Introduction
The mortality rate of cancer patients has been
declining annually owing to advances in diagnostics, surgery, and
medications. However, the estimated number of new cases of gastric
cancer (GC) exceeds one million, ranking fifth among all newly
diagnosed cancers, and many patients experience recurrence after
gastrectomy for GC (1).
Establishing an accurate prediction of its clinical outcomes is
crucial for improving the quality of life of patients with GC and
reducing mortality and medical burden.
Recent research has confirmed that sarcopenia or
systemic inflammatory response markers such as the Glasgow
Prognostic Score (GPS) or Prognostic Nutritional Index (PNI) can
predict survival in patients with GC (2–4).
Osteopenia, a condition of low bone mineral density (BMD), is
associated with the progression of sarcopenia and is independently
associated with poor prognosis in patients with various digestive
tract cancers, including GC (5,6).
Occult vertebral fracture (OVF) are the most common complications
of osteopenia, and more than two-thirds of patients with this
condition are incidentally diagnosed (7). Some reports have demonstrated the
effectiveness of OVF in prognostic prediction in patients with
colorectal liver metastasis and pancreatic cancer (8,9).
However, no study has explored the correlation between OVF and the
clinical outcomes of patients with GC. Therefore, the aim of this
study was to investigate the prognostic impact of OVF in GC
patients undergoing gastrectomy.
Materials and methods
Patients
This retrospective study included 242 patients who
underwent primary gastrectomy for GC between October 2013 and
February 2023 at the Fuji City General Hospital (Shizuoka, Japan).
This study was approved by the Institutional Review Board of Fuji
City General Hospital (approval no. 297: Approved February 15,
2023.). The requirement for acquisition of informed consent from
patients was waived because of the retrospective design of this
study and anonymized data. The inclusion criteria as follows: i)
Patients underwent gastrectomy with Stage I, II, III GC, ii) not
applicable for endoscopic submucosal dissection, and iii) computed
tomography (CT) performed within 30 days before surgery. Patients
who had stage IV disease (n=16), synchronous malignant neoplasms
(n=3), and underwent emergency surgery (n=1) were excluded; the
remaining 222 patients were enrolled in this study.
Treatment and follow-up
The Japanese Gastric Cancer Treatment Guidelines,
including surgical indications, treatment, and selection of
chemotherapy, were used in our treatment strategy for GC (10). Staging and pathological diagnoses
were based on the Japanese Classification of Gastric Carcinoma
(11). Neoadjuvant chemotherapy
with S-1 plus cisplatin was administered to patients with bulky
lymph nodes. Depending the location of the tumor, distal, total, or
proximal gastrectomy was performed. Laparoscopic gastrectomy is
primarily performed in patients with stage I clinical disease. In
patients with clinical stage II or III disease, the attending
surgeon selected between the laparoscopic and open surgical
approaches. Postoperative complications were defined as grade III–V
based on the Clavien-Dindo classification, occurring within 30
postoperative days (12). Patients
with pathological stage II were treated with S-1 alone, and stage
III were treated with S-1 alone or S-1 in combination with
oxaliplatin with adjuvant chemotherapy, if the general condition
was judged to be tolerated based on patients' performance status.
Basic surveillance was conducted until death or 5 years
post-operatively. Patients with stage I disease were followed-up
every 6 months, and those with stage II or III were every 3 months
to check for recurrence by performing blood tests, including those
for the screening of tumor markers. Enhanced CT was performed every
6 months, and upper gastrointestinal endoscopy was performed every
1–2 years. For recurrence, systemic chemotherapy was administered
based on the patient's performance status.
Definition of sarcopenia, osteopenia,
and OVF
Sarcopenia, osteopenia, and OVF were preoperatively
evaluated using CT. Sarcopenia was defined as psoas muscle mass
area (PMA) at the third lumbar vertebra below the sex-specific
median size. The PMA was calculated as follows: Length of the major
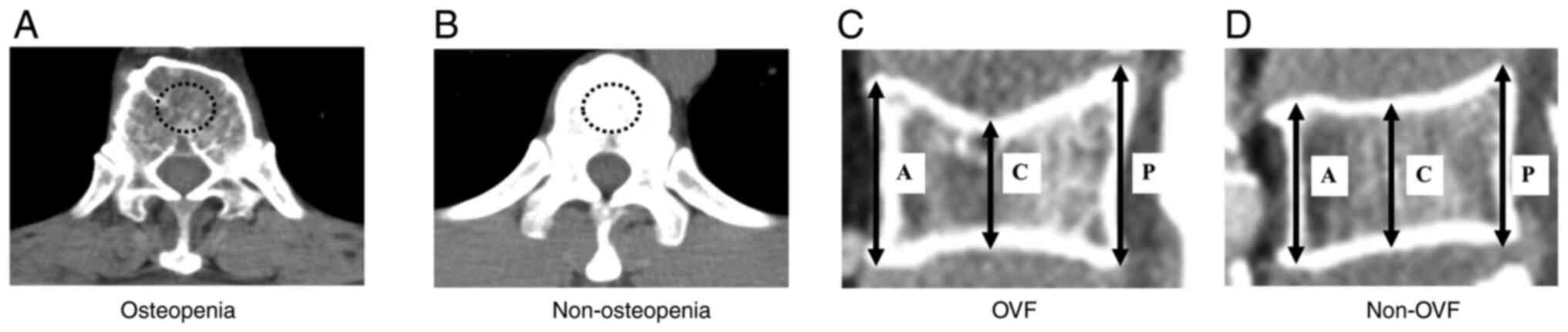
axes × the length of the minor axes × π (13). Osteopenia was defined as a decrease
in the BMD below the standard value. The BMD was measured by bone
mineral density in the midvertebral core of the 11th thoracic
vertebra. The cut-off values of BMD were evaluated based on the
previous reports as follow: Men=308.82–2.49 × age in years,
women=311.84–2.41 × age in years (Fig.
1A and B) (14). OVF was
evaluated using the anterior (A), central (C), and posterior (P)
heights of the vertebrae from the 11th thoracic vertebra to the 5th
lumbar vertebra. The criteria for OVF were C/A <0.8 or C/P
<0.8 in the any of the vertebrae regardless of fracture history
(pathological fractures and symptomatic fractures were excluded)
(Fig. 1C and D) (15).
Measurement of preoperative GPS and
PNI
The GPS was defined as a combination of C-reactive
protein (CRP) and albumin levels. In cases where both levels were
abnormal (CRP >1.0 mg/dl and albumin <3.5 g/dl), the score
was 2; if one level was abnormal, the score was 1; and if neither
level was abnormal, the score was 0 (16). The PNI was calculated as 10× serum
albumin level (g/dl) +0.005× lymphocyte count (17).
Statistical analysis
All statistical analyses were conducted using a
statistical software program (SPSS Statistics for Windows, version
22; IBM Corp., Armonk, N.Y., USA). Quantitative variables are
expressed as median and interquartile range, and differences were
analyzed using the Mann-Whitney U test. Qualitative variables were
compared using the Fisher's exact test. The Kaplan-Meier method was
used to analyze the survival rates, and the log-rank test was used
to compare the differences between the survival rates of the
groups. A Cox proportional hazards regression model was used to
identify the independent prognostic factors associated with
disease-free survival (DFS) and overall survival (OS) rates.
Variables identified as significant in univariate analysis were
included in multivariate analysis. The continuous variables were
classified into two groups. The cutoff value for CEA was set at the
upper normal limit, and the cutoff values for age, PNI, operative
time, and intraoperative blood loss were determined via a receiver
operating characteristic curve using the survival status at the
3-year follow-up. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients' characteristics
The patient characteristics and associations between
clinical variables and OVF are summarized in Table I. The median patient age was 74
years (range: 68–80 years). This study included 170 (77%) men.
Osteopenia, sarcopenia, and OVF were observed in 68 (31%), 110
(50%), and 64 (28%) patients, respectively. The pathological
diagnosis of GC showed that 91 (41%), 55 (25%), and 76 (34%)
patients had stage I, II, and III cancers, respectively.
 | Table I.Patients' characteristics. |
Table I.
Patients' characteristics.
|
|
| OVF |
|
|---|
|
|
|
|
|
|---|
| Variable | Total (n=222) | Yes (n=64) | No (n=158) | P-value |
|---|
| Age,
yearsb | 74 (68–80) | 77 (71–81) | 73 (64–79) | <0.01a |
| Sex,
malec | 170 (77%) | 50 (78%) | 120 (76%) | 0.86 |
| Body mass index,
kg/m2b | 21.7 (19.9–24.4) | 21.7 (19.3–24.2) | 21.7 (20.4–24.5) | 0.57 |
| Serum CEA,
ng/mlb | 3.7 (2.5–5.8) | 3.7 (2.8–5.9) | 3.7 (2.5–5.6) | 0.76 |
| BMD, HUb | 142 (113–176) | 120 (98–145) | 154 (120–187) | <0.01a |
| PMA,
cm2b | 19.1 (13.6–24.6) | 16.8 (12.1–22.9) | 19.8 (14.3–25.1) | 0.02a |
| GPS, 1 or
2c | 64 (29%) | 25 (39%) | 39 (25%) | 0.04a |
| PNIb | 48 (42–52) | 45 (39–51) | 48 (43–52) | 0.01a |
| Osteopenia,
yesc | 68 (31%) | 31 (48%) | 37 (23%) | <0.01a |
| Sarcopenia,
yesc | 110 (50%) | 40 (63%) | 70 (44%) | 0.02a |
| Histological
typec |
|
|
| 0.12 |
|
tub1 | 75 (34%) | 19 (30%) | 56 (35%) |
|
|
tub2 | 35 (16%) | 10 (15%) | 25 (16%) |
|
|
por | 84 (38%) | 29 (45%) | 55 (34%) |
|
|
sig | 19 (8%) | 2 (3%) | 17 (11%) |
|
|
pap | 5 (2%) | 1 (2%) | 4 (3%) |
|
|
muc | 4 (2%) | 3 (5%) | 1 (1%) |
|
| Neoadjuvant
chemotherapy, yesc | 4 (2%) | 1 (2%) | 3 (2%) | >0.99 |
| Operative
approachc |
|
|
| 0.01a |
|
Open | 95 (43%) | 36 (56%) | 59 (37%) |
|
|
Laparoscope | 127 (57%) | 28 (44%) | 99 (63%) |
|
| Operative
procedurec |
|
|
| 0.03a |
| DG | 139 (63%) | 34 (53%) | 105 (67%) |
|
| PG | 6 (3%) | 0 (0%) | 6 (4%) |
|
| TG | 77 (34%) | 30 (47%) | 47 (29%) |
|
| Lymph node
dissectionc |
|
|
| 0.16 |
| D1 | 58 (26%) | 18 (28%) | 40 (25%) |
|
|
D1+ | 87 (39%) | 19 (30%) | 68 (43%) |
|
| D2 | 77 (35%) | 27 (42%) | 50 (32%) |
|
| Operative time,
minb | 270 (230–326) | 271 (236–328) | 265 (229–325) | 0.69 |
| Blood loss,
mlb | 154 (50–415) | 223 (100–453) | 150 (20–400) | 0.10 |
| Postoperative
hospital stay, daysb | 12 (10–20) | 14 (11–22) | 12 (10–19) | 0.06 |
| Postoperative
complication | 25 (11%) | 6 (9%) | 19 (12%) | 0.65 |
| (Clavien-Dindo
grade III–V) c |
|
|
|
|
|
Reoperationc | 8 (3%) | 2 (3%) | 6 (4%) | >0.99 |
| T
factorc |
|
|
|
<0.01a |
| 1 | 84 (38%) | 16 (25%) | 68 (43%) |
|
| 2 | 27 (12%) | 6 (9%) | 21 (13%) |
|
| 3 | 58 (26%) | 15 (24%) | 43 (27%) |
|
| 4 | 53 (24%) | 27 (42%) | 26 (17%) |
|
| Lymph node
metastases, yesc | 99 (45%) | 39 (61%) | 60 (38%) |
<0.01a |
| Stagec |
|
|
|
<0.01a |
| I | 91 (41%) | 17 (26%) | 74 (47%) |
|
| II | 55 (25%) | 14 (22%) | 41 (26%) |
|
|
III | 76 (34%) | 33 (51%) | 43 (27%) |
|
| Adjuvant
chemotherapy, yesc | 52 (23%) | 23 (36%) | 29 (18%) |
<0.01a |
|
Curabilityc |
|
|
| >0.99 |
| R0 | 209 (94%) | 60 (94%) | 149 (94%) |
|
| R1 or
R2 | 13 (6%) | 4 (6%) | 9 (6%) |
|
In the univariate analysis, patients with OVF were
significantly associated with older age (P<0.01), operative
approach (open gastrectomy, P=0.01), T factor (P<0.01), lymph
node metastases (P<0.01), advanced stage (P<0.01), and
adjuvant chemotherapy (P<0.01). In terms of body composition,
patients with OVF had higher GPS (P=0.04) and lower BMD
(P<0.01), PMA (P=0.02), and PNI (P=0.01) than those without OVF.
In addition, postoperative recurrence occurred in 28 (44%) patients
in the OVF group and 25 (16%) patients in the non-OVF group. Of
these, 11 (17%) patients in the OVF group and 12 (8%) patients in
the non-OVF group were administered with postoperative chemotherapy
for recurrence.
Univariate and multivariate DFS
analyses of patients with GC
Table II shows the
prognostic factors for the DFS rates according to Cox proportional
hazard analysis. The univariate analysis of the DFS rates indicated
that osteopenia (P<0.01), sarcopenia (P<0.01), OVF
(P<0.01), GPS score of 1 or 2 (P=0.02), PNI <45 (P=0.04),
intraoperative blood loss ≥227 ml (P=0.01), stage ≥II (P<0.01),
and R1 or R2 (P<0.01) were significant prognostic factors. The
multivariate analysis revealed that OVF [hazard ratio (HR): 2.35,
95% confidence interval (CI): 1.30–4.27; P<0.01], stage ≥II (HR,
6.15; 95% CI, 2.36–16.01; P<0.01), and R1 or R2 (HR, 2.35; 95%
CI, 1.30–4.27; P<0.01) were independent and significant
predictors of DFS.
 | Table II.Univariate and multivariate analyses
of clinicopathological variables in relation to disease-free
survival after gastrectomy for gastric cancer. |
Table II.
Univariate and multivariate analyses
of clinicopathological variables in relation to disease-free
survival after gastrectomy for gastric cancer.
|
|
| DFS univariate
analysis | DFS multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | n | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
|
≥72 | 123 | 1.04 | 0.88 |
|
|
|
<72 | 99 | (0.61–1.79) |
|
|
|
| Sex |
|
|
|
|
|
|
Male | 170 | 0.57 | 0.14 |
|
|
|
Female | 52 | (0.27–1.20) |
|
|
|
| Serum CEA,
ng/ml |
|
|
|
|
|
| ≥5 | 72 | 1.43 | 0.21 |
|
|
|
<5 | 150 | (0.82–2.51) |
|
|
|
| Neoadjuvant
chemotherapy |
|
|
|
|
|
|
Yes | 4 | 0.84 | 1.22 |
|
|
| No | 218 | (0.17–8.84) |
|
|
|
| Osteopenia |
|
|
|
|
|
|
Yes | 68 | 2.20 |
<0.01a | 1.65 | 0.10 |
| No | 154 | (1.27–3.80) |
| (0.91–2.98) |
|
| Sarcopenia |
|
|
|
|
|
|
Yes | 110 | 2.37 |
<0.01a | 1.66 | 0.11 |
| No | 112 | (1.34–4.18) |
| (0.89–3.06) |
|
| OVF |
|
|
|
|
|
|
Yes | 68 | 3.20 |
<0.01a | 2.35 |
<0.01a |
| No | 154 | (1.87–5.50) |
| (1.30–4.27) |
|
| GPS |
|
|
|
|
|
| 1 or
2 | 64 | 1.93 | 0.02a | 1.36 | 0.48 |
| 0 | 158 | (1.10–3.38) |
| (0.60–2.92) |
|
| PNI |
|
|
|
|
|
|
≥45 | 142 | 1.74 | 0.04a | 0.61 | 0.22 |
|
<45 | 80 | (1.01–3.01) |
| (0.27–1.35) |
|
| Operative time,
min |
|
|
|
|
|
|
≥267 | 110 | 0.87 | 0.60 |
|
|
|
<267 | 112 | (0.51–1.49) |
|
|
|
| Intraoperative
blood loss, ml |
|
|
|
|
|
|
≥227 | 100 | 2.06 | 0.01 | 1.56 | 0.14 |
|
<227 | 122 | (1.19–3.56) |
| (0.86–2.81) |
|
| Postoperative
complication |
|
|
|
|
|
| (Clavien-Dindo
grade III–V) |
|
|
|
|
|
|
Yes | 25 | 1.47 | 0.38 |
|
|
| No | 197 | (0.63–3.44) |
|
|
|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
Yes | 52 | 1.69 | 0.07 |
|
|
| No | 170 | (0.97–2.97) |
|
|
|
| Stage |
|
|
|
|
|
| I | 91 | 9.29 |
<0.01a | 6.15 |
<0.01a |
| II or
III | 131 | (3.69–23.37) |
| (2.36–16.01) |
|
| Curability |
|
|
|
|
|
| R1 or
2 | 13 | 8.47 |
<0.01a | 2.35 |
<0.01a |
| R0 | 209 | (3.97–18.05) |
| (1.30–4.27) |
|
Univariate and multivariate OS rate
analyses of patients with GC
Table III shows
the prognostic factors for the OS rates according to Cox
proportional hazard analysis. Univariate analysis of the OS rates
indicated that osteopenia (P=0.01), sarcopenia (P<0.01), OVF
(P<0.01), GPS score of 1 or 2 (P<0.01), PNI <45
(P<0.01), intraoperative blood loss ≥227 ml (P<0.01), stage
≥II (P<0.01), and R1 or R2 (P<0.01) were significant
prognostic factors. The multivariate analysis revealed that OVF
(HR, 2.16; 95% CI, 1.15–4.03; P=0.02), stage ≥II (HR, 5.31; 95% CI,
2.02–13.93; P<0.01), and R1 or R2 (HR, 5.95; 95% CI, 2.47–14.35;
P<0.01) were independent and significant predictors of OS.
 | Table III.Univariate and multivariate analyses
of clinicopathological variables in relation to overall survival
after gastrectomy for gastric cancer. |
Table III.
Univariate and multivariate analyses
of clinicopathological variables in relation to overall survival
after gastrectomy for gastric cancer.
|
|
| OS univariate
analysis | OS multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | n | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
|
≥72 | 123 | 1.22 | 0.49 |
|
|
|
<72 | 99 | (0.69–2.14) |
|
|
|
| Sex |
|
|
|
|
|
|
Male | 170 | 0.64 | 0.24 |
|
|
|
Female | 52 | (0.30–1.46) |
|
|
|
| Serum CEA,
ng/ml |
|
|
|
|
|
| ≥5 | 72 | 1.21 | 0.53 |
|
|
|
<5 | 150 | (0.67–2.20) |
|
|
|
| Neoadjuvant
chemotherapy |
|
|
|
|
|
|
Yes | 4 | 1.43 | 0.72 |
|
|
| No | 218 | (0.20–10.44) |
|
|
|
| Osteopenia |
|
|
|
|
|
|
Yes | 68 | 2.04 | 0.01a | 1.68 | 0.10 |
| No | 154 | (1.15–3.61) |
| (0.91–3.12) |
|
| Sarcopenia |
|
|
|
|
|
|
Yes | 110 | 2.98 |
<0.01a | 1.77 | 0.10 |
| No | 112 | (1.60–5.55) |
| (0.90–3.46) |
|
| OVF |
|
|
|
|
|
|
Yes | 68 | 3.50 |
<0.01a | 2.16 | 0.02a |
| No | 154 | (1.98–6.17) |
| (1.15–4.03) |
|
| GPS |
|
|
|
|
|
| 1 or
2 | 64 | 2.56 |
<0.01a | 1.19 | 0.66 |
| 0 | 158 | (1.45–4.51) |
| (0.54–2.61) |
|
| PNI |
|
|
|
|
|
|
≥45 | 142 | 2.69 |
<0.01a | 0.98 | 0.97 |
|
<45 | 80 | (1.53–4.72) |
| (0.45–2.16) |
|
| Operative time,
min |
|
|
|
|
|
|
≥267 | 110 | 0.75 | 0.32 |
|
|
|
<267 | 112 | (0.43–1.32) |
|
|
|
| Intraoperative
blood loss, ml |
|
|
|
|
|
|
≥227 | 100 | 2.48 |
<0.01a | 1.84 | 0.06 |
|
<227 | 122 | (1.38–4.46) |
| (0.97–3.47) |
|
| Postoperative
complication |
|
|
|
|
|
| (Clavien-Dindo
grade III–V) |
|
|
|
|
|
|
Yes | 25 | 2.05 | 0.08 |
|
|
| No | 197 | (0.92–4.56) |
|
|
|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
Yes | 52 | 1.19 | 0.58 |
|
|
| No | 170 | (0.64–2.21) |
|
|
|
| Stage |
|
|
|
|
|
| I | 91 | 8.89 |
<0.01a | 5.31 |
<0.01a |
| II or
III | 131 | (3.52–22.46) |
| (2.02–13.92) |
|
| Curability |
|
|
|
|
|
| R1 or
2 | 13 | 9.98 |
<0.01a | 5.95 |
<0.01a |
| R0 | 209 | (4.42–22.54) |
| (2.47–14.35) |
|
Impact of OVF on DFS and OS after
gastrectomy for GC
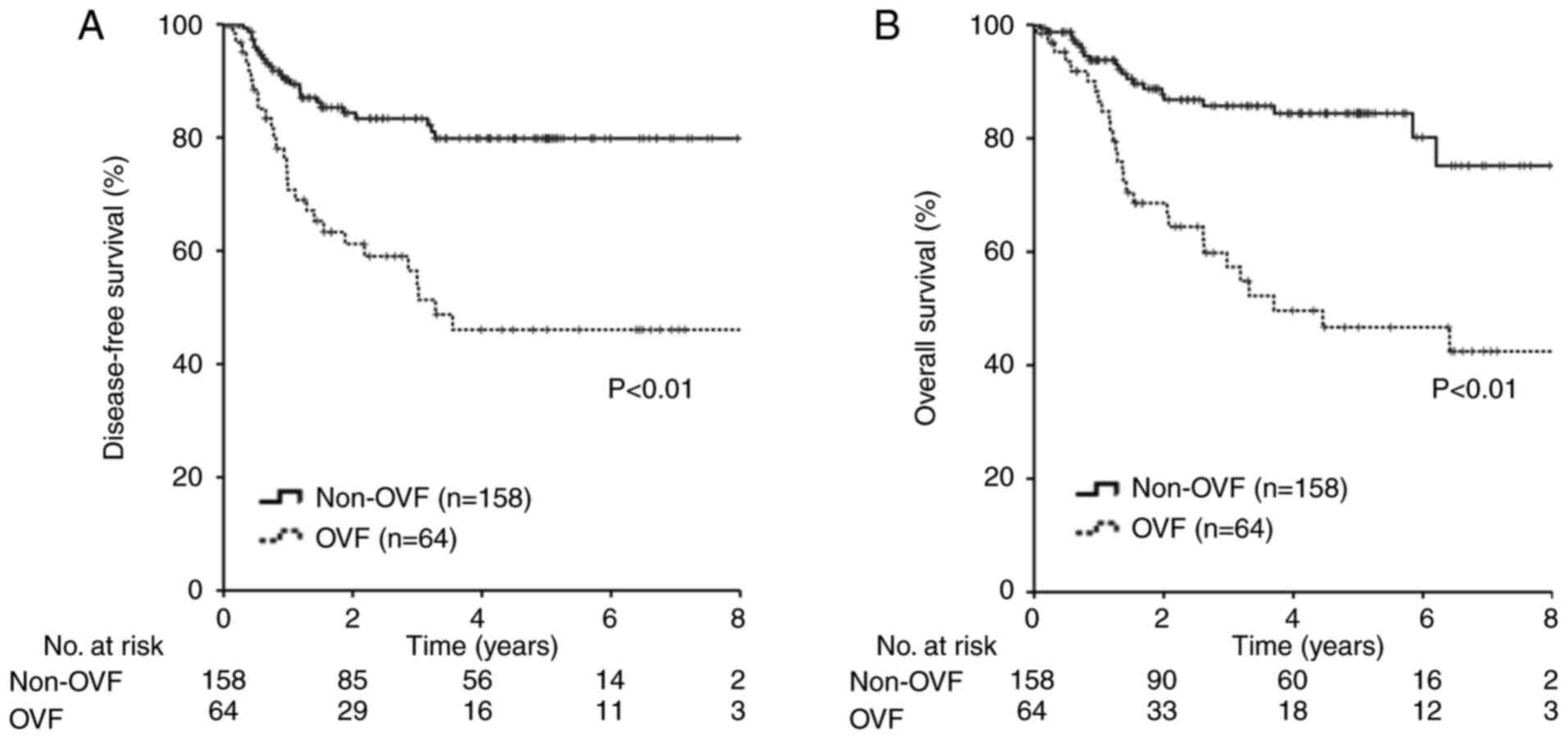
Patients with OVF had significantly lower DFS and OS
rates than those without OVF. (DFS: 5-year survival rate, 46.0 vs.
79.8%, respectively, P<0.01; OS: 5-year survival rate, 46.7 vs.
84.4%, respectively, P<0.01) (Fig.
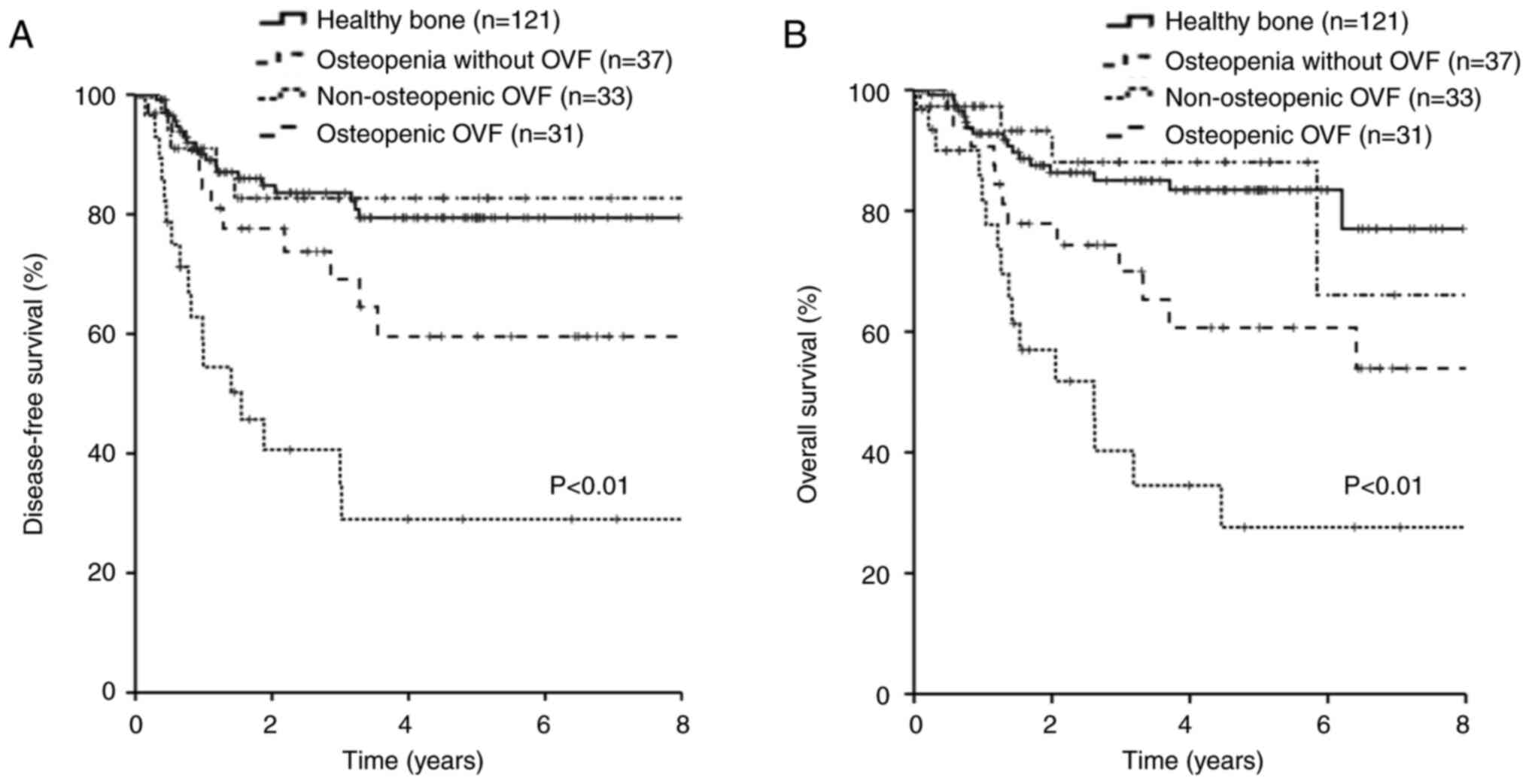
2). In terms of bone status, 121 (55%) patients had healthy
bones, 37 (17%) had osteopenia without OVF, 33 (15%) had
non-osteopenic OVF, and 31 (14%) had osteopenia with OVFs. Patients
with OVF had significantly lower DFS and OS rates than those
without OVF, regardless of osteopenia (P<0.01, P<0.01,
respectively) (Fig. 3). The 5-year
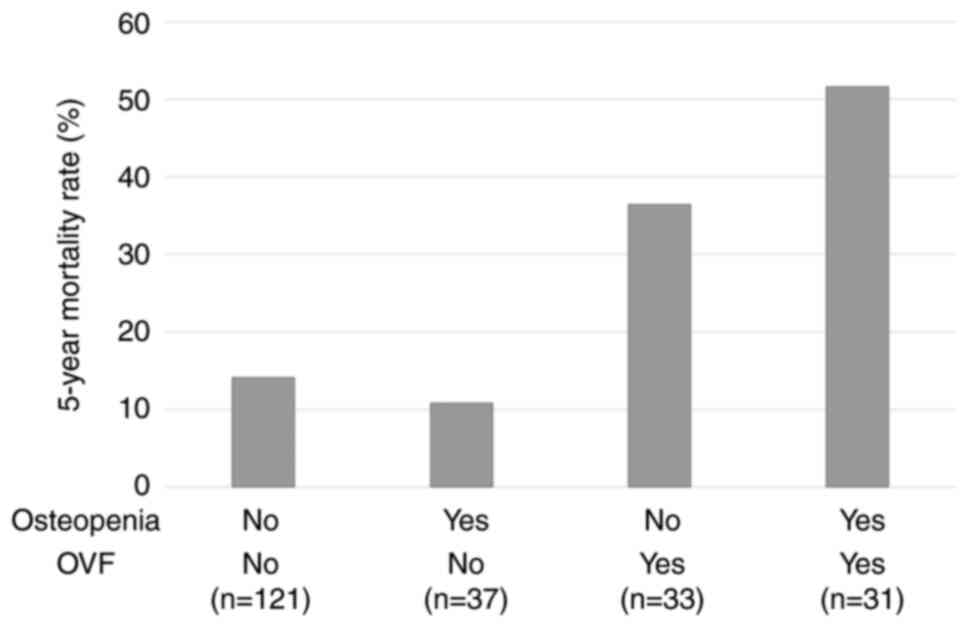
mortality rates after gastrectomy were as follows: healthy bone
(14.1%); osteopenia without OVF (10.8%); non-osteopenic OVF
(36.4%); and osteopenia with OVF (51.6%) (Fig. 4).
Discussion
Our results showed that preoperative OVF was
significantly associated with poor prognosis and recurrence in
patients undergoing gastrectomy for GC. To the best of our
knowledge, this is the first report to demonstrate the impact of
OVF on mortality in patients with GC.
OVF is common and result in acute and chronic pain,
reduced quality of life, and diminished lifespan (7). OVF is a public health problem, with
approximately 750,000 cases occurring annually. The prevalence of
OVF increased from 3% in women aged <60 years to 20% in those
aged >70 years and from 7.5 to 20% in men in the same age group,
increasing in prevalence with age (18). In the present study, the OVF rate
was 26% in women and 29% in men, with a median age of 74. OVF is
the hallmark of osteopenia, which is characterized by a low BMD and
occurs at a higher incidence earlier in life than any other type of
osteoporotic fractures (19). We
have previously shown that osteopenia could be a prognostic factor
in patients with GC (5). However,
in the present study, we found that OVF, a complication of
osteopenia, was a stronger predictor than osteopenia. In addition,
the combination of OVF and osteopenia is associated with worse
prognosis. Thus, this study identified a strong prognostic factor
and is a valuable finding.
The presence of one or more OVF was estimated to
increase the risk of fractures by approximately 5–10 fold.
Furthermore, mortality is reported to increase by approximately
10%, 5 years after OVF (20,21).
If the presence of OVF alone increases mortality, even in the
absence of cancer, it can be inferred that the prognosis of
patients with GC with preoperative OVF is even worse. In the
current study, patients with OVF had significantly worse OS and DFS
rates than those without OVF.
However, biological mechanisms underlying bone
metabolism and malignancy remain unclear. Recently, it was
suggested that the RANK/RANKL system may be associated with bone
metabolism and cancer development. RANKL is a member of the tumor
necrosis factor family that binds to its receptor RANK to control
osteoclast differentiation, activation, and survival.
Proinflammatory and pro-osteolytic cytokines derived from cancer
cells such as tumor necrosis factor-alpha, parathyroid
hormone-related protein, interleukin (IL)-1, IL-6, and IL-8
activates RANK/RANKL signaling mechanism, causing bone loss
(22). Furthermore, the RANK/RANKL
pathway promotes epithelial-mesenchymal transition and metastasis
(23). In the current study, the
OVF group had more advanced disease stage (T-factor; P<0.01;
lymph node metastases; P<0.01; stage, P<0.01).
Various systemic diseases, including metabolic,
genetic, immune, inflammatory, and endocrine diseases, are
associated with an increased risk of OVF; besides, deficiencies of
vitamin D and estrogen are also one of the causes (20). Vitamin D and estrogen have been
reported to be involved in the development of GC. Du et al
have shown that vitamin D and its metabolites inhibit the
viability, growth, and metastasis of GC cells. In addition, vitamin
D metabolites may inhibit Helicobacter pylori infection and
H. pylori-associated GC (24). According to recent reports, vitamin
D may be involved in the anti-cancer mechanism of GC by affecting
the expression of microRNAs, promoting the effects of cisplatin,
and regulating intracellular signal transduction (25). Ge et al reported that
estrogen receptors might be related to the progression and
deterioration of GC (26). Thus,
vitamin D and estrogen may be associated with the occurrence of GC
and OVF; however, further research is needed to assess the
relationship between GC and OVF.
The risk of bone loss after gastrectomy in patients
with GC increases owing to malabsorption and malnutrition. The
absorption of calcium and vitamin D is impaired because most of the
stomach has been removed (27).
Postoperative BMD in patients with GC after gastrectomy is
decreased, and a knowledge of the presence of OVF preoperatively in
patients with GC is more important than in other cancers because
the conditions of patients with lower preoperative BMD are expected
to be worse after gastrectomy (28). Calcium, vitamin D, and
weight-bearing exercises are important for patients with
osteopenia, and bisphosphonates, raloxifene, and nasal calcitonin
have been shown to reduce the incidence of new OVF by 30–50%
(29). OVF is often asymptomatic or
underdiagnosed and under-treated (7). However, the diagnosis of OVF during
routine medical care and appropriate intervention may improve the
prognosis of patients with GC and OVF.
This study has several limitations. First, it was
retrospective and conducted at a single institution with a small
number of patients. Second, the effect of chemotherapy on recurrent
cases is not reflected in the OS, which may lead to bias. Third,
the definitions of sarcopenia, osteopenia, and OVF are
controversial, and the cut-off values vary among studies. Fourth,
we did not consider the diets rich in calcium, patients' medical
histories and medications, such as vitamin D supplements and
bisphosphonates. People with symptomatic fractures are excluded in
the current study, however, those people have interventions such as
vitamin D or bisphosphonate, and may have a good prognosis.
Similarly, people with osteopenia or racial differences who have
bisphosphonates or a high calcium diet may have a better prognosis.
Therefore, we believe that further analysis by these factors will
help to elucidate the mechanism of OVF and GC. Taken together, our
findings need to be validated in large-scale prospective studies.
Furthermore, evaluation in different racial groups is also needed
in the future.
In conclusion, we demonstrated that preoperative OVF
was significantly associated with worse DFS and OS rates in
patients who underwent gastrectomy for GC. In addition, we showed
that the combination of osteopenia and OVF could be a stronger
prognostic indicator than osteopenia and OVF alone.
Acknowledgements
Not applicable.
Funding
This research was supported by JPSP KAKENHI (grant no.
23K16454).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NF, KF, TI, FY and KE conceptualized the study. NF,
KF and FY analysed the data. NF wrote the original draft, and FY,
KE and TI revised and edited the draft. TM and KF performed
statistical analyses. KaT, KeT, MY and MT collected the data and
analyzed the results. NF and KaT confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Fuji City General Hospital (297). The requirement for acquisition
of informed consent from patients was waived because of the
retrospective design of this study and the use of anonymized
data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawamura T, Makuuchi R, Tokunaga M,
Tanizawa Y, Bando E, Yasui H, Aoyama T, Inano T and Terashima M:
Long-term outcomes of gastric cancer patients with preoperative
sarcopenia. Ann Surg Oncol. 25:1625–1632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang DS, Ren C, Qiu MZ, Luo HY, Wang ZQ,
Zhang DS, Wang FH, Li YH and Xu RH: Comparison of the prognostic
value of various preoperative inflammation-based factors in
patients with stage III gastric cancer. Tumor Biol. 33:749–756.
2012. View Article : Google Scholar
|
|
4
|
Li Q, Huang LY and Xue HP: Comparison of
prognostic factors in different age groups and prognostic
significance of neutrophil-lymphocyte ratio in patients with
gastric cancer. World J Gastrointest Oncol. 12:1146–1166. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukushima N, Tsuboi K, Nyumura Y, Hoshino
M, Masuda T, Suzuki T, Kajimoto T, Yano F and Eto K: Prognostic
significance of preoperative osteopenia on outcomes after
gastrectomy for gastric cancer. Ann Gastroenterol Surg. 10:255–264.
2022.PubMed/NCBI
|
|
6
|
Watanabe J, Saitsu A, Miki A, Kotani K and
Sata N: Prognostic value of preoperative low bone mineral density
in patients with digestive cancers: A systematic review and
meta-analysis. Arch Osteoporos. 17:332022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCarthy J and Davis A: Diagnosis and
management of vertebral compression fractures. Am Fam Physician.
94:44–50. 2016.PubMed/NCBI
|
|
8
|
Furukawa K, Haruki K, Taniai T, Yanagaki
M, Tsunematsu M, Tanji Y, Ishizaki S, Shirai Y, Onda S and Ikegami
T: Occult vertebral fracture (OVF) in patients who underwent
hepatectomy for colorectal liver metastasis: Strong association
with oncological outcomes. Cancers (Basel). 15:55132023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishizaki S, Furukawa K, Haruki K,
Tsunematsu M, Shirai Y, Matsumoto M, Okui N, Onda S, Taniai T and
Ikegami T: Prognostic significance of occult vertebral fracture in
patients undergoing pancreatic resection for pancreatic cancer.
Pancreatology. 24:249–254. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma, . 3rd English
edition. Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ochiai A: The 15th Edition of Japanese
Classification of Gastric carcinoma. Japanese Gastric Cancer
Association; Volume 89. Tokyo, Japan: 2017, (In Japanese).
|
|
12
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6,336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masuda T, Shirabe K, Ikegami T, Harimoto
N, Yoshizumi T, Soejima Y, Uchiyama H, Ikeda T, Baba H and Maehara
Y: Sarcopenia is a prognostic factor in living donor liver
transplantation. Liver Transpl. 20:401–407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toshima T, Yoshizumi T, Ikegami T, Harada
N, Itoh S, Mano Y, Motomura T, Soejima Y and Maehara Y: Impact of
osteopenia in liver cirrhosis: Special reference to standard bone
mineral density with age. Anticancer Res. 38:6465–6471. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Orimo H, Hayashi Y, Fukunaga M, Sone T,
Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J,
et al: Diagnostic criteria for primary osteoporosis: Year 1996
revision. J Bone Miner Metab. 14:219–233. 1997.
|
|
16
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Evaluation of cumulative prognostic
scores based on the systemic inflammatory response in patients with
inoperable non-small-cell lung cancer. Br J Cancer. 89:1028–1030.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
18
|
Li Y, Yan L, Cai S, Wang P, Zhuang H and
Yu H: The prevalence and under-diagnosis of vertebral fractures on
chest radiograph. BMC Musculoskelet Disord. 19:2352018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grigoryan M, Guermazi A, Roemer FW, Delmas
PD and Genant HK: Recognizing and reporting osteoporotic vertebral
fractures. Eur Spine J. 12:S104–S112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bazzocchi A and Guglielmi G: Vertebral
fracture identification. Semin Musculoskelet Radiol. 20:317–329.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Watts NB: Osteoporotic vertebral
fractures. Neurosurg Focus. 10:E122001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jones DH, Nakashima T, Sanchez OH,
Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R,
Hojilla CV, et al: Regulation of cancer cell migration and bone
metastasis by RANKL. Nature. 440:692–626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du C, Yang S, Zhao X and Dong H:
Pathogenic roles of alterations in vitamin D and vitamin D receptor
in gastric tumorigenesis. Oncotarget. 8:29474–29486. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shah S, Iqbal Z, Alharbi MG, Kalra HS,
Suri M, Soni N, Okpaleke N, Yadav S and Hamid P: Vitamin D and
gastric cancer: A ray of sunshine? Cureus. 13:e182752021.PubMed/NCBI
|
|
26
|
Ge H, Yan Y, Tian F, Wu D and Huang Y:
Prognostic value of estrogen receptor alpha and estrogen receptor
beta in gastric cancer based on a meta-analysis and the cancer
genome atlas (TCGA) datasets. Int J Surg. 53:24–31. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoo SH, Lee JA, Kang SY, Kim YS, Sunwoo S,
Kim BS and Yook JH: Risk of osteoporosis after gastrectomy in
long-term gastric cancer survivors. Gastric Cancer. 21:720–727.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muszyński T, Polak K, Frątczak A, Miziołek
B, Bergler-Czop B and Szczepanik A: Vitamin D-the nutritional
status of post-gastrectomy gastric cancer patients-systematic
review. Nutrients. 14:27122022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watts NB: Focus on primary care
postmenopausal osteoporosis: An update. Obstet Gynecol Surv.
55:S49–S55. 2000. View Article : Google Scholar : PubMed/NCBI
|