Introduction
Epidermal growth factor receptor (EGFR) gene
mutations account for most cancer-related genomic alterations in
cases of non-small cell lung cancer (NSCLC) in East Asia, as the
incidence rate ranges from 45–55% (1,2). A
single amino acid substitution of leucine to arginine at site 858
in exon 21 (L858R mutation) and an in-frame deletion within exon 19
(exon 19 deletion) account for ~90% of all EGFR mutations in
NSCLC. Other EGFR mutations, including G719X in exon 18,
S768I in exon 20 and L861Q in exon 21, have been detected and
classified as major uncommon EGFR mutations (5–7% of all
EGFR mutations) (3,4). EGFR mutations, including common
(L858R and exon 19 deletion) and major uncommon (G719X, S768I and
L861Q) mutations, alter the activity of the intracellular tyrosine
kinase domain of EGFR and promote downstream pro-survival signaling
pathways in NSCLC (3–7). In the past 2 decades, EGFR-tyrosine
kinase inhibitors (TKIs) have been developed and reported to be
effective at suppressing the growth of human NSCLC cells with
common and major uncommon EGFR mutations (3,4,6–8).
First- and second-generation EGFR-TKIs, including gefitinib,
erlotinib and afatinib, have shown promising anticancer efficacy in
advanced EGFR-mutated NSCLC [60-80% objective response rate
(ORR) and 9–13 months of progression-free survival (PFS)] in
several previous prospective clinical trials and real-world
clinical analyses (6–10). Therefore, first- and
second-generation EGFR-TKIs are used as standard first-line
treatments for patients with advanced NSCLC with EGFR
mutations (3,4,6–10).
Brain metastasis is the main morbidity that
frequently occurs in patients with NSCLC, and 20–40% of patients
with NSCLC experience this complication throughout the course of
their disease (11,12). A previous extensive clinical
analysis reported that patients with NSCLC harboring EGFR
mutations had increased incidence of brain metastasis compared with
those harboring wild-type EGFR (13). Previous studies have reported that
first- and second-generation EGFR-TKIs have good blood-brain
barrier (BBB) permeability and can effectively control the brain
metastasis of EGFR-mutated NSCLC (14,15).
However, in previous large prospective clinical trials, only
asymptomatic or stable symptomatic control patients with previously
treated brain metastases were allowed to be recruited (6–10).
Although the brain metastasis of EGFR-mutated NSCLC has been
included in previous real-world retrospective studies for analysis,
these previous studies reported that brain metastasis was an
unfavorable prognostic factor or reported the effects of additional
local therapies on brain metastasis (7,9,12,15).
In clinical practice, certain patients with brain metastasis of
EGFR-mutated NSCLC have neurological symptoms induced by
brain metastasis and have to receive local therapies (radiation
therapy or neurosurgery) and EGFR-TKIs concurrently (12–15).
In addition, first- and second-generation EGFR-TKIs have been
widely used for the treatment of patients with advanced
EGFR-mutated NSCLC in clinical practice over the past two
decades (6–10).
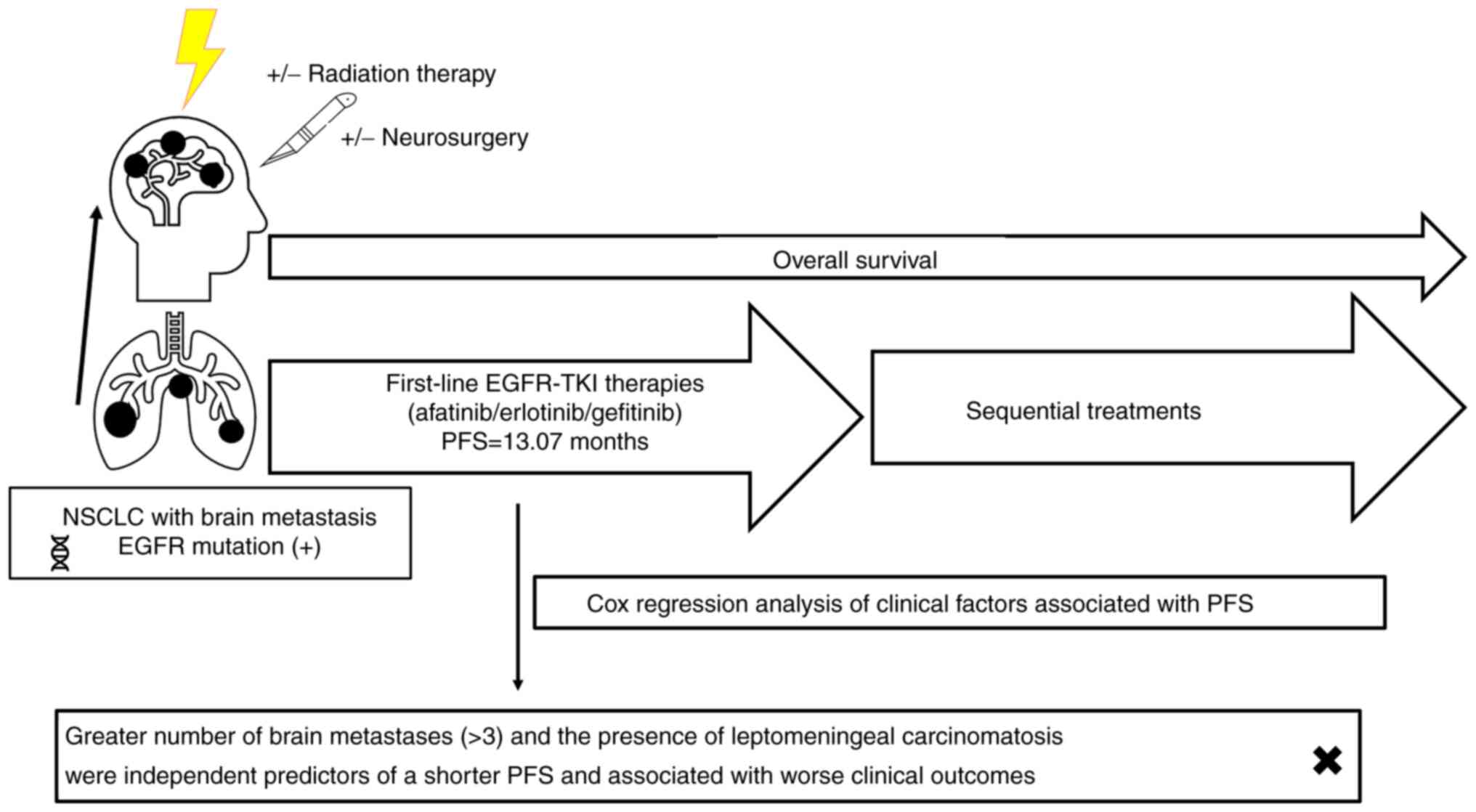
Therefore, the present study aimed to assess the
clinical outcomes of patients with EGFR-mutated NSCLC with
baseline brain metastasis who received first-line first- and
second-generation EGFR-TKIs. In addition, the efficacy of different
EGFR-TKIs were compared and predictive clinical factors associated
with survival outcomes were identified.
Patients and methods
Patients, EGFR mutation detection,
treatment and follow-up
Data was retrieved from the Cancer Center of Chang
Gung Memorial Hospital at Linkou (Taoyuan, Taiwan) database.
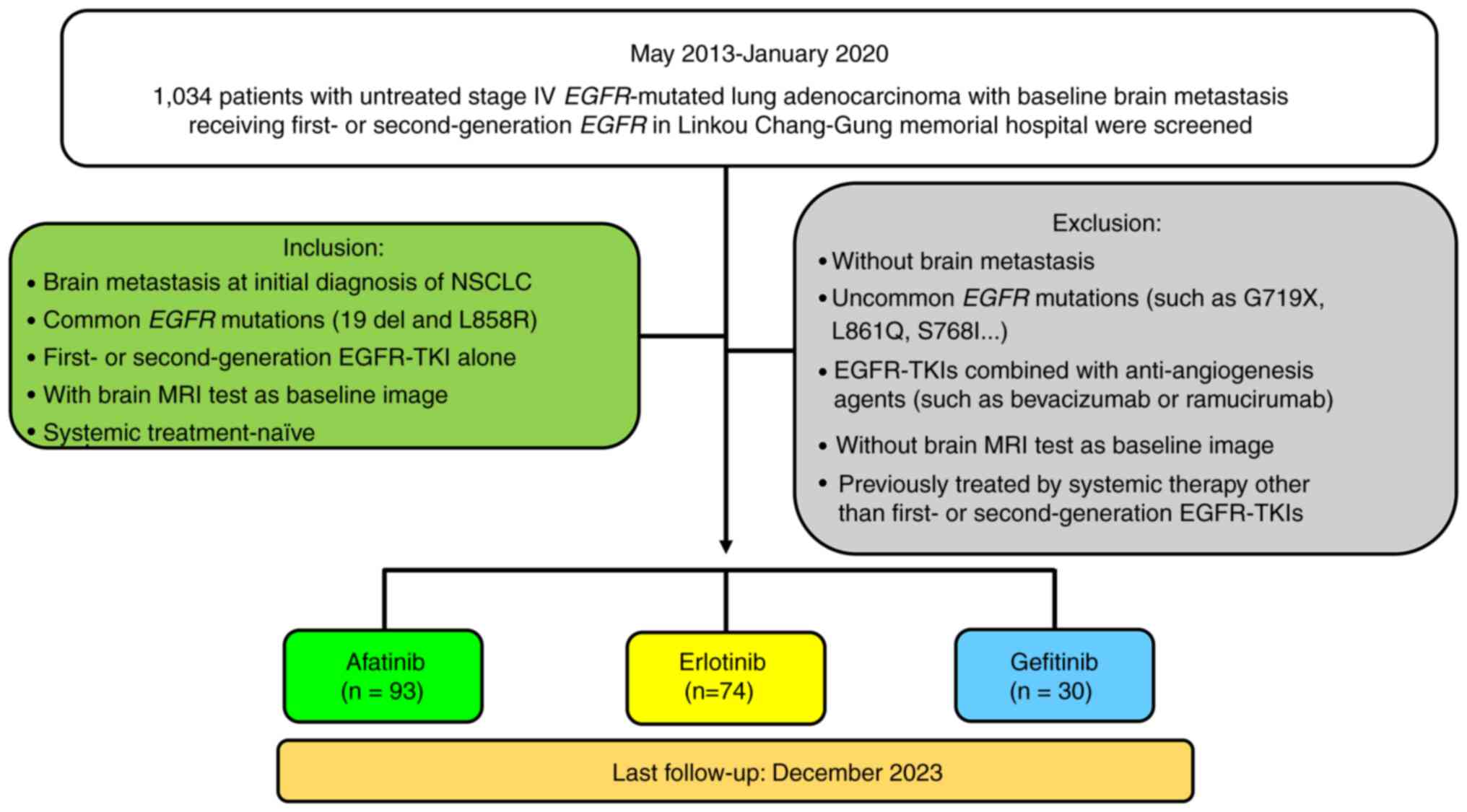
Between May 2013 and January 2020, 1,034 patients with
histologically diagnosed stage IV NSCLC harboring EGFR
mutations who received first-line first- and second-generation
EGFR-TKIs were retrospectively screened. Ultimately, 197 patients
were included in the analysis, and the inclusion criteria for
further analysis were as follows: i) Brain metastasis detected at
the time of the initial diagnosis of NSCLC; ii) common EGFR
mutations (exon 19 deletion and L858R); iii) first- and
second-generation EGFR-TKIs alone used as the first-line therapy
for NSCLC; iv) contrast-enhanced brain magnetic resonance imaging
(MRI) performed at the time of the initial diagnosis of NSCLC that
could be used as a baseline image to assess the size and number of
brain metastases; and v) systemic treatment-naïve status (no
previous systemic treatment such as targeted therapy, chemotherapy
or immunotherapy). Patients meeting the following criteria were
excluded from the analysis in the present study: i) No brain
metastasis at the initial diagnosis of NSCLC; ii) uncommon
EGFR mutations (such as G719X, L861Q and S768I); iii)
treatment with EGFR-TKIs combined with anti-angiogenic agents (such
as bevacizumab or ramucirumab); iv) no contrast-enhanced brain MRI
data available as baseline images; and v) previous treatment with
any systemic therapies, including targeted therapy, chemotherapy or
immunotherapy. The process of selecting study subjects for the
final analysis is summarized in Figure
1.
EGFR mutations of patients [189 patients
(95.9%)] in the present study were mainly detected using an
amplified refractory mutation system-Scorpion assay (ARMS/S). A
number of patients [8 patients (4.1%)] had primary EGFR
mutations detected by direct sequencing, the method of which has
been described previously (16)
(Figs. S1 and S2). The procedures of ARMS/S and direct
sequencing were performed by the Central Molecular Lab of
Department of Pathology (Chang Gung Memorial Hospital), a College
of American Pathologists-accredited laboratory. The DNA used for
EGFR testing were extracted from formalin-fixed paraffin-embedded
tumor tissues or cytology blocks, and DNA extraction were performed
using the DEXPAT kit (Takara Bio, Inc.) following the
manufacturer's instructions. The ABI BigDye Terminator kit version
3.1 (cat. no. 4337458; Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used for EGFR detection via direct
sequencing, and the EGFR Plus RGQ PCR Kit (cat. no. 874601; Qiagen,
Inc.) was used for EGFR detection in ARSMS/S.
The primer sequences used were as follows: Exon 18
forward (F), 5′-TCCAAATGAGCTGGCAAGTG-3′ and reverse (R),
5′-TCCCAAACACTCAGTGAAACAAA-3′; exon 19 F,
5′-TCACAATTGCCAGTTAACGTCT-3′ and R, 5′-CAGCAAAGCAGAAACTCACATC-3′;
exon 20 F, 5′-ACTTCACAGCCCTGCGTAAAC-3′ and R,
5′-ATGGGACAGGCACTGATTTGT-3′; and exon 21 F,
5′-ATGAACTACTTGGAGGACCGTC-3′ and R,
5′-TGCCTCCTTCTGCATGGTATTC-3′.
All patients in the present study underwent brain
MRI to assess the baseline status of brain metastasis, including
the number of metastases, the largest diameter of the metastatic
tumor and the presence or absence of leptomeningeal carcinomatosis
(LMC). All study patients underwent contrast medium-enhanced
computed tomography (CT) and fluorodeoxyglucose (FDG)-positron
emission tomography (PET) to determine the baseline disease status
at diagnosis. Furthermore, they underwent whole-body CT every 3–4
months to evaluate the systemic treatment response to EGFR-TKIs.
Brain MRI was performed on follow-up 3–6 months after treatment in
most study patients [189 patients (95.9%)] to assess the
intracranial treatment response. A small number of study patients
[8 patients (4.1%)] did not receive follow-up brain MRI due to
rapid disease progression and death. Other additional imaging
tests, such as sonograms or FDG-PET, were ordered on the basis of
the needs of clinical physicians to facilitate the evaluation of
disease status.
All the treatment responses were assessed according
to the Response Evaluation Criteria in Solid Tumors (RECIST)
version 3.0 and the responses were classified as complete response
(CR), partial response (PR), stable disease (SD) or progressive
disease (PD). PFS was defined as the period between the date of
first-line EGFR-TKI administration and the first image showing PD.
The duration of brain metastasis-free survival was defined as the
time from the first date of first-line EGFR-TKI treatment to the
first date of progressive brain metastasis revealed by images or
the date of mortality. Overall survival (OS) was measured from the
first date of first-line EGFR-TKI administration to the date of
mortality. If patients survived to the last follow-up time point
(December 2023), PFS, brain metastasis-free survival and OS were
censored at the last date of the clinical visit.
Brain metastasis-related symptoms and first-line
EGFR-TKI treatment-related adverse events (AEs) were retrieved from
electronic chart records and assessed according to the National
Cancer Institute Common Terminology Criteria version 3.0 (17).
Statistical analysis
The baseline demographic characteristics and
treatment modalities of the patients in the present study are
presented as quantitative variables. Cox regression was used to
analyze the predictive clinical variables associated with PFS in
patients receiving first-line EGFR-TKI therapy. Both univariate and
multivariate analyses were performed according to different
clinical variables. For PFS, brain metastasis-free survival and OS
data were analyzed using Kaplan-Meier survival curves. Kaplan-Meier
survival curves with log-rank tests were also used to compare PFS,
brain metastasis-free survival and OS among patients stratified
according to different clinical variables. P<0.05 was considered
to indicate a statistically significant difference. The statistical
analysis in the present study was performed using SPSS Statistics
version 22.0 (IBM Corp.). PFS, brain metastasis-free survival and
OS curves were plotted using GraphPad Prism (version 5.0;
Dotmatics).
Results
Baseline clinical characteristics and
treatment information of all study patients
Among all the patients in the present study, 196
(99.5%) had histologically diagnosed adenocarcinoma and only one
(0.5%) had adenosquamous cell carcinoma. Among the EGFR
mutations, 118 (59.9%) were exon 19 deletion mutations and 79
(40.1%) were L858R mutations. Among the EGFR-TKIs used in
first-line therapy, 30 patients (15.2%) were administered
gefitinib, 74 patients (37.6%) were administered erlotinib and 93
(47.2%) patients were administered afatinib. A total of 17 patients
(8.6%) had LMC at initial diagnosis. Regarding the early
administration of local therapies for brain metastasis
(neurosurgery and radiation therapy within 30 days before or after
initiating first-line EGFR-TKIs), 47 patients (23.9%) had received
neurosurgery and 187 (94.9%) had received radiation therapy to
brain metastases. Radiation therapies and neurosurgery 30 days
after starting first-line EGFR-TKIs were defined as salvage local
therapies. In the present study, 27 patients (13.7%) received
salvage radiation therapies to the brain and 14 (7.1%) received
salvage neurosurgery. Among the 27 patients (13.7%) receiving
salvage radiation therapies, 22 (11.2%) had received early
radiation therapies to the brain and among the 14 patients (7.1%)
receiving salvage neurosurgery, 11 (5.6%) had received early
radiation therapies to the brain.
The main symptoms related to brain metastasis were
recorded in the present study, and the most frequent symptom was
dizziness or vertigo (16.2%), followed by headache (12.2%),
hemiplegia (8.1%), seizure (6.1%), conscious disturbance (3.6%),
gait disturbance (3.0%) and visual disturbance (2.5%).
At the last follow-up in the present study (December
2023), 21 patients were still receiving first-line EGFR-TKI
treatments and 176 patients experienced PD after first-line
EGFR-TKI treatments. Among the 176 patients who experienced PD
after first-line therapy, 103 (52.3%) had undergone tissue
re-biopsies or circulating tumor DNA for secondary T790M mutation
tests. A total of two patients (1%) underwent tissue re-biopsies,
and small cell lung cancer (SCLC) transformation was found using
histology; neither patient underwent EGFR T790M mutation
tests. Among the 103 patients who underwent T790M tests, a total of
66 patients had positive results of EGFR-T790M mutations
(64%). Among the 66 patients (33.5%) with positive T790M mutations,
60 (30.5%) received osimertinib as second-line therapy, 4 patients
(2%) received aumolertinib (HS-10296) in clinical trials and the
remaining 2 (1%) received platinum-based chemotherapy. Among the
patients with negative or unknown T790M mutations, 48 (24.4%)
received platinum-based doublet chemotherapy, 9 (4.6%) received
single agent chemotherapy and 2 (1%) received erlotinib as
second-line systemic therapy. Second-line anti-programmed cell
death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) immune
checkpoint inhibitors (ICIs) were administered to 3 patients
(1.5%), with 2 (1%) receiving ICIs combined with chemotherapy and 1
(0.5%) receiving ICIs alone. A total of 50 patients (25.4%) did not
receive any second-line anticancer agents after first-line EGFR-TKI
PD and received supportive care.
The baseline clinical characteristics and treatment
information of all the study patients are presented in Table I, and the subsequent treatment
modalities after first-line PD treatment are summarized in Table II.
 | Table I.Baseline characteristics and
treatment information for all patients in the present study. |
Table I.
Baseline characteristics and
treatment information for all patients in the present study.
| Characteristic | Patients
(n=197) |
|---|
| Sex |
|
|
Male | 71 (36) |
|
Female | 126 (64) |
| Age, years | 65.3±11.2 |
| ECOG PS |
|
|
0-1 | 156 (79.2) |
| ≥2 | 41 (20.8) |
| Smoking status |
|
|
Non-smoker | 149 (75.6) |
|
Former/current smoker | 48 (24.4) |
| Histology |
|
|
Adenocarcinoma | 196 (99.5) |
|
Adenosquamous | 1 (0.5) |
| EGFR
mutations |
|
| Exon 19
deletion | 118 (59.9) |
|
L858R | 79 (40.1) |
| Concurrent
metastatic sites other than brain metastasis |
|
|
Liver | 36 (18.3) |
|
Bone | 113 (57.1) |
| Number of brain
metastases |
|
| ≤3 | 92 (46.7) |
|
>3 | 105 (53.3) |
| Largest diameter of
brain metastatic tumor, cm |
|
|
<3 | 160 (81.2) |
| ≥3 | 37 (18.8) |
| EGFR-TKI |
|
|
Gefitinib | 30 (15.2) |
|
Erlotinib | 74 (37.6) |
|
Afatinib | 93 (47.2) |
| Early neurosurgery
(within 30 days of initiating first-line EGFR-TKIs) |
|
|
Yes | 47 (23.9) |
| No | 150 (76.1) |
| Early radiation
therapy to brain metastasis (within 30 days of initiating
first-line EGFR-TKIs) |
|
|
Yes | 187 (94.9) |
| No | 10 (5.1) |
| Salvage
neurosurgery (30 days after initiating first-line EGFR-TKIs) | 14 (7.1) |
| Salvage radiation
therapy to brain metastasis (30 days after initiating first-line
EGFR-TKIs) | 27 (13.7) |
| Leptomeningeal
carcinomatosis | 17 (8.6) |
| Neurological
symptoms related to brain metastasis |
|
|
Dizziness or vertigo | 32 (16.2) |
|
Headache | 24 (12.2) |
|
Hemiplegia | 16 (8.1) |
|
Seizure | 12 (6.1) |
|
Conscious disturbance | 7 (3.6) |
| Gait
disturbance | 6 (3.0) |
| Visual
disturbance | 5 (2.5) |
| No
neurological symptoms | 95 (48.2) |
 | Table II.First subsequent treatments after
first-line epidermal growth factor receptor-tyrosine kinase
inhibitors in all patients in the present study. |
Table II.
First subsequent treatments after
first-line epidermal growth factor receptor-tyrosine kinase
inhibitors in all patients in the present study.
| A, Therapies and
tests |
|---|
|
|---|
| Variable | Value |
|---|
| First-line EGFR-TKI
therapy administered | 21 (10.7) |
| Progressive disease
after first-line EGFR-TKI therapy | 176 (89.3) |
| EGFR T790M
mutation tests (by tissue sample re-biopsy or ctDNA analysis) |
|
|
Yes | 103 (52.3) |
| No | 73 (37.1) |
| Small cell
transformation | 2 (1) |
| EGFR T790M
mutation |
|
|
Positive | 66 (33.5) |
|
Negative | 47 (23.9) |
| EGFR T790M
mutation rate | 64 |
|
| B, Treatments
after first-line EGFR-TKIs |
|
|
Variable | Value |
|
| T790M
mutation-positive |
|
|
Third-generation
EGFR-TKIs | 64 (32.5) |
|
Osimertinib | 60 (30.5) |
|
Aumolertinib (HS-10296) | 4 (2) |
|
Platinum-base doublet
chemotherapy | 2 (1) |
| T790M
mutation-negative and unknown |
|
|
Erlotinib | 2 (1.0) |
|
Platinum-base doublet
chemotherapy | 48 (24.4) |
| Single
agent chemotherapy | 9 (4.6) |
|
Anti-PD-1/PD-L1 immune
checkpoint inhibitors | 3 (1.5) |
| Anti-angiogenesis
agent |
|
|
Bevacizumab | 5 (2.5) |
|
Supportive care | 50 (25.4) |
Efficacy of first-line EGFR therapies
in systemic responses and brain metastasis
Among all 197 study patients who received first-line
afatinib treatment, 1 (0.5%) achieved CR, 147 (74.6%) achieved PR,
26 (13.2%) achieved SD and 23 (11.7%) experienced PD. The systemic
ORR and disease control rate (DCR) were 75.1 and 88.3%,
respectively (Table III). In
terms of the intracranial treatment response to first-line EGFR-TKI
therapies, 26 patients (13.2%) achieved CR, 124 (62.9%) achieved
PR, 33 (16.8%) achieved SD, 11 (5.6%) achieved PD and 3 were not
evaluated. The intracranial ORR and DCR were 76.1 and 92.9%,
respectively (Table III).
 | Table III.Systemic and brain metastasis
treatment responses to first-line epidermal growth factor
receptor-tyrosine kinase inhibitors in all patients in the present
study. |
Table III.
Systemic and brain metastasis
treatment responses to first-line epidermal growth factor
receptor-tyrosine kinase inhibitors in all patients in the present
study.
| Variable | Patients
(n=197) |
|---|
| Systemic treatment
response |
|
|
Complete response | 1 (0.5) |
| Partial
response | 147 (74.6) |
| Stable
disease | 26 (13.2) |
|
Progressive disease | 23 (11.7) |
|
Objective response rate | 75.1 |
| Disease
control rate | 88.3 |
| Intracranial
treatment response |
|
|
Complete response | 26 (13.2) |
| Partial
response | 124 (62.9) |
| Stable
disease | 33 (16.8) |
|
Progressive disease | 11 (5.6) |
| Not
evaluated | 3 (1.5) |
|
Objective response rate | 76.1 |
| Disease
control rate | 92.9 |
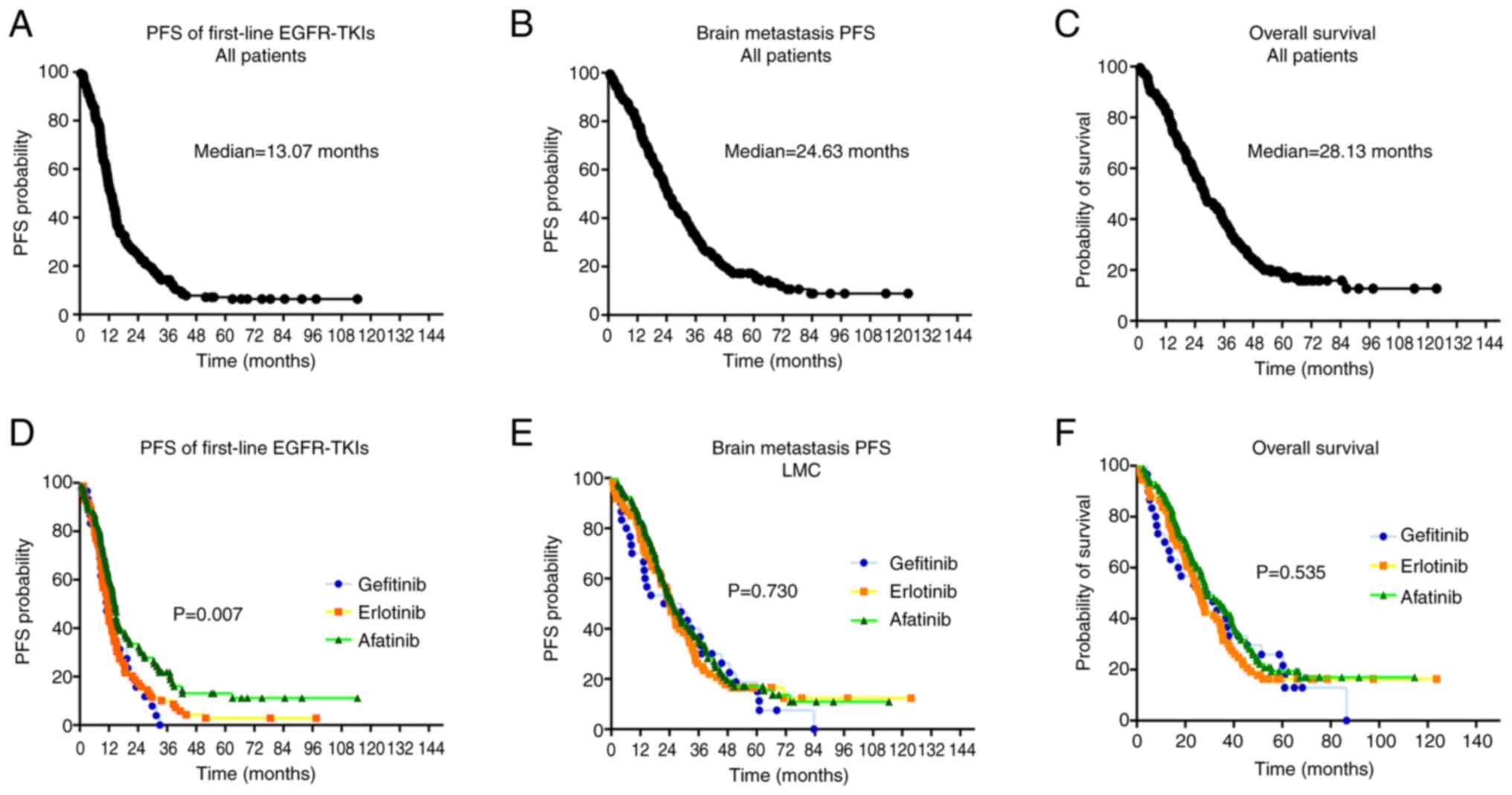
For the study patients overall, the median PFS of
first-line EGFR-TKI treatments was 13.07 months [95% confidence
interval (CI), 11.43–14.70; Fig.
2A], the median brain metastasis PFS (BMPFS) was 24.63 months
(95% CI, 20.98–28.28; Fig. 2B) and
the median OS was 28.13 months (95% CI, 23.53–32.74; Fig. 2C). The median PFS, BMPFS and OS
among patients receiving different first-line EGFR-TKIs (gefitinib,
erlotinib and afatinib) were analyzed. The median PFS times were
11.13 months (95% CI, 7.51–14.76), 11.53 months (95% CI,
10.06–13.01) and 14.63 months (95% CI, 13.19–16.07) for patients
receiving first-line gefitinib, erlotinib and afatinib,
respectively (log-rank test, P=0.007; Fig. 2D). The median BMPFS rates were 21.77
months (95% CI, 0.03–44.18), 24.40 months (95% CI, 20.76–28.38) and
25.83 months (95% CI, 20.41–31.26) for patients receiving
first-line gefitinib, erlotinib and afatinib, respectively
(log-rank test, P=0.730; Fig. 2E).
The median OS rates were 28.80 months (95% CI, 9.03–48.57), 26.23
months (95% CI, 21.76–30.71) and 29.10 months (95% CI, 22.14–36.06)
for patients receiving first-line gefitinib, erlotinib and
afatinib, respectively (log-rank test, P=0.535; Fig. 2F).
The results indicate that first- and
second-generation EGFR-TKIs were effective as first-line therapies
in patients with EGFR-mutated NSCLC with baseline brain
metastasis and that treatment with afatinib yielded significantly
longer PFS compared with gefitinib and erlotinib.
Cox regression analysis of the
clinical predictive factors associated with PFS after first-line
EGFR-TKI treatment
The median PFS of patients receiving first-line
EGFR-TKI treatment according to different clinical variables was
analyzed using Cox regression, and the results are presented in
Table IV. According to the
univariate analysis, the clinical factors of the absence of
baseline bone metastasis, fewer brain metastases (≤3), first-line
afatinib use, early neurosurgery, early radiation therapy to the
brain and the absence of baseline LMC were significantly associated
with longer PFS. No significant difference in PFS was recorded
among patients with and without salvage local therapies (salvage
radiation therapies and neurosurgery). Using multivariate analysis,
fewer brain metastases (≤3) and the absence of a baseline LMC were
revealed to be independent predictors of longer PFS. In the present
analysis, increased metastatic number (>3) and the presence of
LMC were demonstrated to be independent unfavorable clinical
factors associated with PFS in patients with EGFR-mutated
NSCLC with baseline brain metastasis who received first- and
second-generation EGFR-TKIs.
 | Table IV.Cox regression analysis of clinical
factors associated with progression-free survival in patients
receiving first-line epidermal growth factor receptor-tyrosine
kinase inhibitors. |
Table IV.
Cox regression analysis of clinical
factors associated with progression-free survival in patients
receiving first-line epidermal growth factor receptor-tyrosine
kinase inhibitors.
|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variable | n | Median PFS,
months | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age |
|
| 0.952
(0.694–1.307) | 0.762 | - | - |
| ≤60
years | 62 | 12.23 |
|
|
|
|
| >60
years | 135 | 13.27 |
|
|
|
|
| Sex |
|
| 1.056
(0.775–1.440) | 0.729 | - | - |
|
Male | 71 | 11.37 |
|
|
|
|
|
Female | 126 | 13.43 |
|
|
|
|
| ECOG PS |
|
| 1.215
(0.848–1.740) | 0.290 | - | - |
|
0-1 | 156 | 13.07 |
|
|
|
|
| ≥2 | 41 | 13.27 |
|
|
|
|
| Smoking status |
|
| 0.987
(0.695–1.401) | 0.940 | - | - |
|
Non-smoker | 149 | 13.43 |
|
|
|
|
|
Former/current smoker | 48 | 11.83 |
|
|
|
|
| EGFR
mutations |
|
| 0.792
(0.584–1.075) | 0.135 | - | - |
|
L858R | 79 | 11.63 |
|
|
|
|
| Exon 19
deletion | 118 | 14.63 |
|
|
|
|
| Liver
metastases |
|
| 1.356
(0.931–1.976) | 0.111 | - | - |
|
Yes | 36 | 11.53 |
|
|
|
|
| No | 161 | 13.40 |
|
|
|
|
| Bone
metastases |
|
| 1.504
(1.110–2.038) | 0.008 | - | - |
|
Yes | 113 | 11.63 |
|
|
|
|
| No | 84 | 14.97 |
|
|
|
|
| Number of brain
metastases |
|
| 2.099
(1.537–2.865) | <0.001 | 1.782
(1.537–2.488) | 0.001 |
| ≤3 | 92 | 15.67 |
|
|
|
|
|
>3 | 105 | 10.97 |
|
|
|
|
| Largest diameter of
brain metastatic tumor |
|
| 0.938
(0.644–1.365) | 0.738 | - | - |
| <3
cm | 160 | 12.13 |
|
|
|
|
| ≥3
cm | 37 | 14.97 |
|
|
|
|
| EGFR-TKIs |
|
| 0.754
(0.615–0.923) | 0.006 | - | - |
|
Gefitinib | 30 | 11.13 |
|
|
|
|
|
Erlotinib | 74 | 11.53 |
|
|
|
|
| Afatinib | 93 | 14.63 |
|
|
|
|
| Early
neurosurgery |
|
| 0.647
(0.452–0.926) | 0.016 | - | - |
| No | 150 | 12.07 |
|
|
|
|
|
Yes | 47 | 16.50 |
|
|
|
|
| Early radiation
therapy |
|
| 0.527
(0.277–0.999) | 0.050 | - | - |
| No | 10 | 8.63 |
|
|
|
|
|
Yes | 187 | 13.27 |
|
|
|
|
| Salvage
neurosurgery |
|
| 1.003
(0.570–1.767) | 0.991 | - | - |
| No | 183 | 12.57 |
|
|
|
|
|
Yes | 14 | 14.63 |
|
|
|
|
| Salvage radiation
therapy |
|
| 0.972
(0.636–1.485) | 0.894 | - | - |
| No | 170 | 13.07 |
|
|
|
|
|
Yes | 27 | 13.07 |
|
|
|
|
| Leptomeningeal
carcinomatosis |
|
| 3.105
(1.857–5.193) | <0.001 | 2.451
(1.433–4.184) | 0.001 |
| No | 180 | 8.20 |
|
|
|
|
|
Yes | 17 | 13.43 |
|
|
|
|
Comparisons of PFS, BMPFS and OS based
on the number of brain metastases and LMC status
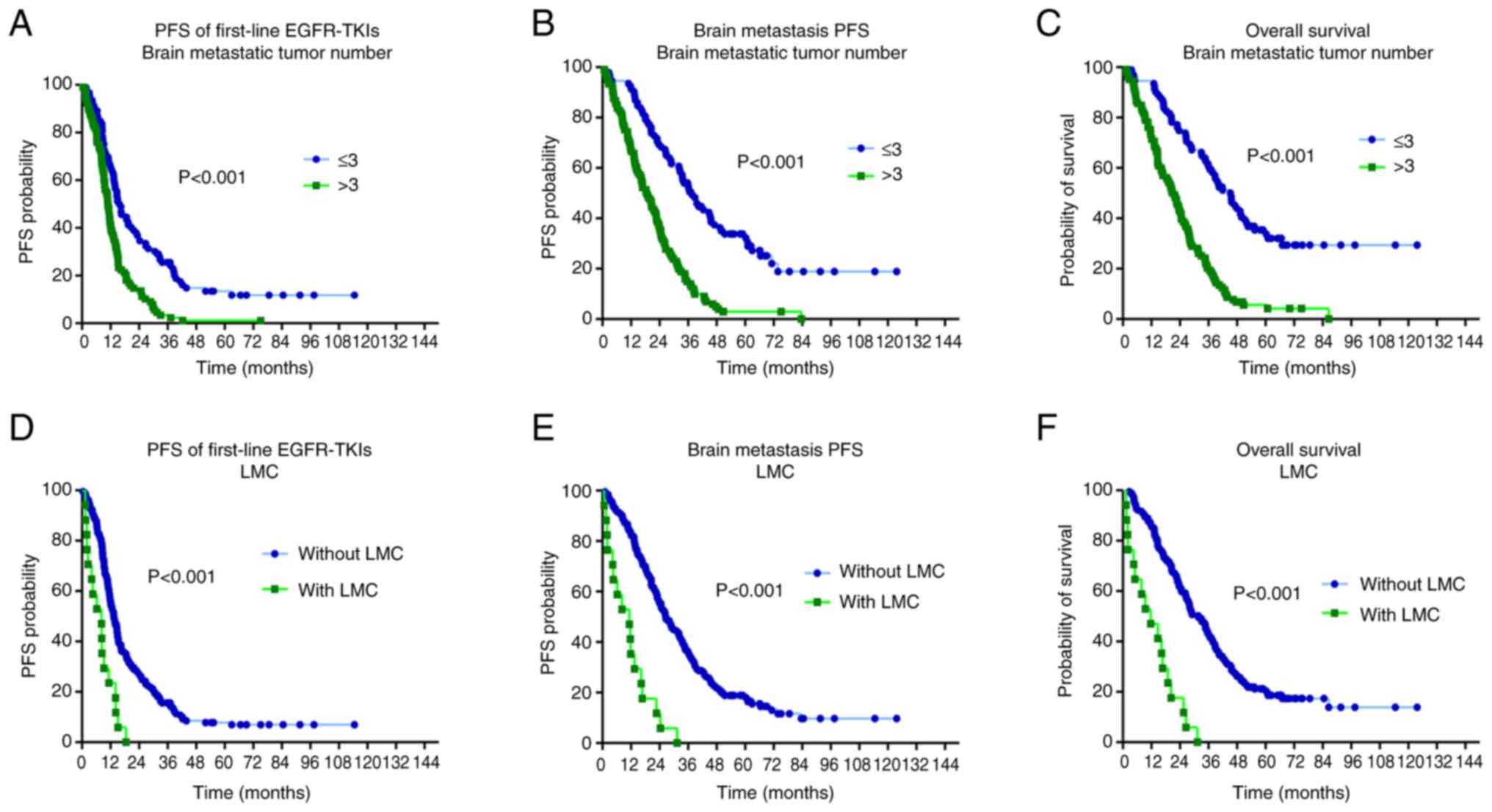
Patients were categorized according to the number of
brain metastatic tumors (≤3 and >3) and those with or without
baseline data to compare PFS, BMPFS and OS. Patients with fewer
baseline brain metastatic tumors (≤3) had significantly longer PFS
[15.67 vs. 10.97 months; hazard ratio (HR)=0.465; CI, 0.339–0.637;
P<0.001; Fig. 3A], BMPFS (37.73
vs. 18.90 months; HR=0.325; CI, 0.235–0.451; P<0.001; Fig. 3B) and OS (45.50 vs. 21.70 months;
HR=0.309; CI, 0.222–0.431; P<0.001; Fig. 3C) compared with those with more
brain metastatic tumors (>3). After first-line EGFR-TKI
treatment, patients without LMC had significantly longer PFS (13.43
vs. 8.20 months; HR=0.149; CI, 0.066–0.339, P<0.001; Fig. 3D), BMPFS survival (26.63 vs. 11.67
months; HR=0.047; CI, 0.018–0.124; P<0.001; Fig. 3E) and OS (31.80 vs. 11.30 months;
HR=0.046; CI, 0.017–0.122, P<0.001; Fig. 3F) compared with those with LMC. The
results of the present study demonstrate that patients with an
increased number of metastases (>3) or LMC have significantly
worse clinical outcomes compared with those with fewer metastases
(≤3) or without LMC in terms of PFS, BMPFS and OS. A summary of the
aforementioned results is presented in Fig. 4.
First-line first- and
second-generation EGFR-TKI-related AEs
The AEs associated with first-line EGFR-TKIs are
summarized in Table V. Among the
197 patients in the present study, skin toxicities such as rash and
acne were the most common AEs (87.8%), followed by paronychia
(61.9%), diarrhea (57.4%), stomatitis (45.2%), anorexia (34.0%) and
nausea and vomiting (16.2%). Grade 3 AEs included skin toxicity
(8.6%), diarrhea (5.1%), paronychia (1.5%) and stomatitis (1.1%).
All grade 3 AEs in the present study were controlled by reducing
the dose of EGFR-TKIs or temporally interrupting EGFR-TKI therapies
and administering medicines for symptomatic relief (such as topical
agents for skin toxicity and anti-diarrheal agents for diarrhea).
No permanent discontinuation of EGFR-TKI due to severe AEs occurred
in the present study. No cases of EGFR-TKI treatment-related
mortality were recorded in the present study.
 | Table V.Treatment-related adverse events
associated with first- and second-generation epidermal growth
factor receptor-tyrosine kinase inhibitors. |
Table V.
Treatment-related adverse events
associated with first- and second-generation epidermal growth
factor receptor-tyrosine kinase inhibitors.
|
|
| Grade |
|---|
|
|
|
|
|---|
| Adverse event | All (n=197) | 1-2 | 3 | 4 |
|---|
| Skin rash/acne | 173 (87.8) | 156 (79.2) | 17 (8.6) | 0 (0.0) |
| Paronychia | 122 (61.9) | 119 (60.4) | 3 (1.5) | 0 (0.0) |
| Diarrhea | 113 (57.4) | 103 (52.3) | 10 (5.1) | 0 (0.0) |
| Stomatitis | 89 (45.2) | 87 (44.2) | 2 (1.1) | 0 (0.0) |
| Anorexia (decreased
appetite) | 67 (34.0) | 67 (34.0) | 0 (0.0) | 0 (0.0) |
| Nausea or
vomiting | 32 (16.2) | 32 (16.2) | 0 (0.0) | 0 (0.0) |
Overall, the safety of the first- and
second-generation EGFR-TKIs in patients with EGFR-mutated
NSCLC with brain metastasis is acceptable, and the AEs are
manageable.
Discussion
The results of the present study demonstrate that
first- and second-generation EGFR-TKIs are effective and safe
first-line treatments for patients with EGFR-mutated NSCLC
with baseline brain metastasis. The PFS of patients treated with
the second-generation EGFR-TKI afatinib was significantly longer
compared with patients treated with the first-generation EGFR-TKIs
gefitinib and erlotinib, and afatinib was equally effective at
controlling brain metastasis, as indicated by brain metastasis-free
survival. A greater number of brain metastatic tumors (>3) and
the presence of LMCs were revealed to be independent factors
associated with shorter PFS after first-line EGFR-TKI treatment. In
addition, at baseline, a greater number of brain metastatic tumors
(>3) or the presence of LMC negatively affected brain
metastasis-free survival and OS. The frequent AEs including skin
toxicities, paronychia, diarrhea, stomatitis and gastrointestinal
upset associated with first- and second-generation EGFR-TKIs in the
present study are in line with previous studies, and the AEs in the
present study were manageable (6–10). No
AE-related permanent discontinuation or treatment-related mortality
occurred in the present study.
Previous clinical studies have reported that first-
and second-generation EGFR-TKIs, including gefitinib, erlotinib and
afatinib, have improved effects on EGFR-mutated NSCLC brain
metastases than conventional chemotherapy (18). In most previous clinical trials
assessing EGFR-TKIs in patients with advanced EGFR-mutated
NSCLC, patients with baseline brain metastasis were required to be
asymptomatic or treated and stable before they entered the clinical
trial (18). In real-world clinical
practice, certain patients with EGFR-mutated NSCLC with
baseline brain metastasis have neurological symptoms and have to
receive additional radiation therapy or neurosurgery before or
during EGFR-TKI treatment (12,18).
An analysis of two previous retrospective studies suggested that
brain metastasis resection surgery provided a survival benefit to
patients with EGFR-mutated NSCLC receiving EGFR-TKI
treatments (12,19). Our previous study revealed that
neurosurgery had a median of 2 years of BMPFS for patients with
brain metastasis with NSCLC who were candidates for surgery
(12). Radiation therapies such as
whole-brain radiotherapy and stereotactic body radiation therapy
are important therapeutic modalities that are frequently
administered to patients with NSCLC with brain metastasis. Previous
studies have reported that brain radiation therapy, in addition to
neurosurgery and targeted therapies, reduces the recurrence rate of
brain metastasis and may improve OS in patients with brain
metastatic NSCLC (19–22). Given local therapies (radiation
therapies and neurosurgery) in addition to EGFR-TKIs benefit brain
metastasis control (19–22), the majority of patients (94.9%) in
the present study had received early brain radiation therapies
(within 30 days of initiating first-line EGFR-TKI treatments).
Certain patients (23.9%) had received early neurosurgery (within 30
days of initiating first-line EGFR-TKI treatments). The median
BMPFS of 24.63 months was also comparable to the results reported
in previous clinical studies (12,19–22).
Osimertinib is a third-generation EGFR-TKI that has
been reported to have improved BBB permeability than first- and
second-generation EGFR-TKIs in a previous preclinical study
(23). In a pivotal clinical trial
(FLAURA), osimertinib was reported to be associated with
significantly longer PFS compared with gefitinib or erlotinib in
untreated patients with EGFR-mutated NSCLC with central
nervous system (CNS) metastasis (15.2 vs. 9.6 months) (24). The results of the FLAURA trial
indicate that osimertinib may have improved efficacy against CNS
metastasis compared with first-generation EGFR-TKIs (24). According to the protocol of the
FLAURA trial, patients with CNS metastasis who were neurologically
stable were eligible. In addition, any local treatment for brain
metastasis or systemic steroid therapy was required to be completed
>2 weeks before the initiation of trial treatment. Therefore,
patients eligible for the FLAURA trial were relatively more
neurologically stable than those in real-world practice (24). Moreover, a previous retrospective
study by Huang et al (25)
reported that osimertinib was not significantly superior to
afatinib in terms of PFS from first-line therapy in patients with
metastatic EGFR-mutated NSCLC. In the same study, patients
with baseline brain metastasis treated with osimertinib had
markedly longer PFS than patients treated with afatinib (25). The results of the study by Huang
et al (25) suggest that
osimertinib could be superior to afatinib in untreated patients
with EGFR-mutated NSCLC with baseline brain metastasis.
Given the concern about the cost-effectiveness of first- to
third-generation EGFR-TKIs, osimertinib is not always affordable
for patients or covered by a national insurance policy, although
osimertinib has preferred efficacy and toxicity in the treatment of
patients with advanced EGFR-mutated NSCLC (26,27).
To the best of our knowledge, the present study is
the first to directly compare first- and second-generation
EGFR-TKIs in untreated patients with EGFR-mutated NSCLC with
baseline brain metastasis. The results revealed that afatinib was
associated with a significantly longer median PFS than
first-generation EGFR-TKIs (14.63 vs. 11.53 and 11.13 months). The
assessment of the efficacy of EGFR-TKIs using PFS indicates
systemic disease control, not only in the CNS. The results further
demonstrated BMPFS and suggested that gefitinib, erlotinib and
afatinib may have equal efficacy in controlling brain metastasis.
The PFS of patients receiving first-line afatinib therapy was
longer than that reported in a previous study by Huang et al
(25) (10.9 months), in which 28
patients with baseline brain metastasis received afatinib, which
was less than the number of patients included in the present study
(n=93). In addition, information on local therapies such as
radiation therapy or neurosurgery was not provided in the same
study; therefore, these factors may have contributed to the
differences between the two studies (25).
A greater number of intracranial metastatic tumors
(>3) and the presence of LMC were identified as predictive
factors associated with poor prognosis in the present study.
Patients with these two factors were also demonstrated to have
significantly shorter BMPFS and OS times. According to the analysis
of two previous studies, a greater number of intracranial
metastatic tumors was reported to be associated with unfavorable
outcomes in patients with NSCLC with brain metastasis (28,29).
Leptomeningeal metastasis has been reported to be a severe
complication in patients with advanced NSCLC in previous studies,
and the appearance of LMC is associated with extremely poor
prognosis (30,31). The life expectancy of patients with
untreated LMC can be as short as 4–6 weeks (30,31).
Although a greater number of intracranial metastatic tumors (>3)
and the presence of LMC had been previously reported as clinical
factors associated with poor clinical outcomes, the patients with
NSCLC in the previous studies were non-selective and received
heterogenous systemic treatment agents (28–31).
In comparison with these previous studies, the patients included in
the present study were specifically those with common EGFR
mutations and baseline brain metastasis receiving first-line first-
and second-EGFR-TKIs.
More therapeutic strategies for patients with NSCLC
with unfavorable factors, such as more intracranial metastatic
tumors or LMC, need to be developed and explored. For example, the
anti-angiogenic agent bevacizumab in combination with EGFR-TKIs has
been reported to increase the efficacy of controlling intracranial
disease progression in previous studies (32,33).
In patients with NSCLC with LMC, a recent study reported that the
administration of the chemotherapy regimen pemetrexed intrathecally
improved OS (31). In addition,
third-generation osimertinib may be a preferred EGFR-TKI due to its
improved efficacy regarding CNS penetration compared with first-
and second-generation EGFR-TKIs in those with a greater number of
intracranial metastatic tumors (>3) or the presence of LMC.
Taken together, for patients with EGFR-mutated NSCLC with
brain metastasis, the addition of bevacizumab and intrathecal
pemetrexed to the regimen should be considered, and the choice of
osimertinib can be considered for those with unfavorable
outcomes.
Certain limitations of the present study should be
mentioned and clarified. Firstly, the common EGFR mutations
in the present study were exon 19 deletion and L858R, but no
patients with major uncommon EGFR mutations, including
G719X, S768I or L861Q, were included. The results of previous
studies have demonstrated that second- and third-generation
EGFR-TKIs are more effective than first-generation EGFR-TKIs in
patients with advanced NSCLC harboring major uncommon EGFR
mutations (G719X, S768I and L861Q) (4,34).
Regarding the unequal efficacy of first- and second-generation
EGFR-TKIs, patients with uncommon EGFR mutations were
excluded from the present study. Secondly, the impact of the de
novo T790M mutation on patients with NSCLC with baseline brain
metastasis is unknown according to the results of the present study
as no patients with this mutation were included. In addition, the
efficacy of first-line osimertinib therapy was not assessed.
Osimertinib has been conditionally reimbursed by the Taiwan Health
Insurance Bureau since April 2020, and the timepoint of osimertinib
reimbursement was later than the timepoint of inclusion in this
study (4,35). Very few patients with advanced
EGFR-mutated NSCLC in Taiwan received osimertinib as
first-line therapy before national reimbursement. This explains why
those receiving first-line osimertinib were not included for
analysis and comparison in the present study. Although osimertinib
has been suggested as a preferred first-line therapy for advanced
EGFR-mutated NSCLC, certain patients may experience
intolerable toxicities induced by osimertinib which may require
permanent discontinuation of osimertinib (36,37).
In the analysis of previous studies, patients with
EGFR-mutated NSCLC with baseline brain metastasis were
reported to have notably shorter PFS of EGFR-TKI treatments
compared with those without brain metastasis (38,39).
However, the previous studies did not report data on performing
radiation therapies or neurosurgery in those patients with baseline
brain metastasis (37,38). A previous study performed by Gu
et al (40) reported that
upfront brain radiation therapy in addition to first-line EGFR-TKIs
improved intracranial disease control with a median BMPFS of 28.9
months. However, the systemic PFS of first-line EGFR-TKI treatments
combined with upfront brain radiation therapy were not reported in
the same previous study (39). The
results of the present study are the first to demonstrate both
systemic PFS and BMPFS in patients with EGFR-mutated NSCLC
with baseline brain metastasis receiving first- and
second-generation EGFR-TKIs, to the best of our knowledge. The
median PFS of first-line EGFR-TKIs in the present study was 13.07
months with most patients receiving early brain radiation therapy,
and the PFS of first-line EGFR-TKIs is in line with that reported
by previous studies (6–10). Certain patients in the present study
received salvage brain radiation therapy or neurosurgery, but no
significant difference in PFS of first-line EGFR-TKIs was recorded
between patients with and without salvage local therapies to brain
metastasis. The analysis suggests that early brain radiation
therapy may benefit the clinical outcomes of patients with
EGFR-mutated NSCLC with baseline brain metastasis receiving
first- and second-generation EGFR-TKIs. The results also indicate
that for patients with EGFR-mutated NSCLC with baseline
brain metastasis who are intolerant of osimertinib-related
toxicities, first- and second-generation EGFR-TKIs combined with
early local therapies are feasible therapeutic choices.
In conclusion, first- and second-generation
EGFR-TKIs are effective and safe for treating untreated patients
with EGFR-mutated NSCLC with baseline brain metastasis and
remain feasible therapies if osimertinib is not available or if it
causes intolerable toxicity. For patients whose unfavorable
factors, such as a greater number of brain metastases and LMCs, are
associated with worse clinical outcomes, combination therapeutic
strategies and procedures should be considered.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study received funding support from the Taiwan
Ministry of Science and Technology (grant no. 112-2314-B-182-067)
and the Chang Gung Medical Research Project (grant no.
CMRPG3N1331).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CCH and PCH wrote and revised this manuscript. CTY,
CCH, LCC and PCH were responsible for study conception and design.
CCH, LCC, HWK, SCHK, JSJ and ACCH collected the data. HWK, CEW,
SCHK, CTY and PCH provided the study materials and patients. CCH,
LCC, CEW and CCW analyzed and interpreted the data. CCW and CTY
confirm the authenticity of all the raw data. PCH, LCC and HWK
supervised the study. All authors read and approved the final
version of the manuscript.
Ethical approval and consent to
participate
The present retrospective study was approved by the
Ethics Committee of Chang Gung Medical Foundation (approval no.
201901341A3). The study utilized the Chang Gung Research Database,
and the Chang Gung Medical Foundation Ethics Committee granted a
waiver of informed consent due to its retrospective nature. No
identifiable data of the study patients, such as dates of birth or
personal IDs, are presented in the present manuscript. The present
study was performed in accordance with the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu SV, Elkins IB, Feldman J and Goldberg
SB: EGFR mutations are not all the same: The importance of
biomarker testing in non-small cell lung cancer (NSCLC)-A podcast
discussion between patients and oncologists. Oncol Ther.
11:419–431. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li K, Yang M, Liang N and Li S:
Determining EGFR-TKI sensitivity of G719X and other uncommon EGFR
mutations in non-small cell lung cancer: Perplexity and solution
(Review). Oncol Rep. 37:1347–1358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsu PC, Lee SH, Chiu LC, Lee CS, Wu CE,
Kuo SC, Ju JS, Huang AC, Li SH, Ko HW, et al: Afatinib in untreated
stage IIIB/IV lung adenocarcinoma with major uncommon epidermal
growth factor receptor (EGFR) mutations (G719X/L861Q/S768I): A
multicenter observational study in Taiwan. Target Oncol.
18:195–207. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valley CC, Arndt-Jovin DJ, Karedla N,
Steinkamp MP, Chizhik AI, Hlavacek WS, Wilson BS, Lidke KA and
Lidke DS: Enhanced dimerization drives ligand-independent activity
of mutant epidermal growth factor receptor in lung cancer. Mol Biol
Cell. 26:4087–4099. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsu PC, Chang JW, Chang CF, Huang CY, Yang
CT, Kuo CS, Fang YF and Wu CE: Sequential treatment in advanced
non-small cell lung cancer harboring EGFR mutations. Ther Adv
Respir Dis. 16:175346662211327312022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin CY, Chou YT, Su PL, Lin CC, Chang JW,
Huang CY, Fang YF, Chang CF, Kuo CS, Hsu PC, et al: Generation and
validation of a predictive model for estimating survival among
patients with EGFR-mutant non-small cell lung cancer. Am J Cancer
Res. 13:4208–4221. 2023.PubMed/NCBI
|
|
8
|
Morimoto K, Yamada T, Takeda T, Shiotsu S,
Date K, Tamiya N, Goto Y, Kanda H, Chihara Y, Kunimatsu Y, et al:
Clinical efficacy and safety of first- or second-generation
EGFR-TKIs after osimertinib resistance for EGFR mutated lung
cancer: A prospective exploratory study. Target Oncol. 18:657–665.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang AC, Huang CH, Ju JS, Chiu TH, Tung
PH, Wang CC, Liu CY, Chung FT, Fang YF, Guo YK, et al: First- or
second-generation epidermal growth factor receptor tyrosine kinase
inhibitors in a large, real-world cohort of patients with non-small
cell lung cancer. Ther Adv Med Oncol. 13:175883592110357102021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marin-Acevedo JA, Pellini B, Kimbrough EO,
Hicks JK and Chiappori A: Treatment strategies for non-small cell
lung cancer with common EGFR mutations: A review of the history of
EGFR TKIs approval and emerging data. Cancers (Basel). 15:6292023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu PC, Miao J, Huang Z, Yang YL, Xu Z,
You J, Dai Y, Yeh CC, Chan G, Liu S, et al: Inhibition of
yes-associated protein suppresses brain metastasis of human lung
adenocarcinoma in a murine model. J Cell Mol Med. 22:3073–3085.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu PC, Chiu LC, Chen KT, Wang CC, Wu CT,
Wu CE, Ko HW, Kuo SC, Lin YC, Wang CC and Yang CT: Clinical outcome
analysis of non-small cell lung cancer patients with brain
metastasis receiving metastatic brain tumor resection surgery: A
multicenter observational study. Am J Cancer Res. 13:3607–3617.
2023.PubMed/NCBI
|
|
13
|
Shin DY, Na II, Kim CH, Park S, Baek H and
Yang SH: EGFR mutation and brain metastasis in pulmonary
adenocarcinomas. J Thorac Oncol. 9:195–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park SJ, Kim HT, Lee DH, Kim KP, Kim SW,
Suh C and Lee JS: Efficacy of epidermal growth factor receptor
tyrosine kinase inhibitors for brain metastasis in non-small cell
lung cancer patients harboring either exon 19 or 21 mutation. Lung
Cancer. 77:556–560. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li SH, Liu CY, Hsu PC, Fang YF, Wang CC,
Kao KC, Tseng LC and Yang CT: Response to afatinib in
treatment-naïve patients with advanced mutant epidermal growth
factor receptor lung adenocarcinoma with brain metastases. Expert
Rev Anticancer Ther. 18:81–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang SF, Liu HP, Li LH, Ku YC, Fu YN,
Tsai HY, Chen YT, Lin YF, Chang WC, Kuo HP, et al: High frequency
of epidermal growth factor receptor mutations with complex patterns
in non-small cell lung cancers related to gefitinib responsiveness
in Taiwan. Clin Cancer Res. 10:8195–8203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Cancer Institute Common
Terminology, . Criteria for adverse events (version 3.0).
|
|
18
|
Baik CS, Chamberlain MC and Chow LQL:
Targeted therapy for brain metastases in EGFR-mutated and
ALK-rearranged non-small-cell lung cancer. J Thorac Oncol.
10:1268–1278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng Y, Su X, Zhao Y, Zhou Y, Guo T, Chu
X, Chu L, Yang X, Ni J and Zhu Z: Rationale and value of
consolidative cranial local therapy in EGFR-mutant non-small cell
lung cancer patients with baseline brain metastasis treated with
first-line EGFR-TKIs. Ther Adv Med Oncol. 15:175883592311699752023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mahajan A, Ahmed S, McAleer MF, Weinberg
JS, Li J, Brown P, Settle S, Prabhu SS, Lang FF, Levine N, et al:
Post-operative stereotactic radiosurgery versus observation for
completely resected brain metastases: A single-centre, randomised,
controlled, phase 3 trial. Lancet Oncol. 18:1040–1048. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsui DCC, Camidge DR and Rusthoven CG:
Managing central nervous system spread of lung cancer: The state of
the art. J Clin Oncol. 40:642–660. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Linh DM, Thinh TH, Hieu NV and Duc NM:
Treatment outcomes of EGFR-TKI with or without locoregional brain
therapy in advanced EGFR-mutant non-small cell lung cancer patients
with brain metastases. Contemp Oncol (Pozn). 27:71–79.
2023.PubMed/NCBI
|
|
23
|
Colclough N, Chen K, Johnström P,
Strittmatter N, Yan Y, Wrigley GL, Schou M, Goodwin R, Varnäs K,
Adua SJ, et al: Preclinical comparison of the blood-brain barrier
permeability of osimertinib with other EGFR TKIs. Clin Cancer Res.
27:189–201. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang YH, Hsu KH, Tseng JS, Yang TY, Chen
KC, Su KY, Yu SL, Chen JJW and Chang GC: The difference in clinical
outcomes between osimertinib and afatinib for first-line treatment
in patients with advanced and recurrent EGFR-mutant non-small cell
lung cancer in Taiwan. Target Oncol. 17:295–306. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holleman MS, Al MJ, Zaim R, Groen HJM and
Uyl-de Groot CA: Cost-effectiveness analysis of the first-line
EGFR-TKIs in patients with non-small cell lung cancer harbouring
EGFR mutations. Eur J Health Econ. 21:153–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan H, Wang C, Chen C, Han S and Zhao Z:
Cost-effectiveness of 12 first-line treatments for patients with
advanced EGFR mutated NSCLC in the United Kingdom and China. Front
Oncol. 12:8196742022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shah PP, Franke JL, Medikonda R, Jackson
CM, Srivastava S, Choi J, Forde PM, Brahmer JR, Ettinger DS,
Feliciano JL, et al: Mutation status and postresection survival of
patients with non-small cell lung cancer brain metastasis:
Implications of biomarker-driven therapy. J Neurosurg. 136:56–66.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perng PS, Hsu HP, Lee PH, Huang CC, Lin CC
and Lee JS: Correlation of EGFR mutation subtypes and survival in
surgically treated brain metastasis from non-small-cell lung
cancer. Asian J Surg. 46:269–276. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng H and Perez-Soler R: Leptomeningeal
metastases in non-small-cell lung cancer. Lancet Oncol. 19:e43–e55.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hong Y, Miao Q, Zheng X, Xu Y, Huang Y,
Chen S, Huang Z, Xu H, Jiang K, Zhong Q, et al: Effects of
intrathecal pemetrexed on the survival of patients with
leptomeningeal metastasis from lung adenocarcinoma: A propensity
score matching analysis. J Neurooncol. 165:301–312. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng PH, Chen KY, Huang YC, Luo CS, Wu SM,
Chen TT, Lee CN, Yeh CT, Chuang HC, Han CL, et al: Bevacizumab
reduces S100A9-positive MDSCs linked to intracranial control in
patients with EGFR-mutant lung adenocarcinoma. J Thorac Oncol.
13:958–9567. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee SH, Lin YC, Chiu LC, Ju JS, Tung PH,
Huang AC, Li SH, Fang YF, Chen CH, Kuo SC, et al: Comparison of
afatinib and erlotinib combined with bevacizumab in untreated stage
IIIB/IV epidermal growth factor receptor-mutated lung
adenocarcinoma patients: A multicenter clinical analysis study.
Ther Adv Med Oncol. 14:175883592211132782022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bar J, Peled N, Schokrpur S, Wolner M,
Rotem O, Girard N, Aboubakar Nana F, Derijcke S, Kian W, Patel S,
et al: UNcommon EGFR Mutations: International Case Series on
Efficacy of Osimertinib in Real-Life Practice in First-LiNe Setting
(UNICORN). J Thorac Oncol. 18:169–180. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hsu PC, Chang JW, Chiu LC, Yang CT, Kuo
SC, Fang YF and Wu CE: Analysis of genomic alternations in
epidermal growth factor receptor (EGFR)-T790M-mutated non-small
cell lung cancer (NSCLC) patients with acquired resistance to
osimertinib therapy. Clin Transl Oncol. Sep 24;doi:
10.1007/s12094-024-03727-7 (Epub ahead of print).
|
|
36
|
Shalata W, Abu Jama A, Dudnik Y, Abu Saleh
O, Shalata S, Tourkey L, Sheva K, Meirovitz A and Yakobson A:
Adverse events in osimertinib treatment for EGFR-Mutated
Non-Small-Cell lung cancer: Unveiling Rare Life-threatening
myelosuppression. Medicina (Kaunas). 60:12702024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou S, Kishi N, Alerasool P and Rohs NC:
Adverse event profile of epidermal growth factor receptor tyrosine
kinase inhibitors for Non-small cell lung cancer: An updated
Meta-analysis. Target Oncol. 19:547–564. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marrett E, Kwong WJ and Chow LQ: Factors
associated with time to EGFR TKI treatment in patients with
non-squamous metastatic non-small-cell lung cancer. Future Oncol.
18:1535–1544. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ju JS, Huang AC, Tung PH, Huang CH, Chiu
TH, Wang CC, Ko HW, Chung FT, Hsu PC, Fang YF, et al: Brain
metastasis, EGFR mutation subtype and generation of EGFR-TKI
jointly influence the treatment outcome of patient with EGFR-mutant
NSCLC. Sci Rep. 13:203232023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gu Y, Xu Y, Zhuang H, Jiang W, Zhang H, Li
X, Liu Y, Ma L, Zhao D, Cheng Y, et al: Value and significance of
brain radiation therapy during first-line EGFR-TKI treatment in
lung adenocarcinoma with EGFR sensitive mutation and synchronous
brain metastasis: Appropriate timing and technique. Thorac Cancer.
12:3157–3168. 2021. View Article : Google Scholar : PubMed/NCBI
|