Introduction
Helicobacter pylori is a bacterium that is
capable of thriving at the low oxygen and acidic conditions of the
stomach, and infection is closely related to various
gastrointestinal disorders, such as peptic ulcer disease and
non-ulcer dyspepsia. (1). The
bacterium produces the enzyme, urease, which decomposes urea to
generate ammonia thereby neutralizing the surrounding acid and
facilitating its survival in the highly acidic stomach mucosa
(2). This property notably
contributes to the issue of drug resistance of H. pylori,
and the application of novel nanomaterials for the treatment of
drug-resistant bacteria represents a promising avenue (3–5). The
risk associated with H. pylori infection stems from its
ability to induce chronic inflammation, which is a significant
factor in tumorigenesis. Chronic inflammation can lead to genetic
mutations in gastric mucosal cells and increase the risk of gastric
cancer (6). In recent years, with
the in-depth research on the association between H. pylori
infection and cancer, increasing evidence suggests that H.
pylori infection is not only associated with the development of
gastric cancer, but also potentially associated with other types of
cancer such as pancreatic cancer (7,8).
Pancreatic cancer is a highly malignant tumor
characterized by subtle early symptoms that can be easily
overlooked or misdiagnosed resulting in a mid-to-late stage
diagnosis and a missed opportunity for the most effective treatment
(9). In addition, the complex
biological behavior of pancreatic cancer makes it a challenging
tumor to treat (10). According to
the 2022 Global Cancer Statistics, there were ~511,000 new cases of
pancreatic cancer and 467,000 pancreatic cancer-associated deaths.
Pancreatic cancer has one of the worst prognoses, ranking sixth
among the causes of cancer-related deaths in both men and women,
and accounting for ~5% of all cancer-related deaths worldwide. The
incidence is approximately four times higher in countries with a
higher Human Development Index (HDI) compared with those with a
lower HDI (11).
The etiology of pancreatic cancer is complex and is
not yet fully understood. Existing studies suggested that
pancreatic cancer may be associated with several factors such as
genetic factors, dietary factors, smoking, alcoholism, chronic
pancreatitis, pancreatic stones, obesity and metabolic syndrome.
Among them, mutations in BRCA1, BRCA2 and CDKN2A are associated
with an increased risk of pancreatic cancer (12,13).
Smoking and alcohol abuse may lead to DNA damage and gene mutations
in pancreatic cells and are therefore considered to be important
risk factors for pancreatic cancer (14).
Although the etiology of pancreatic cancer has not
yet been fully elucidated, the potential carcinogenic role of H.
pylori infection has attracted increased attention from
researchers. There have been numerous attempts to study the
association between H. pylori infection and pancreatic
cancer risk. However, the studies have revealed notable
heterogeneity and even contradictory results (15). Huang et al (16) conducted a nested case-control study
of 448 pancreatic cancer cases and 447 individually matched control
subjects; the authors demonstrated that there was no marked
association between H. pylori infection and pancreatic
cancer risk in Western European populations [odds ratio (OR), 0.96;
95% confidence interval (CI), 0.70–1.31]. By contrast, in a
population-based case-control study, Risch et al (17) found an association between
pancreatic cancer and CagA− H. pylori
colonization, especially for individuals in the non-O blood group
(OR, 2.78; 95% CI, 1.49–5.20). Even meta-analyses that combined
several studies have shown varying results. A meta-analysis by Xiao
et al (18) showed a notable
association between H. pylori infection and pancreatic
cancer development in Europe and East Asia, but this association
was weak in North America. A meta-analysis by Zhou et al
(19) indicated that there was no
sufficient evidence to support an association between H.
pylori infection and increased risk of pancreatic cancer, with
similar results for the CagA+ H. pylori infection
subgroup. A quantitative synthesis of 10 studies conducted by
Schulte et al (20) revealed
that CagA+ H. pylori infection may be a
protective factor for pancreatic cancer development (OR, 0.78; 95%
CI, 0.67–0.91), whereas CagA− strain infection may be a
potential risk factor (OR, 1.30; 95% CI, 1.02–1.65). This
heterogeneity may stem from a variety of factors, including
differences in study design, region, ethnicity, H. pylori
strains, inconsistencies in diagnostic criteria for pancreatic
cancer and limitations in sample size.
Given the limitations and uncertainties of existing
studies, additional in-depth and systematic studies are necessary.
The present review aimed to collect additional abundant and
standardized data, including prospective and retrospective studies,
through rigorous inclusion criteria and more comprehensive
statistical analyses to overcome the controversies and limitations
in the existing studies and to clarify the association between
H. pylori infection and the risk of pancreatic cancer,
providing new ideas for the prevention and management strategies of
pancreatic cancer and H. pylori infection.
Materials and methods
Registration protocol
The present review followed the Preferred Reporting
Items for Systematic Reviews and Meta-Analyses guidelines (21) and was registered on PROSPERO
(https://www.crd.york.ac.uk/prospero/;
registration no. CRD42024520782) to ensure the completeness and
traceability of the study design, analysis and results. The
registration information includes the study purpose, study design,
key indicators and the plan for data collection and analysis.
Search strategy
The PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/) and Cochrane Library
(https://www.cochranelibrary.com/)
databases were searched, and the search scope was confined to
studies published from the inception of the database up to August
31st, 2023. When searching PubMed, subject terms were selected
according to the Medical Subject Headings (MeSH) subject term list,
and when searching Embase, Emtree was used to check and adjust the
terms. The pattern of subject terms plus free words were used while
searching, and the terms were mainly from the fields of pancreatic
cancer and H. pylori. For example, the following search
strategy was used in the PubMed database: [(‘Helicobacter
pylori’ (MeSH Terms) OR ‘helicobacter pylori’ (All
Fields) OR ‘H. pylori’ (All Fields)] AND [‘Pancreatic
neoplasms’ (MeSH Terms) OR ‘pancreatic neoplasms’ (All Fields) OR
‘pancreatic cancer’ (All Fields) OR ‘pancreatic adenocarcinoma’
(All Fields)] AND [‘1,000/1/1’ (Date-Publication): ‘2023/8/31’
(Date-Publication)]. The search terms used in the Embase and
Cochrane Library databases were similar to those used in PubMed.
The search strategy was developed after discussion among all
authors and modified by several rounds of adjustments. In addition,
a manual citation search was performed on the included studies.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i)
Case-control or cohort study; ii) human study object; iii)
investigation of the relationship between H. pylori
infection and pancreatic cancer risk; iv) H. pylori
infection with or without CagA+ status as determined by
serology [such as enzyme linked immunosorbent assay (ELISA) or
western blotting] or any other reliable method; v) diagnosis of
pancreatic cancer (exocrine pancreatic cancer or pancreatic duct
cancer) pathologically confirmed or from reliable documentation;
vi) available detailed data on the status of H. pylori
infection in pancreatic cancer cases and control groups; and vii)
literature published in the English language.
The exclusion criteria were as follows: i)
Unavailable abstracts or full texts; ii) unavailable detailed data
such as positive rate of H. pylori infection status; iii)
study types such as reviews, conferences, guidelines and
meta-analyses; iv) topic unrelated to the association between H.
pylori infection and pancreatic cancer risk; v) low quality
studies such as those with a too small a sample size or notable
flaws in the study design; vi) study data overlapped with data from
other studies; and vii) outcome indicators unrelated to pancreatic
cancer.
Two authors read the literature separately and
selected the studies strictly according to the aforementioned
inclusion and exclusion criteria. Differences were resolved through
discussion.
Data extraction
Data extraction was separately performed by two
authors with a unified data table. The results were cross-checked
and differences were resolved through discussion. Data were
extracted based on first author, publication year, study location,
study design type, sample size, mean age, diagnostic criteria for
H. pylori infection and pancreatic cancer, as well as CagA
status.
Literature quality evaluation
The quality of the methodology section of the
included studies was assessed according to the Newcastle-Ottawa
Scale (NOS) (22). This scale is
applicable to case-control and cohort studies. The contents of the
evaluation can be divided into three categories: i) Selection of
the study population: Definition of cases, representativeness of
case groups, selection of controls and definition of controls; ii)
comparability: Comparability between the control and case groups;
and iii) exposure: Determination of exposure, consistency of
exposure determination methods between groups and the non-response
rate. The evaluation was completed according to the scores of these
items. The item for between-group comparability can be awarded 2
points, while other items receive 1 point each, with a maximum
possible score of 9 points. The higher the score, the higher the
quality of the methodology section of the assessed study. A score
of >7 was considered to indicate a high-quality study in the
present analysis.
Statistical analysis
Stata (version 14.0; http://www.stata.com/) was used for statistical
analysis. An overall meta-analysis of all included studies was
performed to determine the association between H. pylori
infection and pancreatic cancer risk. In addition, several subgroup
analyses were performed, including a meta-analysis that included
only high-quality studies, and subgroup analyses sorted by study
design, geographical distribution and diagnostic criteria for H.
pylori infection. Subgroup analyses of the association between
CagA+ H. pylori infection and CagA−
H. pylori infection were also conducted.
OR was used as the combined effect size. OR and 95%
CI were used as statistical measures of the strength of
association. Heterogeneity between studies was measured by the
I2 value based on χ2 tests, and the
heterogeneity was considered to be significant if I2 was
>50% (23). Considering that
there is always heterogeneity in intervention effects across
multiple studies from different groups and geographical locations,
a random effects model was used to calculate the combined effect
sizes. The funnel plot method, Begg's rank correlation and Egger's
linear regression test were used to detect potential publication
bias. P<0.05 was considered to indicate a statistically
significant publication bias (24,25).
The effect of publication bias on the merged results was assessed
using the trim and fill method (26). Leave-one-out sensitivity analyses
were performed to check the robustness of the combined results and
to avoid a significant influence of extreme data from a single
study on the combined results.
Results
Literature search and characteristics
of the included studies
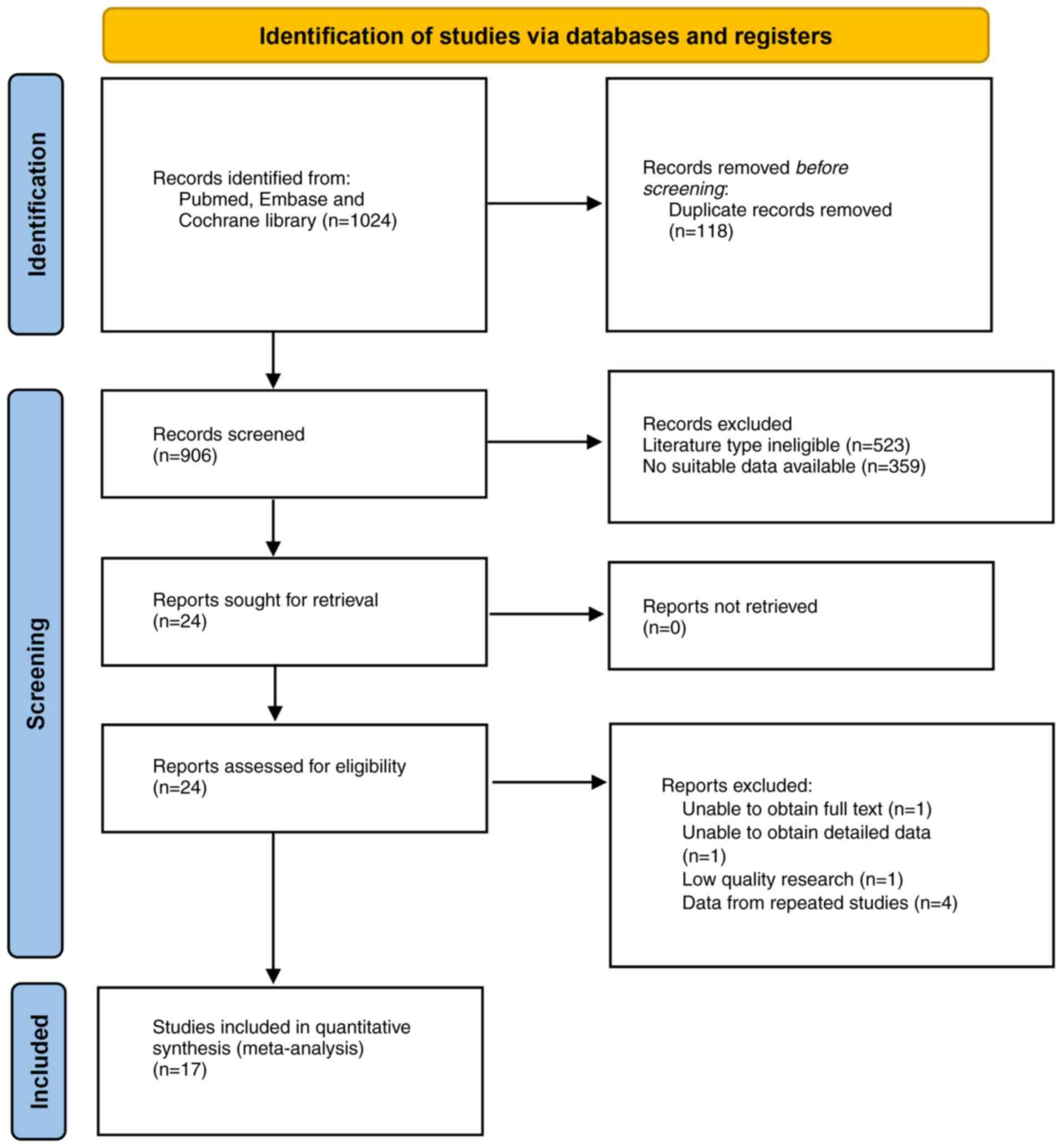
The search of the three databases (PubMed, Embase
and Cochrane Library) and the manual citation search yielded 1,024
articles, leaving 906 articles after screening for duplicates.
Further screening of titles, abstracts and full text yielded 17
suitable articles for the present study (16,17,20,27–40).
The selection process is shown in Fig.
1. These studies involved 67,910 participants (3,358 patients
with pancreatic cancer and 64,372 control group members) and
included 9 case-control studies, 5 nested case-control studies and
3 cohort studies. Of these studies, 7 were conducted in Asia, 5 in
Europe, 4 in North America and 1 in Oceania. The sample size range
of the studies was 53–30,110 (Table
I). Additionally, 16 studies used serological markers as the
diagnostic criteria for H. pylori infection, and only Hsu
et al (36) used
histopathological examination to diagnose H. pylori
infection. This histopathological approach may depend largely on
the level of expertise of the examiner and may not identify
previous infection. A total of 11 studies further tested for CagA
antibodies, while 1 study employed multiple serology to
simultaneously test for CagA, Vacuolating Cytotoxin A (VacA) and
other virulence factors (31).
 | Table I.Main characteristics of included
studies in the meta-analysis. |
Table I.
Main characteristics of included
studies in the meta-analysis.
| First author,
year | Region | Mean age or age
rangea | Study design | H. pylori
(+) in cancer group, n | H. pylori
(−) in cancer group, n | H. pylori
(+) in control group, n | H. pylori
(−) in cancer group, n | Sample size, n | H. pylori
detection method | Ascertainment of
pancreatic cancer | NOS score | (Refs.) |
|---|
| Raderer et
al, 1998 | Europe | 21-85 | Case-control | 60 | 32 | 13 | 14 | 119 | ELISA | Histopathological
diagnosis | 7 | (33) |
| Stolzenberg-Solomon
et al, 2001 | Europe | 50-69 | Nested
case-control | 99 | 22 | 165 | 61 | 347 | ELISA | Histopathological
diagnosis | 8 | (27) |
| de Martel et
al, 2008 | North America | 49.5/50.3 | Nested
case-control | 51 | 53 | 155 | 107 | 366 | ELISA | Tumor registry
reports | 7 | (30) |
| Lindkvist et
al, 2008 | Europe | 47.9/47.5 | Nested
case-control | 39 | 48 | 100 | 163 | 350 | ELISA | Diagnostic
histopathology or imaging | 8 | (34) |
| Risch et al,
2010 | North America | 66.9/68.3 | Case-control | 80 | 293 | 120 | 570 | 1,063 | ELISA | Medical report | 7 | (17) |
| Shimoyama et
al, 2010 | Asia | 66.9/61.6 | Case-control | 16 | 3 | 29 | 5 | 53 |
E-plateb | - | 4 | (38) |
| Hsu et al,
2014 | Asia | 51.1/51.0 | Cohort | 11 | 11 | 6,011 | 24,077 | 30,110 | Pathological
diagnosis | Tumor registry
reports | 5 | (36) |
| Risch et al,
2014 | Asia | 64.9/64.9 | Case-control | 233 | 528 | 327 | 467 | 1,555 | ELISA | Medical report | 8 | (29) |
| Ai et al,
2015 | Asia | 56.8/54.6 | Case-control | 31 | 25 | 16 | 44 | 116 | ELISA | Histopathology or
clinical diagnosis | 7 | (35) |
| Schulte et
al, 2015 | Oceania | 66.5/67.4 | Case-control | 113 | 443 | 119 | 489 | 1,164 | ELISA | Histopathology or
clinical diagnosis | 8 | (20) |
| Chen et al,
2016 | Europe | 50-75 | Cohort | 27 | 19 | 4,738 | 4,766 | 9,550 | ELISA | Tumor registry
reports | 6 | (37) |
| Huang et al,
2017 | Europe | 57.8 | Nested
case-control | 196 | 250 | 206 | 241 | 893 | ELISA | Tumor registry
reports | 8 | (16) |
| Hirabayashi et
al, 2019 | Asia | 40-69 | Cohort | 83 | 36 | 13,669 | 6,328 | 20,116 | ELISA | Tumor registry
reports | 7 | (32) |
| Permuth et
al, 2021 | North America | 67.6/59.0 | Case-control | 13 | 118 | 16 | 115 | 262 | Multiplex
serologyc | Histopathological
diagnosis | 8 | (31) |
| Laya et al,
2022 | Asia | 55.85/53.21 | Case-control | 34 | 27 | 42 | 52 | 155 | ELISA | Diagnostic
histopathology or imaging | 5 | (39) |
| Osaki et al,
2022 | Asia | 68.8/73.6 | Case-control | 20 | 39 | 11 | 14 | 84 | ELISA | - | 4 | (40) |
| Lee et al,
2023 | North America | 63.9/62 | Nested
case-control | 150 | 335 | 377 | 745 | 1,607 | ELISA | Medical report | 8 | (28) |
It is noteworthy that the study populations of
Stolzenberg-Solomon et al (27) and Yu et al (41) were both derived from the Finnish
ATBC cohort study, which was designed to identify the role of
α-tocopherol or β-carotene in reducing cancer incidence in male
smokers. The study by Yu et al (41) had a larger sample size and a longer
follow-up period but was not group-matched according to
interventions in the ATBC study, indicating that the results may
have been influenced by interventions in the ATBC cohort study.
Therefore, the study by Stolzenberg-Solomon et al (27) was finally included in the present
analysis. Some meta-analyses included both articles (19) indicating that there was likely some
duplication in the study population which could affect the
credibility of the results.
Literature quality evaluation
The NOS scores of the 17 included studies ranged
from 4 to 8, with an mean score of 6.8. The results of the
literature quality assessment are presented in Table I. A total of 12 studies were
determined to be high quality based on the NOS scores.
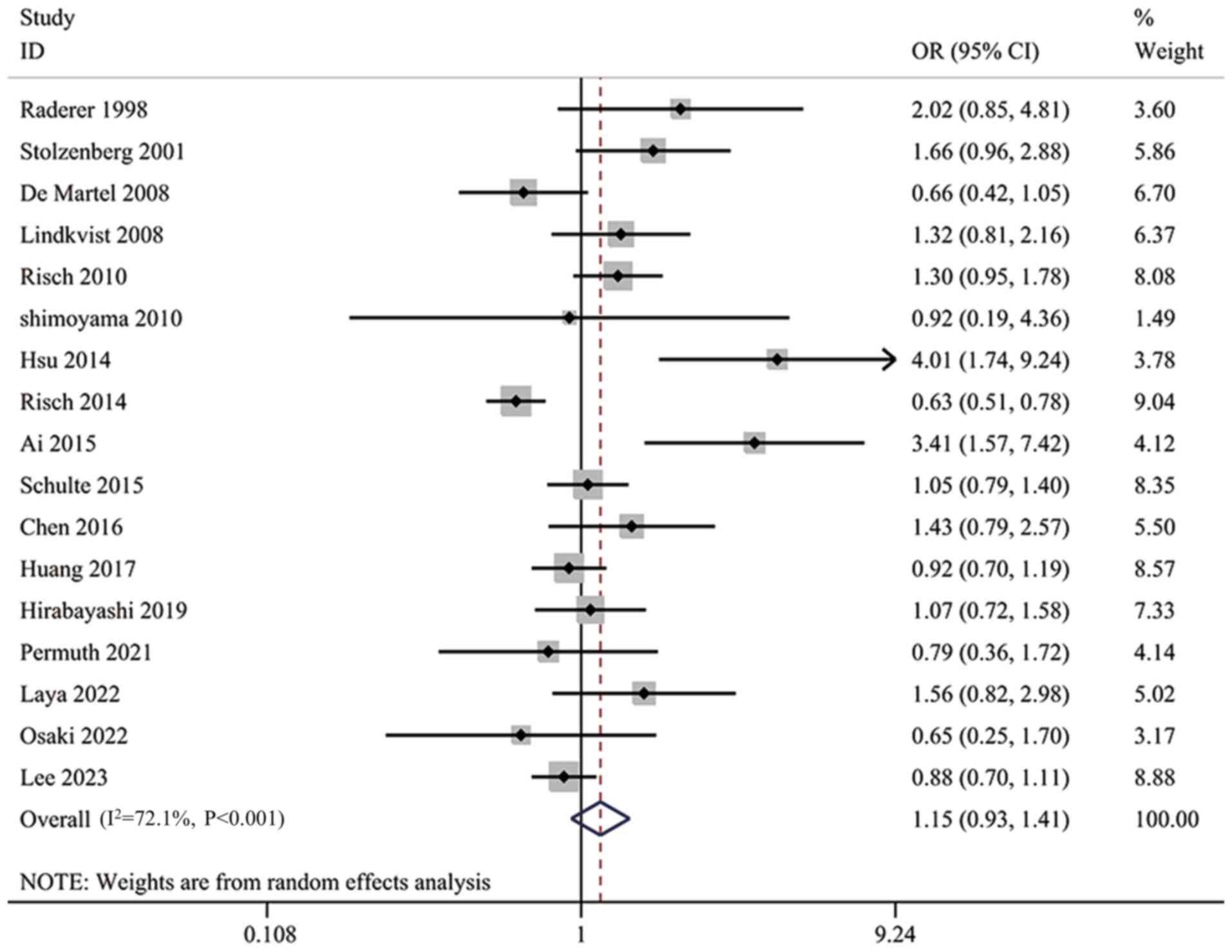
Overall analysis
All 17 studies were included in the analysis. The
heterogeneity test showed a significant heterogeneity among studies
(I2=72.1%; P<0.001; Fig.
2). The results of the meta-analysis suggested that H.
pylori infection was not significantly associated with the risk
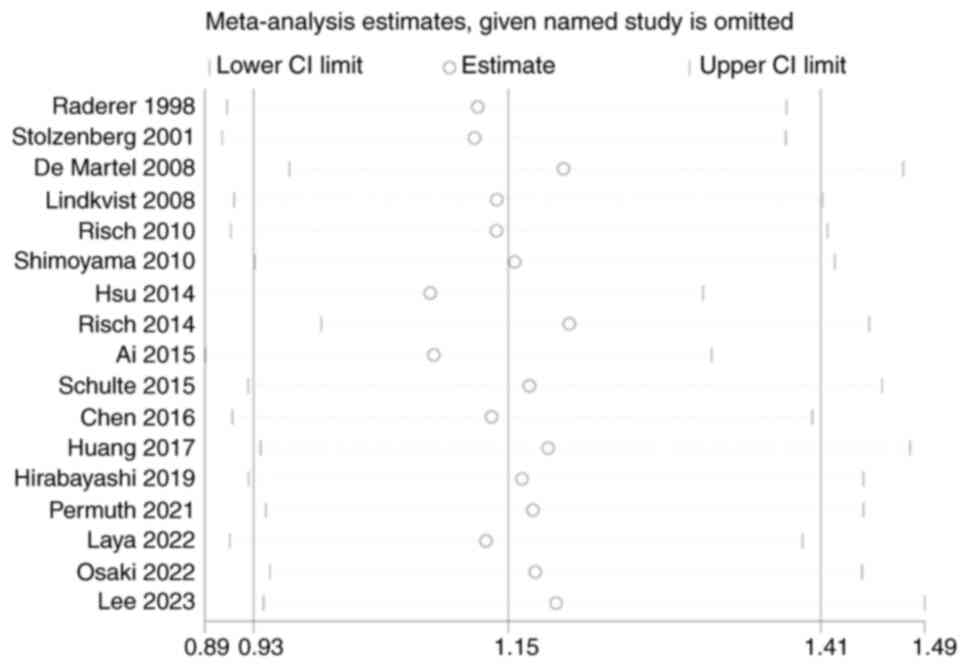
of pancreatic cancer (OR, 1.15; 95% CI, 0.93–1.41; Fig. 2). A leave-one-out sensitivity
analysis was performed to verify the reliability of the combined
results. The findings indicated that the combined results were
stable and not affected by the extremes of a single study (Fig. 3).
Subgroup analyses
To explore potential sources of heterogeneity and
identify key factors influencing the combined results, subgroup
analyses were conducted, where studies were grouped and analyzed
based on their quality, geographical region, study design,
diagnostic criteria and the subtype of H. pylori.
Subgroup analysis of high-quality
studies
To reduce the potential impact of low-quality
studies on the combined outcomes, only 12 high-quality studies
(16,17,20,27–35)
were included in this subgroup. The heterogeneity of this subgroup
was still significant (I2=73.3%; P<0.001). The
analysis showed no significant association between H. pylori
infection and pancreatic cancer risk (OR, 1.06; 95% CI, 0.86–1.31;
Fig. S1). However, in this
subgroup, the results were closer to the line of null effect and
their 95% CIs were narrower.
Subgroup analysis on study region
All 17 studies were grouped according to the region
of the study population. Among them, 7 studies were assigned to the
Asian group, 5 studies to the European group, 4 studies to the
North American group and 1 study to the Oceania group. The results
in the European group (OR, 1.28; 95% CI, 0.95–1.72), the Asian
group (OR, 1.34; 95% CI, 0.77–2.34) and the Oceania group (OR,
1.05; 95% CI, 0.79–1.40) suggested that H. pylori infection
was a risk factor for pancreatic cancer (Fig. S2). By contrast, in North America
(OR, 0.92; 95% CI, 0.69–1.23), H. pylori infection was a
protective factor for pancreatic cancer. However, none of these
associations were statistically significant, which may suggest that
there were small regional differences in the association between
H. pylori infection and pancreatic cancer, but that these
differences did not have a decisive effect.
Subgroup analysis on study design
The types of the studies included were case-control
studies, nested case-control studies and cohort studies, which may
have different implementation pathways and levels of evidence in
evidence-based medicine. Therefore, the studies were analyzed in
subgroups according to study design to identify potential sources
of heterogeneity (42,43). The analysis showed that there was no
significant difference between the results of the case-control
study group (OR, 1.07; 95% CI, 0.78–1.48), the nested case-control
study group (OR, 1.05; 95% CI, 0.82–1.34) and the cohort study
group (OR, 1.68; 95% CI, 0.86–3.29), which suggests that study
design may not be a major source of heterogeneity (Fig. S3).
Subgroup analysis on diagnostic
criteria
The original studies employed a variety of
diagnostic methods for H. pylori infection. Therefore, the
original studies were analyzed in groups based on the diagnostic
criteria. A total of 14 studies used ELISA-based Hp-IgG positivity
as a diagnostic criterion for H. pylori infection, and the
analysis of this group still suggested no significant association
between H. pylori infection and the risk of pancreatic
cancer (OR, 1.10; 95% CI, 0.90–1.35; Fig. S4). By contrast, the remaining three
diagnostic methods (E-Plate, multiple serology and histopathology)
had all been used in a single study and had limited significance
for a combined analysis.
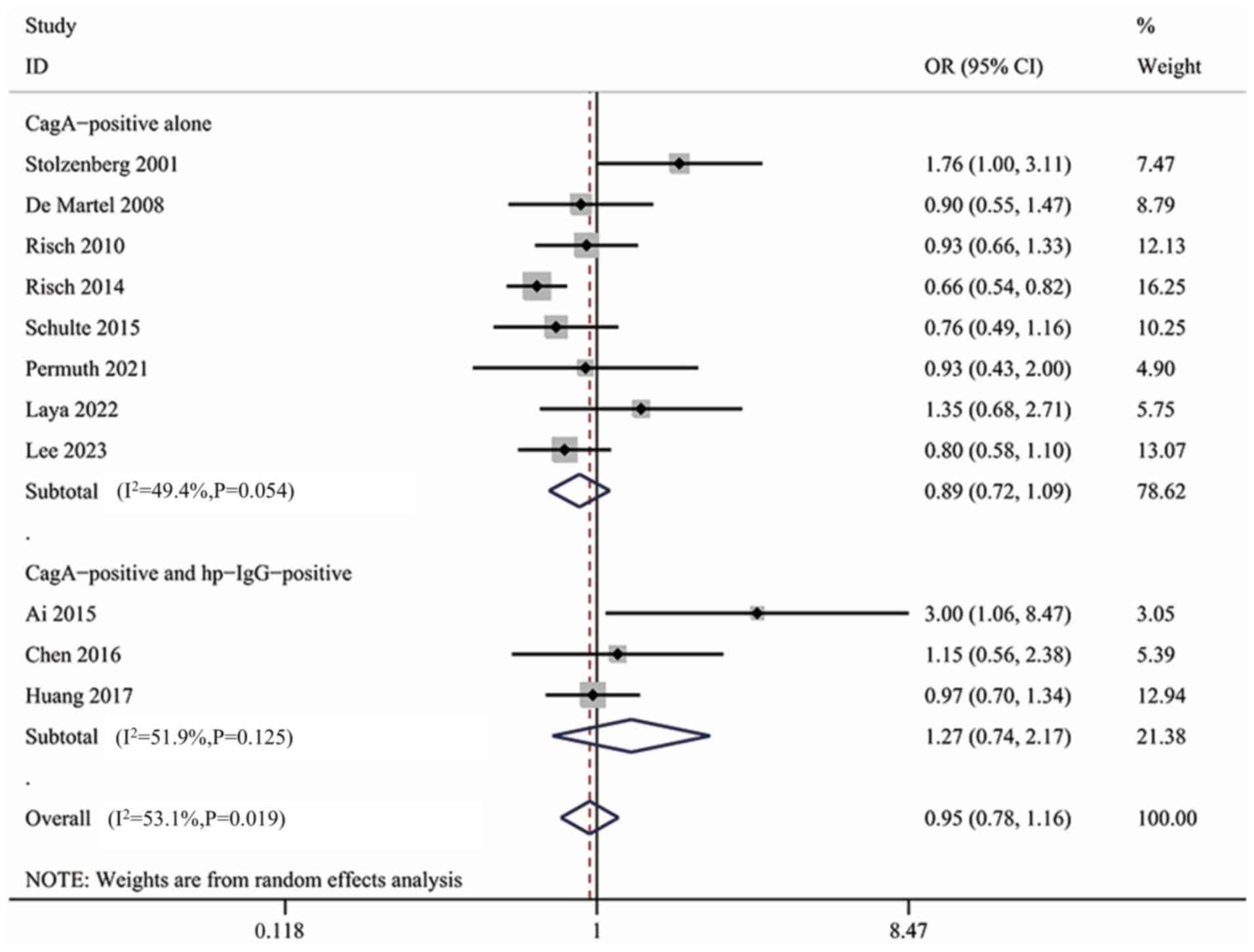
Subgroup analysis of CagA+
H. pylori infection
CagA is a crucial virulence factor of H.
pylori that is associated with tumorigenic risk and
CagA+ H. pylori is typically considered to
possess higher virulence (6). A
total of 11 studies (9 studies using ELISA, 1 using an immunoblot
test and 1 using multiple serology) additionally examined the CagA
status of H. pylori, all of which were included in the
present subgroup. The result showed no significant association
between CagA+ H. pylori infection and the risk of
pancreatic cancer (OR, 0.95; 95% CI, 0.78–1.16; Fig. S5). However, the diagnostic criteria
for CagA+ H. pylori infection varied among
studies. For example, a study in the United States in 2023
determined CagA positivity based on the detection of CagA only
(28), whereas a cohort study in
Germany in 2016 interpreted the results of CagA testing on the
basis of Hp-IgG positivity (37).
The different diagnostic criteria likely affected the reliability
of the results. For both diagnostic criteria, a subgroup analysis
was performed, although no significant association was found in
both the CagA+ group alone (OR, 0.89; 95% CI, 0.72–1.09)
and the Hp-IgG+ + CagA+ group (OR, 1.27; 95%
CI, 0.74–2.17) (Fig. 4). Therefore,
the conclusions did not change. In this subgroup, further subgroup
analyses were performed based on quality, study region and study
design, but none yielded meaningful results (data not shown). The
results of the subgroup analysis of CagA+ H.
pylori infection were reliable and not abnormally affected by a
single extreme result, as demonstrated by sensitivity analysis
(Fig. S6).
Subgroup analysis of CagA−
H. pylori infection
A total of 7 studies additionally analyzed
CagA− H. pylori infection, all of which were
included in the subgroup analysis. The test for heterogeneity
indicated that inter-study heterogeneity was not significant
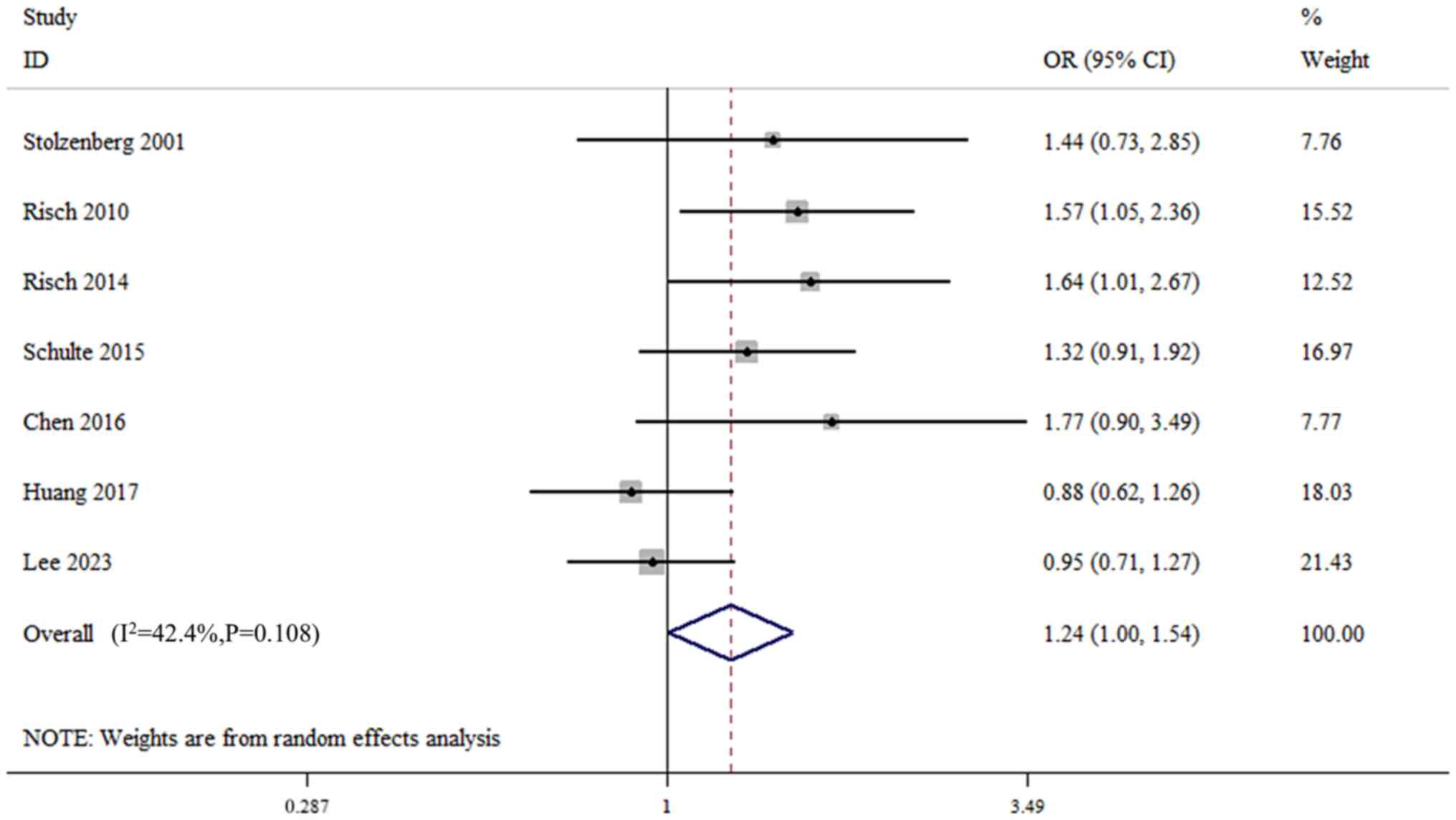
(I2=42.4%; P=0.108; Fig.
5). The quantitative synthesis results showed that
CagA− H. pylori infection was associated with the
risk of pancreatic cancer (OR, 1.24; 95% CI, 1.00–1.54; Fig. 5). The results suggested that
CagA− H. pylori infection could be a risk factor
for pancreatic cancer. However, the corresponding sensitivity
analysis suggested that this result was not very stable (Fig. S7).
Publication bias
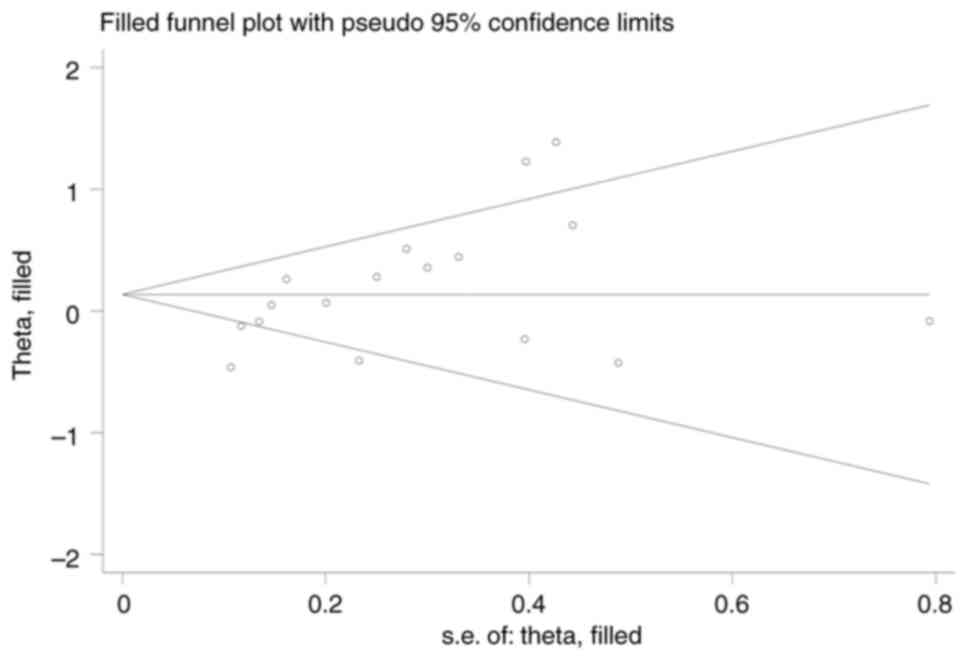
The funnel plot results were slightly asymmetric
(Fig. S8). Begg's test did not
identify publication bias (P=0.077), but Egger's test suggested
some publication bias (P=0.014). The trim and fill analysis allows
the modelling of results that may be absent due to publication
bias, thus assessing the impact of publication bias on the results
and providing an adjusted effect value. A trim-and-fill analysis
was performed, and the results showed that no studies were trimmed
or filled (Fig. 6), and the
adjusted results were consistent with the original results. The
results demonstrated that there was no significant publication bias
and its influence on the results of the meta-analysis was weak.
Discussion
Although the potential oncogenic role of H.
pylori infection has received widespread attention, its
association with pancreatic cancer risk is unclear and study
findings are controversial. The present study aimed to examine the
existing literature and assess the association between different
types of H. pylori infection and pancreatic cancer risk and
to explore possible causes.
In the present study, the predetermined inclusion
and exclusion criteria were strictly followed to select
high-quality original studies. By excluding non-compliant or
low-quality literature and including those studies that met the
criteria, the present study enhances the generalizability of the
selected research population and the universality of the research
conclusions. In addition, the selected original studies included a
variety of study types, such as case-control, nested case-control
and cohort studies, providing a multidimensional perspective that
contributed to a comprehensive assessment of the association
between H. pylori infection and pancreatic cancer risk. The
largest sample size to date (a total of 67,910 subjects) was
included, which notably enhanced the statistical efficacy of the
present meta-analysis and reduced randomization error, thus
providing more robust and reliable conclusions.
For statistical analysis, a comprehensive analytical
strategy was used to investigate the complex relationship between
H. pylori infection and pancreatic cancer risk. Through
subgroup analyses, the effects of study region, design, diagnostic
criteria and CagA status on the results were examined, which helped
to identify potential heterogeneity among different subgroups and
provide valuable clues for future studies. In addition, to ensure
the robustness of the findings, sensitivity analyses were further
performed to assess the impact of individual studies on the overall
effect estimates and the trim-and-fill method was employed to
adjust for potential publication bias. The use of these analytical
tools has increased the confidence in the study's conclusions.
The present analysis showed no significant
association between H. pylori infection and pancreatic
cancer risk (OR, 1.15; 95% CI, 0.93–1.41). Although there was some
publication bias and significant heterogeneity, the result of the
sensitivity analysis and the trim-and-fill analysis demonstrated
that the results are stable and reliable. Meta-analyses by Zhou
et al (19) and Schulte
et al (20) also showed
similar results. Trikudanathan et al (44) and Xiao et al (18) included 6 and 9 original studies,
respectively, and their results suggested a statistically
significant association between H. pylori infection and
pancreatic cancer risk (OR, 1.38; 95% CI, 1.08–1.75 and OR, 1.47;
95% CI, 1.22–1.77, respectively). However, building on their
original study, several newly published papers were also included
in the present study, including 3 cohort studies, which enhanced
the credibility of the results. Xiao et al (18) also performed a subgroup analysis of
high-quality studies, in which 4 original studies that were
considered high-quality were analyzed and found statistically
significant results (OR, 1.28; 95% CI, 1.01–1.63). Although this
result still suggested that H. pylori infection was a risk
factor, the OR and 95% CI of the high-quality subgroup were closer
to 1 compared with the results of their overall analysis (OR, 1.47;
95% CI, 1.22–1.77), suggesting that the results of the overall
analysis were somewhat influenced by the other studies. By
contrast, the high-quality subgroup analysis in the present study
involved 12 articles, including all 4 articles used by Xiao et
al (18) and 8 new high-quality
articles. The results suggested no significant association between
H. pylori infection and pancreatic cancer risk (OR, 1.06;
95% CI, 0.86–1.31), consistent with the results of the overall
analysis of the present study. Similarly, the OR and 95% CI of the
high-quality subgroup analysis were closer to 1 than those of the
overall analysis.
Regional subgroup analyses were performed in the
present study, and no statistically significant results were found
in the European, Asian or North American groups. The results of the
study by Zhou et al (19)
are consistent with the findings of the present study. By contrast,
Xiao et al (18) found
statistically significant results in the European and East Asian
groups (OR, 1.56; 95% CI, 1.15–2.10 and OR, 2.01; 95% CI,
1.33–3.02, respectively). Compared with the study by Xiao et
al, the regional subgroup analyses in the present study
additionally included 8 newly published articles (including 3
cohort studies) and did not include 3 studies published in
languages other than English. Consequently, the regional subgroup
analyses in the present study incorporated more recent data and
larger sample sizes.
Subgroup analyses on the diagnostic criteria and
study design did not reveal significant heterogeneity between
groups. Of the four diagnostic methods for H. pylori
infection included in the present study, three were used in only 1
study. Therefore, interpretation of diagnostic criteria subgroup
results were limited by sample size.
CagA protein is an important virulence factor of
H. pylori. CagA interferes with cell signal transduction by
binding to various receptors of host cells, thus affecting cell
proliferation, migration and apoptosis (6). The present study comprehensively
analyzed the role of the CagA protein based on existing data, and
the findings indicated no significant association between
CagA+ H. pylori infection and the risk of
pancreatic cancer. Certain previous meta-analyses corroborate this
finding (18,19). CagA− H. pylori
infection was significantly associated with the risk of pancreatic
cancer (OR, 1.24; 95% CI, 1.00–1.54) in the present study. Compared
with the study of Zhou et al (19) (OR, 1.22; 95% CI, 1.00–1.49), the
present study additionally included a 2016 cohort study from
Germany (37) and a 2023 nested
case-control study from the United States (28). By introducing these 2 new original
studies, narrower confidence intervals were obtained and therefore
the results showed statistical significance. In terms of
CagA− H. pylori infection, several meta-analyses
are consistent with the findings of the present study (20,37,45),
but the present study had the largest sample size and the narrowest
confidence intervals. However, the corresponding sensitivity
analysis showed that the combined results were not very stable.
After several critical studies were excluded individually (17,20,29,37),
the results were no longer statistically significant.
VacA is also a major virulence factor produced by
H. pylori. As a cytotoxin, VacA can interact with host cell
membranes to form transmembrane channels that disrupt membrane
integrity. This damage results in the leakage of intracellular
material and loss of cellular function, which in turn may trigger
cell death (46,47). This mechanism of VacA makes it one
of the key factors related to H. pylori infection, gastric
mucosal injury and inflammation. However, only 1 study examined
VacA status, and therefore quantitative synthetic analyses could
not be performed in the present study (31).
The association between CagA− H.
pylori infection and pancreatic cancer risk may involve
multiple biological mechanisms. First, H. pylori infection
itself may cause damage to pancreatic cells through a chronic
inflammatory response, and this inflammatory environment may
promote the development of pancreatic cancer. Chronic inflammation
is recognized as an important cancer-promoting factor that can lead
to DNA damage, cell proliferation and immune escape, thereby
increasing the risk of pancreatic cancer (48,49).
For example, a study found that H. pylori infection was
associated with elevated markers of inflammation in patients with
pancreatic cancer, suggesting that inflammation may play a role in
the development of pancreatic cancer (18). H. pylori infection may
elevate inflammation levels and promote β-catenin accumulation by
inducing spermine oxidase, which metabolizes the polyamine,
spermine, into spermidine and H2O2 (50). There is evidence that gastric
polyamine levels are positively associated with gastritis in H.
pylori-infected gerbils (51).
An association between colonic spermidine levels and histological
damage was also observed in a wild-type mouse model of
Citrobacter rodentium infection (52). The Wnt/β-catenin signaling pathway
is pivotal in carcinogenesis (53,54).
H. pylori induces nuclear accumulation of β-catenin in
gastric epithelial cells, facilitating the development of cells
exhibiting cancer stem cell-like characteristics (55).
Second, H. pylori infection may affect the
immune surveillance and immune escape mechanisms of pancreatic
cancer by affecting the immune microenvironment of the pancreas and
altering the distribution and function of immune cells (6). It has been shown that H. pylori
infection has the capacity to upregulate the expression of
indoleamine 2,3-dioxygenase in macrophages, thereby inducing M2
polarization (56). M2 macrophages
promote cancer initiation and malignant progression by enhancing
angiogenesis and increasing tumor migration, invasion and
intravasation, while also inhibiting antitumor immunity (57). Guo et al (58) showed that M2 macrophages shield
tumor-initiating cells from immune elimination and are essential
for tumorigenesis. In addition, M2 macrophages are able to promote
tumor cell colonization and growth by regulating the interaction
between tumor cells and surrounding cells, as well as by remodeling
the stroma surrounding tumor cells (59).
H. pylori infection is also associated with
oxidative stress and extensive DNA damage related to chronic
inflammation (60). It is well
known that H. pylori causes neutrophil infiltration and
elevated de novo synthesis of reactive oxygen species (ROS)
by epithelial cells both in vivo and in vitro
(61,62). ROS are oxygen-containing chemicals
that are highly reactive in living organisms and, under normal
physiological conditions, they are produced by cellular metabolism
and are involved in cell signaling processes (63,64).
In turn, the increase in ROS leads to DNA damage and genetic
instability and may even activate tumorigenic signals (64–66).
Hardbower et al (60)
inhibited DNA damage induced by oxidative stress in mouse and
gerbil models infected by H. pylori, which were found to
exhibit a decrease in heterotopic hyperplasia and carcinoma.
CagA− H. pylori infection
exhibits enhanced survivability in highly acidic conditions, which
may mean that these strains are more likely to infect or colonize
highly acidic individuals (67).
The highly acidic trait coupled with infection by H. pylori
may induce a strong stimulation of the pancreas (68,69).
Pancreatic cells found in a highly secretory active state for a
long period are more prone to malignancy (70,71).
Contrary to CagA− strains, CagA+ strains are
generally considered to be more virulent and capable of inducing
more severe gastric mucosal atrophy, intestinal epithelial
hyperplasia and inflammatory cell infiltration (72). Consequently, a reduction in gastric
acidity may be more prevalent among the long-term effects of
CagA+ H. pylori, which may instead alleviate the
burden on pancreatic cells. This may explain why CagA−
H. pylori infection is more dangerous in terms of pancreatic
cancer risk. Moreover, in addition to CagA and VacA, H.
pylori possesses an extensive array of virulence factors,
including dupA, iceA and htrA (73–75).
Subgroup analysis based only on CagA status overlooks the role of
these virulence factors, and taking these virulence factors into
account helps to explain the relationship between H. pylori
infection and pancreatic cancer more scientifically.
Lifestyle and genetic susceptibility are also
significant factors influencing pancreatic cancer risk (13). For instance, smoking, high BMI and
diabetes are often regarded as risk factors for pancreatic cancer
(76–78), while mutations of various genes
(such as CDKN2A, BRCA2, ATM and BRCA1) have been shown to be
associated with pancreatic cancer (79,80).
Certain studies matched for fundamental confounders such as age,
sex, smoking and alcohol intake, thereby eliminating their
influence on the results (16,27).
Nonetheless, regarding dietary structure and genetic
susceptibility, which are more difficult indicators to count, only
a few studies have controlled their distribution across groups
(20,31). Therefore, more high-quality studies
are required to elucidate the association between H. pylori
infection and pancreatic cancer risk.
The present study also has some limitations.
High-performance assays for H. pylori infection, such as
tissue culture and nested PCR (81), were infrequently employed in the
studies included in the analysis, and the majority of original
studies used serology for diagnosing H. pylori infection,
which is among the most prevalent diagnostic procedures (82,83).
The lesions resulting from H. pylori infection exhibit
marked variability across individuals (84,85).
The extent of chronic inflammation due to H. pylori
infection was not assessed, nor were the changes in the acidity of
the stomach (which stimulates the pancreas) in the case of
diagnosis using serology. This deficiency reveals the shortcomings
in the degree of refinement of the subgroups of H. pylori
infection. Furthermore, studies have demonstrated that the
conversion rate of serum CagA antibodies was considerably lower
than that of Hp-IgG antibodies, and that the inclusion of CagA
antibodies in the diagnostic criteria could facilitate the
detection of remote H. pylori infection with greater
efficacy (86,87). Therefore, some studies have chosen
to use the results of CagA antibodies to correct for the results of
Hp-IgG antibodies (17,20,27–29).
However, some studies neglected to do so, and some did not test for
CagA antibodies, which likely contributed to the underestimation of
the H. pylori infected population. In the case-control
studies covered in the present study, there was often a long
interval between specimen collection and testing, and it has been
found that the level of serologic markers might change after
prolonged storage (30), which
could be avoided by higher-quality study designs.
As only one of the original studies included tested
VacA status using multiple serological methods (31), it was not possible to perform a
meta-analysis on the association between VacA and pancreatic cancer
risk. Furthermore, since the original studies included in the
present study included just 3 cohort studies (32,36,37),
the degree of evidence for the original data should be raised. The
emergence of more rigorously designed studies with higher levels of
evidence will help to address these issues.
In conclusion, the results of the present study
suggested that H. pylori infection, including
CagA+ H. pylori infection, did not significantly
increase the risk of pancreatic cancer. However, CagA−
H. pylori infection is a risk factor that warrants caution.
Although study region, diagnostic methods, study design and
virulence of strains all had some impact on the results, this
impact did not affect the conclusions.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study received funding from Anhui Province Higher Education
Institutions Natural Science Research Key Project (grant no.
2024AH050739) and Anhui Medical University Clinical and Early
Discipline Co-construction Project.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MZ, CQ and CX conceived of the study; MZ, CQ, CX
and WX participated in the design of the study; CQ and ZZ
participated in data collection; CQ, XS and XW analyzed and
interpreted the data; CQ and CX drafted the manuscript; CX revised
and edited the manuscript. MZ and WX confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crowe SE: Helicobacter pylori infection. N
Engl J Med. 380:1158–1165. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marshall BJ and Warren JR: Unidentified
curved bacilli in the stomach of patients with gastritis and peptic
ulceration. Lancet. 1:1311–1315. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Cui Y, Wei X, Zang Y, Chen X,
Cheng L and Wang X: CuCo2O4 nanoflowers with
multiple enzyme activities for treating bacterium-infected wounds
via cuproptosis-like death. ACS Nano. 18:15845–15863. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xing J, Shan J, Xue H, Zhang H, Cheng L,
Hao J and Wang X: Multifunctional adaptable injectable TiN-based
hydrogels for antitumor and antidrug-resistant bacterial therapy.
Adv Healthc Mater. 13:e24002972024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Z, Shan J, Jin X, Sun W, Cheng L, Chen
XL and Wang X: Nanoarchitectonics of in situ antibiotic-releasing
acicular nanozymes for targeting and inducing cuproptosis-like
death to eliminate drug-resistant bacteria. ACS Nano.
18:24327–24349. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peek RM Jr and Blaser MJ: Helicobacter
pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer.
2:28–37. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malfertheiner P, Megraud F, O'Morain CA,
Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton
J, Graham DY, et al: Management of helicobacter pylori
infection-the maastricht V/Florence consensus report. Gut. 66:6–30.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang C, Chen Y, Long Y, Zheng H, Jing J
and Pan W: Helicobacter pylori and gastrointestinal cancers: Recent
advances and controversies. Clin Med Insights Oncol.
18:117955492412346372024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stoffel EM, Brand RE and Goggins M:
Pancreatic cancer: Changing epidemiology and new approaches to risk
assessment, early detection, and prevention. Gastroenterology.
164:752–765. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Whitcomb DC, Shelton CA and Brand RE:
Genetics and genetic testing in pancreatic cancer.
Gastroenterology. 149:1252–1264.e4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klein AP: Pancreatic cancer epidemiology:
Understanding the role of lifestyle and inherited risk factors. Nat
Rev Gastroenterol Hepatol. 18:493–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bosetti C, Lucenteforte E, Silverman DT,
Petersen G, Bracci PM, Ji BT, Negri E, Li D, Risch HA, Olson SH, et
al: Cigarette smoking and pancreatic cancer: An analysis from the
international pancreatic cancer case-control consortium (Panc4).
Ann Oncol. 23:1880–1888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franceschi F, Tortora A, Gasbarrini G and
Gasbarrini A: Helicobacter pylori and extragastric diseases.
Helicobacter. 19 (Suppl 10):S52–S58. 2014. View Article : Google Scholar
|
|
16
|
Huang J, Zagai U, Hallmans G, Nyrén O,
Engstrand L, Stolzenberg-Solomon R, Duell EJ, Overvad K, Katzke VA,
Kaaks R, et al: Helicobacter pylori infection, chronic corpus
atrophic gastritis and pancreatic cancer risk in the European
prospective investigation into cancer and nutrition (EPIC) cohort:
A nested case-control study. Int J Cancer. 140:1727–1735. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Risch HA, Yu H, Lu L and Kidd MS: ABO
blood group, Helicobacter pylori seropositivity, and risk of
pancreatic cancer: A case-control study. J Natl Cancer Inst.
102:502–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao M, Wang Y and Gao Y: Association
between Helicobacter pylori infection and pancreatic cancer
development: A meta-analysis. PLoS One. 8:e755592013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou BG, Mei YZ, Wang JS, Xia JL, Jiang X,
Ju SY and Ding YB: Is Helicobacter pylori infection associated with
pancreatic cancer? A systematic review and meta-analysis of
observational studies. Ther Adv Chronic Dis.
14:204062232311551192023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schulte A, Pandeya N, Fawcett J, Fritschi
L, Risch HA, Webb PM, Whiteman DC and Neale RE: Association between
Helicobacter pylori and pancreatic cancer risk: A meta-analysis.
Cancer Causes Control. 26:1027–1035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Egger M, Smith GD, Schneider M and Minder
C: Bias in meta-analysis detected by a simple, graphical test. BMJ.
315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duval S and Tweedie R: Trim and fill: A
simple funnel-plot-based method of testing and adjusting for
publication bias in meta-analysis. Biometrics. 56:455–463. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stolzenberg-Solomon RZ, Blaser MJ, Limburg
PJ, Perez-Perez G, Taylor PR, Virtamo J and Albanes D; ATBC Study,
: Helicobacter pylori seropositivity as a risk factor for
pancreatic cancer. J Natl Cancer Inst. 93:937–941. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee AA, Wang QL, Kim J, Babic A, Zhang X,
Perez K, Ng K, Nowak J, Rifai N, Sesso HD, et al: Helicobacter
pylori seropositivity, ABO blood type, and pancreatic cancer risk
from 5 prospective cohorts. Clin Transl Gastroenterol.
14:e005732023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Risch HA, Lu L, Kidd MS, Wang J, Zhang W,
Ni Q, Gao YT and Yu H: Helicobacter pylori seropositivities and
risk of pancreatic carcinoma. Cancer Epidemiol Biomarkers Prev.
23:172–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Martel C, Llosa AE, Friedman GD,
Vogelman JH, Orentreich N, Stolzenberg-Solomon RZ and Parsonnet J:
Helicobacter pylori infection and development of pancreatic cancer.
Cancer Epidemiol Biomarkers Prev. 17:1188–1194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Permuth JB, Rahman S, Chen DT, Waterboer T
and Giuliano AR: A case control study of the seroprevalence of
helicobacter pylori proteins and their association with pancreatic
cancer risk. J Pancreat Cancer. 7:57–64. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirabayashi M, Inoue M, Sawada N, Saito E,
Abe SK, Hidaka A, Iwasaki M, Yamaji T, Shimazu T and Tsugane S:
Helicobacter pylori infection, atrophic gastritis, and risk of
pancreatic cancer: A population-based cohort study in a large
Japanese population: The JPHC study. Sci Rep. 9:60992019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raderer M, Wrba F, Kornek G, Maca T,
Koller DY, Weinlaender G, Hejna M and Scheithauer W: Association
between Helicobacter pylori infection and pancreatic cancer.
Oncology. 55:16–19. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lindkvist B, Johansen D, Borgström A and
Manjer J: A prospective study of Helicobacter pylori in relation to
the risk for pancreatic cancer. BMC Cancer. 8:3212008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ai F, Hua X, Liu Y, Lin J and Feng Z:
Preliminary study of pancreatic cancer associated with Helicobacter
pylori infection. Cell Biochem Biophys. 71:397–400. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsu WY, Lin CH, Lin CC, Sung FC, Hsu CP
and Kao CH: The relationship between Helicobacter pylori and cancer
risk. Eur J Intern Med. 25:235–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen XZ, Schöttker B, Castro FA, Chen H,
Zhang Y, Holleczek B and Brenner H: Association of helicobacter
pylori infection and chronic atrophic gastritis with risk of
colonic, pancreatic and gastric cancer: A ten-year follow-up of the
ESTHER cohort study. Oncotarget. 7:17182–17193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimoyama T, Takahashi R, Abe D, Mizuki I,
Endo T and Fukuda S: Serological analysis of Helicobacter hepaticus
infection in patients with biliary and pancreatic diseases. J
Gastroenterol Hepatol. 25 (Suppl 1):S86–S89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Laya GB, Anandhi A, Gurushankari B, Mandal
J and Kate V: Association between helicobacter pylori and
periampullary and pancreatic cancer: A case-control study. J
Gastrointest Cancer. 53:902–907. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Osaki T, Lin Y, Sasahira N, Ueno M,
Yonezawa H, Hojo F, Okuda M, Matsuyama M, Sasaki T, Kobayashi S, et
al: Prevalence estimates of helicobacter species infection in
pancreatic and biliary tract cancers. Helicobacter. 27:e128662022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu G, Murphy G, Michel A, Weinstein SJ,
Männistö S, Albanes D, Pawlita M and Stolzenberg-Solomon RZ:
Seropositivity to Helicobacter pylori and risk of pancreatic
cancer. Cancer Epidemiol Biomarkers Prev. 22:2416–2419. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Sackett DL, Rosenberg WM, Gray JA,
Haynes RB and Richardson WS: Evidence based medicine: What it is
and what it isn't. BMJ. 312:71–72. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guyatt GH, Oxman AD, Vist GE, Kunz R,
Falck-Ytter Y, Alonso-Coello P and Schünemann HJ; GRADE Working
Group, : GRADE: An emerging consensus on rating quality of evidence
and strength of recommendations. BMJ. 336:924–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Trikudanathan G, Philip A, Dasanu CA and
Baker WL: Association between Helicobacter pylori infection and
pancreatic cancer. A cumulative meta-analysis. JOP. 12:26–31.
2011.PubMed/NCBI
|
|
45
|
Liu H, Chen YT and Wang R XZ: Helicobacter
pylori infection, atrophic gastritis, and pancreatic cancer risk: A
meta-analysis of prospective epidemiologic studies. Medicine
(Baltimore). 96:e781112017.
|
|
46
|
Cover TL and Blanke SR: Helicobacter
pylori VacA, a paradigm for toxin multifunctionality. Nat Rev
Microbiol. 3:320–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Necchi V, Sommi P, Vanoli A, Fiocca R,
Ricci V and Solcia E: Natural history of Helicobacter pylori VacA
toxin in human gastric epithelium in vivo: Vacuoles and beyond. Sci
Rep. 7:145262017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Posselt G, Backert S and Wessler S: The
functional interplay of Helicobacter pylori factors with gastric
epithelial cells induces a multi-step process in pathogenesis. Cell
Commun Signal. 11:772013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiménez-Soto LF and Haas R: The CagA toxin
of Helicobacter pylori: Abundant production but relatively low
amount translocated. Sci Rep. 6:232272016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sierra JC, Piazuelo MB, Luis PB, Barry DP,
Allaman MM, Asim M, Sebrell TA, Finley JL, Rose KL, Hill S, et al:
Spermine oxidase mediates Helicobacter pylori-induced gastric
inflammation, DNA damage, and carcinogenic signaling. Oncogene.
39:4465–4474. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chaturvedi R, de Sablet T, Asim M,
Piazuelo MB, Barry DP, Verriere TG, Sierra JC, Hardbower DM,
Delgado AG, Schneider BG, et al: Increased Helicobacter
pylori-associated gastric cancer risk in the Andean region of
Colombia is mediated by spermine oxidase. Oncogene. 34:3429–3440.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gobert AP, Al-Greene NT, Singh K, Coburn
LA, Sierra JC, Verriere TG, Luis PB, Schneider C, Asim M, Allaman
MM, et al: Distinct immunomodulatory effects of spermine oxidase in
colitis induced by epithelial injury or infection. Front Immunol.
9:12422018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: Function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu
C, Wang C and Ye L: Wnt/β-catenin signaling in cancers and targeted
therapies. Signal Transduct Target Ther. 6:3072021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo
G, Hu CJ, Dong H and Yang SM: Helicobacter pylori upregulates Nanog
and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem
cell-like properties in human gastric cancer. Cancer Lett.
374:292–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Peng R, Xu C, Zhang L, Liu X, Peng D, Chen
X, Liu D and Li R: M2 macrophages participate in ILC2 activation
induced by Helicobacter pylori infection. Gut Microbes.
16:23470252024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cassetta L and Pollard JW: Targeting
macrophages: Therapeutic approaches in cancer. Nat Rev Drug Discov.
17:887–904. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guo X, Zhao Y, Yan H, Yang Y, Shen S, Dai
X, Ji X, Ji F, Gong XG, Li L, et al: Single tumor-initiating cells
evade immune clearance by recruiting type II macrophages. Genes
Dev. 31:247–259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Doak GR, Schwertfeger KL and Wood DK:
Distant relations: Macrophage functions in the metastatic niche.
Trends Cancer. 4:445–459. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hardbower DM, de Sablet T, Chaturvedi R
and Wilson KT: Chronic inflammation and oxidative stress: The
smoking gun for Helicobacter pylori-induced gastric cancer? Gut
Microbes. 4:475–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Davies GR, Banatvala N, Collins CE, Sheaff
MT, Abdi Y, Clements L and Rampton DS: Relationship between
infective load of Helicobacter pylori and reactive oxygen
metabolite production in antral mucosa. Scand J Gastroenterol.
29:419–424. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bagchi D, Bhattacharya G and Stohs SJ:
Production of reactive oxygen species by gastric cells in
association with Helicobacter pylori. Free Radic Res. 24:439–450.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Granger DN and Kvietys PR: Reperfusion
injury and reactive oxygen species: The evolution of a concept.
Redox Biol. 6:524–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Franco R and Vargas MR: Redox biology in
neurological function, dysfunction, and aging. Antioxid Redox
Signal. 28:1583–1586. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Srinivas US, Tan BWQ, Vellayappan BA and
Jeyasekharan AD: ROS and the DNA damage response in cancer. Redox
Biol. 25:1010842019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Karita M and Blaser MJ: Acid-tolerance
response in Helicobacter pylori and differences between cagA+ and
cagA- strains. J Infect Dis. 178:213–219. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kunovsky L, Dite P, Jabandziev P, Dolina
J, Vaculova J, Blaho M, Bojkova M, Dvorackova J, Uvirova M, Kala Z
and Trna J: Helicobacter pylori infection and other bacteria in
pancreatic cancer and autoimmune pancreatitis. World J Gastrointest
Oncol. 13:835–844. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Risch HA: Etiology of pancreatic cancer,
with a hypothesis concerning the role of N-nitroso compounds and
excess gastric acidity. J Natl Cancer Inst. 95:948–960. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Storz P: Acinar cell plasticity and
development of pancreatic ductal adenocarcinoma. Nat Rev
Gastroenterol Hepatol. 14:296–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Marstrand-Daucé L, Lorenzo D, Chassac A,
Nicole P, Couvelard A and Haumaitre C: Acinar-to-Ductal metaplasia
(ADM): On the road to pancreatic intraepithelial neoplasia (PanIN)
and pancreatic cancer. Int J Mol Sci. 24:99462023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sozzi M, Valentini M, Figura N, De Paoli
P, Tedeschi RM, Gloghini A, Serraino D, Poletti M and Carbone A:
Atrophic gastritis and intestinal metaplasia in Helicobacter pylori
infection: The role of CagA status. Am J Gastroenterol. 93:375–379.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sharndama HC and Mba IE: Helicobacter
pylori: An up-to-date overview on the virulence and pathogenesis
mechanisms. Braz J Microbiol. 53:33–50. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kusters JG, van Vliet AH and Kuipers EJ:
Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev.
19:449–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yamaoka Y: Mechanisms of disease:
Helicobacter pylori virulence factors. Nat Rev Gastroenterol
Hepatol. 7:629–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lynch SM, Vrieling A, Lubin JH, Kraft P,
Mendelsohn JB, Hartge P, Canzian F, Steplowski E, Arslan AA, Gross
M, et al: Cigarette smoking and pancreatic cancer: A pooled
analysis from the pancreatic cancer cohort consortium. Am J
Epidemiol. 170:403–413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bosetti C, Rosato V, Li D, Silverman D,
Petersen GM, Bracci PM, Neale RE, Muscat J, Anderson K, Gallinger
S, et al: Diabetes, antidiabetic medications, and pancreatic cancer
risk: An analysis from the international pancreatic cancer
case-control consortium. Ann Oncol. 25:2065–2072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chari ST, Leibson CL, Rabe KG, Ransom J,
de Andrade M and Petersen GM: Probability of pancreatic cancer
following diabetes: A population-based study. Gastroenterology.
129:504–511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hu C, Hart SN, Polley EC, Gnanaolivu R,
Shimelis H, Lee KY, Lilyquist J, Na J, Moore R, Antwi SO, et al:
Association between inherited germline mutations in cancer
predisposition genes and risk of pancreatic cancer. JAMA.
319:2401–2409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhen DB, Rabe KG, Gallinger S, Syngal S,
Schwartz AG, Goggins MG, Hruban RH, Cote ML, McWilliams RR, Roberts
NJ, et al: BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial
pancreatic cancer: A PACGENE study. Genet Med. 17:569–577. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Patel SK, Pratap CB, Jain AK, Gulati AK
and Nath G: Diagnosis of Helicobacter pylori: What should be the
gold standard? World J Gastroenterol. 20:12847–12859. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hooi JKY, Lai WY, Ng WK, Suen MMY,
Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu
JCY, et al: Global prevalence of helicobacter pylori infection:
Systematic review and meta-analysis. Gastroenterology. 153:420–429.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li Y, Choi H, Leung K, Jiang F, Graham DY
and Leung WK: Global prevalence of Helicobacter pylori infection
between 1980 and 2022: A systematic review and meta-analysis.
Lancet Gastroenterol Hepatol. 8:553–564. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mitchell H and Katelaris P: Epidemiology,
clinical impacts and current clinical management of Helicobacter
pylori infection. Med J Aust. 204:376–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shah SC, Halvorson AE, Lee D, Bustamante
R, McBay B, Gupta R, Denton J, Dorn C, Wilson O, Peek R Jr, et al:
Helicobacter pylori Burden in the United States according to
individual demographics and geography: A nationwide analysis of the
veterans healthcare system. Clin Gastroenterol Hepatol.
22:42–50.e26. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kist M, Strobel S, Kirchner T and Dammann
HG: Impact of ELISA and immunoblot as diagnostic tools one year
after eradication of Helicobacter pylori in a multicentre treatment
study. FEMS Immunol Med Microbiol. 24:239–242. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lu CY, Kuo CH, Lo YC, Chuang HY, Yang YC,
Wu IC, Yu FJ, Lee YC, Jan CM, Wang WM and Wu DC: The best method of
detecting prior Helicobacter pylori infection. World J
Gastroenterol. 11:5672–5676. 2005. View Article : Google Scholar : PubMed/NCBI
|