes,
and increased cancer progression. Diabetes Metab J. 45:799–812.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poznyak A, Grechko AV, Poggio P,
Myasoedova VA, Alfieri V and Orekhov AN: The diabetes
mellitus-atherosclerosis connection: The role of lipid and glucose
metabolism and chronic inflammation. Int J Mol Sci. 21:18352020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gado M, Tsaousidou E, Bornstein SR and
Perakakis N: Sex-based differences in insulin resistance. J
Endocrinol. 261:e2302452024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bagnardi V, Rota M, Botteri E, Tramacere
I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, et
al: Alcohol consumption and site-specific cancer risk: A
comprehensive dose-response meta-analysis. Br J Cancer.
112:580–593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saladi RN, Nektalova T and Fox JL:
Induction of skin carcinogenicity by alcohol and ultraviolet light.
Clin Exp Dermatol. 35:7–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu J, Liu W, Liu X, Zhou X and Li G:
Alcohol drinking, smoking, and cutaneous melanoma risk: Mendelian
randomization analysis. Gac Sanit. 37:1023512023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gandini S, Masala G, Palli D, Cavicchi B,
Saieva C, Ermini I, Baldini F, Gnagnarella P and Caini S: Alcohol,
alcoholic beverages, and melanoma risk: A systematic literature
review and dose-response meta-analysis. Eur J Nutr. 57:2323–2332.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reaven GM: Banting lecture 1988. Role of
insulin resistance in human disease. Diabetes. 37:1595–1607. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan K, Chlebowski RT, Mortimer JE, Gunter
MJ, Rohan T, Vitolins MZ, Adams-Campbell LL, Ho GYF, Cheng TD and
Nelson RA: Insulin resistance and breast cancer incidence and
mortality in postmenopausal women in the women's health initiative.
Cancer. 126:3638–3647. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin G, Sun Z, Jin Y, Ren X, Zhang Z, Wang
S, Zhou G, Huang K, Zhao H and Jiang X: The association between the
triglyceride-glucose index and prognosis in postoperative renal
cell carcinoma patients: A retrospective cohort study. Front
Endocrinol (Lausanne). 15:13017032024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang AMY, Wellberg EA, Kopp JL and
Johnson JD: Hyperinsulinemia in obesity, inflammation, and cancer.
Diabetes Metab J. 45:285–311. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lien EC, Lyssiotis CA and Cantley LC:
Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer.
Recent Results Cancer Res. 207:39–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Belfiore A, Frasca F, Pandini G, Sciacca L
and Vigneri R: Insulin receptor isoforms and insulin
receptor/insulin-like growth factor receptor hybrids in physiology
and disease. Endocr Rev. 30:586–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang AMY, Magrill J, de Winter TJJ, Hu X,
Skovsø S, Schaeffer DF, Kopp JL and Johnson JD: Endogenous
hyperinsulinemia contributes to pancreatic cancer development. Cell
Metab. 30:403–404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stolzenberg-Solomon RZ, Graubard BI, Chari
S, Limburg P, Taylor PR, Virtamo J and Albanes D: Insulin, glucose,
insulin resistance, and pancreatic cancer in male smokers. JAMA.
294:2872–2878. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lv Z and Guo Y: Metformin and its benefits
for various diseases. Front Endocrinol (Lausanne). 11:1912020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gwinn DM, Shackelford DB, Egan DF,
Mihaylova MM, Mery A, Vasquez DS, Turk BE and Shaw RJ: AMPK
phosphorylation of raptor mediates a metabolic checkpoint. Mol
Cell. 30:214–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Podhorecka M, Ibanez B and Dmoszyńska A:
Metformin-its potential anti-cancer and anti-aging effects. Postepy
Hig Med Dosw (Online). 71:170–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vallianou NG, Evangelopoulos A and Kazazis
C: Metformin and cancer. Rev Diabet Stud. 10:228–235. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frasca F, Pandini G, Scalia P, Sciacca L,
Mineo R, Costantino A, Goldfine ID, Belfiore A and Vigneri R:
Insulin receptor isoform A, a newly recognized, high-affinity
insulin-like growth factor II receptor in fetal and cancer cells.
Mol Cell Biol. 19:3278–3288. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhong W, Wang X, Wang Y, Sun G, Zhang J
and Li Z: Obesity and endocrine-related cancer: The important role
of IGF-1. Front Endocrinol (Lausanne). 14:10932572023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang S, Wang N, Yu B, Cao M, Wang Y, Guo
Y, Zhang Y, Zhang P, Yu X, Wang S, et al: Circulating IGF-1
promotes prostate adenocarcinoma via FOXO3A/BIM signaling in a
double-transgenic mouse model. Oncogene. 38:6338–6353. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yakar S, Pennisi P, Zhao H, Zhang Y and
LeRoith D: Circulating IGF-1 and its role in cancer: Lessons from
the IGF-1 gene deletion (LID) mouse. Novartis Found Symp. 262:3–18.
265–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Malaguarnera R, Sacco A, Voci C, Pandini
G, Vigneri R and Belfiore A: Proinsulin binds with high affinity
the insulin receptor isoform A and predominantly activates the
mitogenic pathway. Endocrinology. 153:2152–2163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ortega-Molina A and Serrano M: PTEN in
cancer, metabolism, and aging. Trends Endocrinol Metab. 24:184–189.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng H, Liu P, Zhang F, Xu E, Symonds L,
Ohlson CE, Bronson RT, Maira SM, Di Tomaso E, Li J, et al: A
genetic mouse model of invasive endometrial cancer driven by
concurrent loss of Pten and Lkb1 is highly responsive to mTOR
inhibition. Cancer Res. 74:15–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen J, Zhang XD and Proud C: Dissecting
the signaling pathways that mediate cancer in PTEN and LKB1
double-knockout mice. Sci Signal. 8:pe12015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang XL, Zhang L, Youker K, Zhang MX, Wang
J, LeMaire SA, Coselli JS and Shen YH: Free fatty acids inhibit
insulin signaling-stimulated endothelial nitric oxide synthase
activation through upregulating PTEN or inhibiting Akt kinase.
Diabetes. 55:2301–2310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu J, Shen J, Sun TT, Zhang X and Wong N:
Obesity, insulin resistance, NASH and hepatocellular carcinoma.
Semin Cancer Biol. 23:483–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leachman SA, Lucero OM, Sampson JE,
Cassidy P, Bruno W, Queirolo P and Ghiorzo P: Identification,
genetic testing, and management of hereditary melanoma. Cancer
Metastasis Rev. 36:77–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Olefsky JM and Glass CK: Macrophages,
inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kershaw EE and Flier JS: Adipose tissue as
an endocrine organ. J Clin Endocrinol Metab. 89:2548–2556. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vozarova B, Weyer C, Hanson K, Tataranni
PA, Bogardus C and Pratley RE: Circulating interleukin-6 in
relation to adiposity, insulin action, and insulin secretion. Obes
Res. 9:414–417. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L and Karin M: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gonullu G, Ersoy C, Ersoy A, Evrensel T,
Basturk B, Kurt E, Oral B, Gokgoz S and Manavoglu O: Relation
between insulin resistance and serum concentrations of IL-6 and
TNF-alpha in overweight or obese women with early stage breast
cancer. Cytokine. 31:264–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu CT, Chen MF, Chen WC and Hsieh CC: The
role of IL-6 in the radiation response of prostate cancer. Radiat
Oncol. 8:1592013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bulló M, García-Lorda P, Peinado-Onsurbe
J, Hernández M, Del Castillo D, Argilés JM and Salas-Salvadó J:
TNFalpha expression of subcutaneous adipose tissue in obese and
morbid obese females: Relationship to adipocyte LPL activity and
leptin synthesis. Int J Obes Relat Metab Disord. 26:652–658. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Michalaki V, Syrigos K, Charles P and
Waxman J: Serum levels of IL-6 and TNF-alpha correlate with
clinicopathological features and patient survival in patients with
prostate cancer. Br J Cancer. 90:2312–2316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cruceriu D, Baldasici O, Balacescu O and
Berindan-Neagoe I: The dual role of tumor necrosis factor-alpha
(TNF-α) in breast cancer: Molecular insights and therapeutic
approaches. Cell Oncol (Dordr). 43:1–18. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pfalzer AC, Leung K, Crott JW, Kim SJ, Tai
AK, Parnell LD, Kamanu FK, Liu Z, Rogers G, Shea MK, et al:
Incremental elevations in TNFα and IL6 in the human colon and
procancerous changes in the mucosal transcriptome accompany
adiposity. Cancer Epidemiol Biomarkers Prev. 27:1416–1423. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zheng YJ, Ho W, Sanlorenzo M, Vujic I,
Daud A, Algazi A, Rappersberger K and Ortiz-Urda S: Melanoma risk
during immunomodulating treatment. Melanoma Res. 32:411–418. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Molinelli E, Ceccarelli G, Fantone S, Di
Mercurio E, Gambini D, Maurizi A, Perugini J, Tossetta G,
Brisigotti V, De Simoni E, et al: Melanoma and subcutaneous adipose
tissue: Role of peritumoral adipokines in disease characterization
and prognosis. Pigment Cell Melanoma Res. 36:423–430. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sims EK, Carr ALJ, Oram RA, DiMeglio LA
and Evans-Molina C: 100 Years of insulin: Celebrating the past,
present and future of diabetes therapy. Nat Med. 27:1154–1164.
2021. View Article : Google Scholar : PubMed/NCBI
|