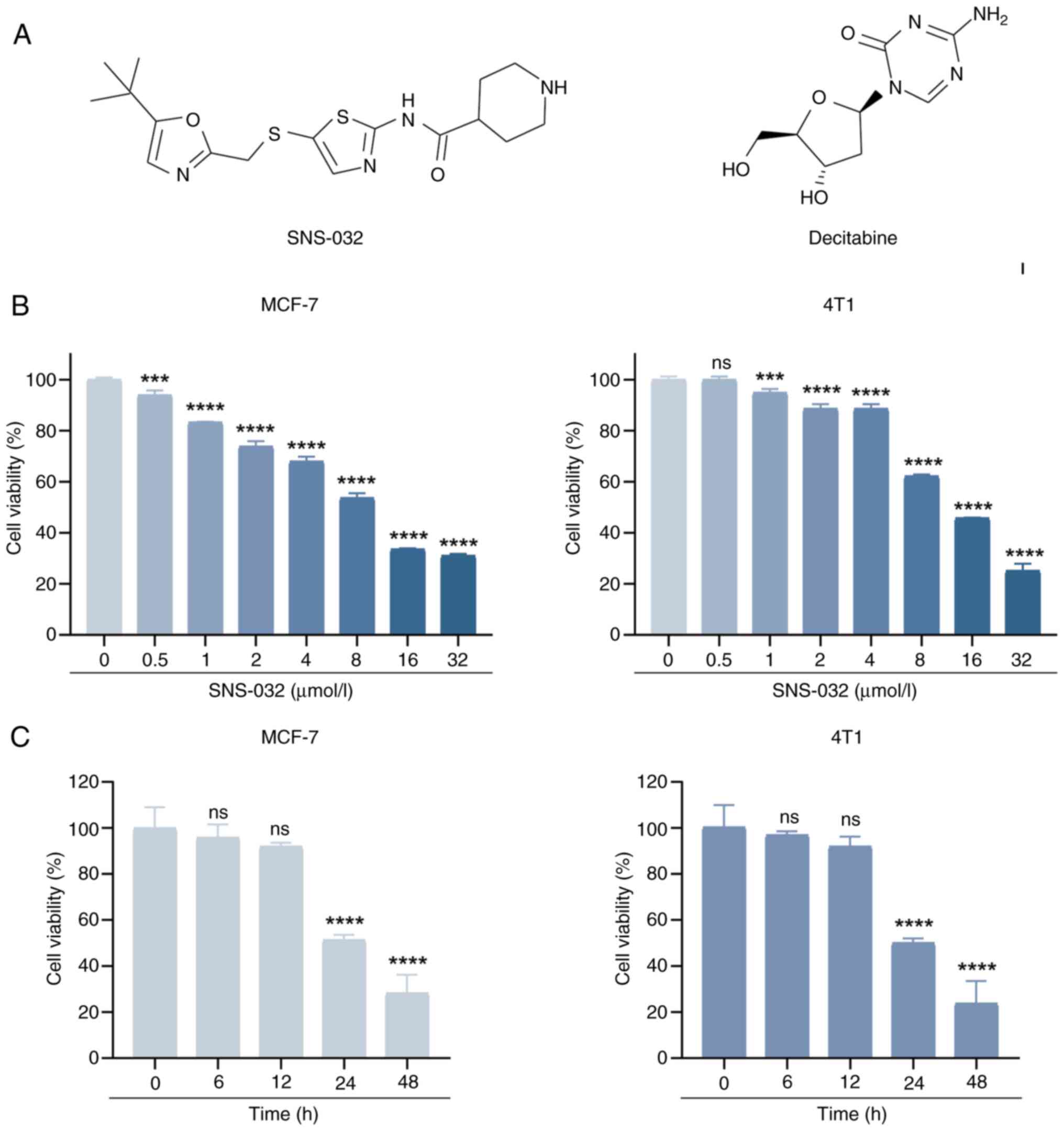
Introduction
Breast cancer (BC) is the most prevalent malignant
tumor in women worldwide. According to the World Health
Organization, BC accounts for the majority of cancer related
mortalities among women globally, which seriously impacts the
physical and psychological health of patients (1–3). The
5-year survival rate of BC has reached 90% because of advances in
drug therapy and the establishment of a precise diagnosis, with the
treatment level of BC improving annually (4,5).
Chemotherapy remains an essential component of the comprehensive
treatment of BC because it can substantially increase the 5-year
survival rate and improve prognosis (6). However, issues with treatment such as
drug resistance, drug-related toxicity and poor tolerance to
traditional chemotherapeutic drugs must be resolved. Therefore, the
development of new drugs has become a priority.
Pyroptosis is a novel form of programmed cell death
(PCD) mediated by members of the gasdermin (GSDM) family that
occurs after autophagy and apoptosis (7). It is morphologically characterized by
cell swelling, dissolution and vesicular protrusions, followed by
cell membrane rupture, which leads to the release of
proinflammatory cytokines and cell contents into the extracellular
space. These include high mobility group B protein (HMGB)14, IL-18,
IL-1, adenosine 5′-triphosphate (ATP) and HMGB1, all of which
trigger an inflammatory response (7–9). This
is closely associated with immune system activation (10). The GSDM family of proteins, which
are effectors of pyroptosis, can be cleaved by the caspase family
and granzyme (GZM) to release the active, membrane pore-forming
N-terminal domain and split the junction domain between the N- and
C-terminus (9). The N-terminus of
GSDM family proteins forms large pores in the cell membrane,
breaking osmotic pressure causing cell expansion and producing
large vacuoles (11–13).
GSDME, a protein belonging to the GSDM family, is
distinct from GSDMD and specifically cleaved by caspase-3 (14,15).
Previously, GSDME has been found to serve a role in the occurrence
and development of malignant tumors, including lung cancer,
colorectal cancer and BC (16–18).
In addition, paclitaxel accelerates pyroptosis in small cell lung
cancer cells, and increasing the expression of GSDME can
effectively enhance their sensitivity to paclitaxel (19). There appears to be an association
between low survival rate and low expression of GSDME in patients
with BC, indicating that GSDME might serve as a tumor suppressor in
BC (20).
GSDME is highly expressed in normal cell lines;
however, most tumor cell lines have methylated promoters that lower
GSDME expression compared with that in normal cells, making it more
difficult to activate pyroptosis in tumor cells (14,15).
As a result, when treating malignant tumors, appropriate
chemotherapy drugs could be chosen based on the expression level of
GSDME. Drugs which can increase GSDME expression may increase the
sensitivity of cells to chemotherapy drugs and achieve a more
potent anticancer effect (21). The
DNA methyltransferase inhibitor, decitabine (DAC; Fig. 1A), can induce functional
re-expression of the abnormally silenced GSDME gene in cancer
(22,23); therefore, DAC can be used to
increase the expression of GSDME in BC cells with low GSDME
expression, thereby enhancing the killing of tumor cells through
pyroptosis. Therefore, the combination of DAC and GSDME-inducing
pyroptosis agents for cancer treatment may be a promising strategy
for BC treatment.
SNS-032 (formerly BMS-387032; Fig. 1A) was originally designed to obtain
a selective cyclin-dependent kinase 2 (CDK2) inhibitor by the
Bristol-Myers Squibb Pharmaceutical Research Institute (Stamford,
USA) (24). Subsequent studies have
found that the anticancer activity of this compound is mainly
dependent on the inhibition of CDK7 and CDK9 (24–26),
which effectively kill chronic lymphocytic leukemia (CLL) cells
in vitro by blocking RNA polymerase II phosphorylation and
inhibiting RNA synthesis (24).
Several studies have demonstrated that SNS-032 has a strong
anticancer effect on hematological malignancies (24–27)
and solid tumors, such as uveal melanoma (25), cervical cancer (28) and non-small cell lung cancer
(29). In addition, SNS-032 has
been reported in phase I clinical studies in patients with
metastatic refractory solid tumors (30), advanced CLL and multiple myeloma
(31). However, to date, there have
been no further reports of SNS-032 in clinical trials. SNS-032 not
only inhibits tumor proliferation through the cell cycle, but also
induces apoptosis, leading to tumor cell death in melanoma
(25), BC (32) and esophageal squamous cell carcinoma
(33). However, no studies have
reported the activation of the pyroptosis pathway to inhibit
tumors.
SNS-032 combined with the demethylating drug, DAC,
is a promising treatment strategy for patients with BC. The present
study aimed to establish the mechanism of SNS-032 to inhibit tumors
and to determine whether the combination with DAC can improve
inhibition of tumors, providing a new approach for BC
treatment.
Materials and methods
Cell culture
MCF-7 cells, a human BC cell line from Procell Life
Science & Technology Co., Inc., were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.). The mouse BC cell line 4T1,
derived from BALB/c mice, were from cell bank of the Typical
Culture Preservation Committee of the Chinese Academy of Sciences
(Beijing, China) and cultured in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.). All media were supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (cat. no G4003; Wuhan Servicebio Technology
Co., Ltd.). All cell lines were cultured in a humidified incubator
at 37°C and 5% CO2. The cells were seeded 24 h later.
Trypsin/EDTA at 0.25% (w/v) and phosphate-buffered saline (PBS)
were purchased from Thermo Fisher Scientific, Inc.
Materials
SNS-032 (cat. no S1145) and DAC (cat. no S1200) were
purchased from Selleck Chemicals and dissolved in dimethyl
sulfoxide (DMSO) at a concentration of 5 mM. The
pan-caspase-specific inhibitor Z-VAD-FMK (cat. no HY-16658B),
necroptosis inhibitor Necrostatin-1 (cat. no HY-15760) and specific
cell caspase-3 inhibitor Z-DEVD-FMK (cat. no HY-12466) were
purchased from MedChemExpress and dissolved in DMSO at
concentrations of 10, 5 and 10 mM, respectively. The lactate
dehydrogenase (LDH) Release Assay kit (cat. no C0017),
bicinchoninic acid assay (cat. no P0009) and ATP Assay kit (cat. no
S0027) were purchased from Beyotime Institute of Biotechnology. The
Annexin-V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
Test kit (cat no. 556547) was purchased from BD Biosciences.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8; Dojindo
Laboratories, Inc.) was used to determine the viability of the BC
cell lines. MCF-7 and 4T1 cells were pretreated with or without DAC
(5 µmol/l) for 24 h at 37°C seeded in 96-well plates at 3,000
cells/well and treated with different doses (0, 1, 2, 4, 8, 16 and
32 µmol/l) of SNS-032. According to the manufacturer's
instructions, 100 µl working solution was added to each well after
24 h SNS-032 treatment and incubated for 2 h. The absorbance at 450
nm was measured using a Synergy 2 microplate reader (BioTek;
Agilent Technologies, Inc.) and cell viability was determined using
the following formula: Cell viability (%)=(experimental group
OD450-blank control OD450)/(control group
OD450-blank control OD450) ×100.
Colony formation assay
MCF-7 and 4T1 cells were seeded in six-well plates
(1,000 cells/well), incubated overnight and treated with DAC (5
µmol/l) and SNS-032 (10 µmol/l in MCF-7 and 15 µmol/l in 4T1 cells)
at 37°C. The culture was allowed to continue for 7 days, with
medium changes every 3 days. The cells were fixed with 4%
paraformaldehyde for 10 min (cat. no S1101; Wuhan Servicebio
Technology Co., Ltd.), stained with 0.1% crystal violet for 15 min
and thoroughly washed at room temperature. Finally, the number of
colonies (>50 cells/colony) in each well was counted using
ImageJ (V1.8.0.112; National Institutes of Health) and the colony
formation efficiency was calculated as a percentage relative to the
control (100%).
LDH assay
LDH release was detected using an LDH release Assay
Kit. The supernatants were collected by centrifugation of cells at
400 × g for 5 min at 4°C after treatment with SNS-032 (10 µmol/l in
MCF-7 and 15 µmol/l in 4T1 cells) for 24 h with or without DAC (5
µmol/l) pretreatment for 24 h at 37°C. According to the
manufacturer's protocol, after the working detection reagent was
prepared, 60 µl LDH detection reagent and 100 µl sample supernatant
were added into 96 wells quickly. After 30 min incubation, a
Synergy 2 microplate reader was used to measure the absorbance at
490 nm in the dark and the data were processed according to the
following formula: LDH release (%)=(experimental group
OD490-blank control OD490)/(maximum release
group OD490-blank control OD490) ×100.
ATP assay
An ATP Assay Kit was used for quantitative
determination of ATP. The medium was discarded and the lysis buffer
was added to the BC cells after treatment with SNS-032 (10 µmol/l
in MCF-7 and 15 µmol/l in 4T1 cells) for 24 h with or without DAC
(5 µmol/l) pretreatment for 24 h at 37°C. The cell lysate was
collected and centrifuged at 12,000 × g for 5 min at 4°C to obtain
the supernatants. According to the manufacturer's protocol, 100 µl
ATP detection reagent was added to a black 96-well plate and
incubated at room temperature for 5 min. Subsequently, 20 µl each
sample supernatant was added immediately into the black 96-well
plate at room temperature. Finally, a Synergy 2 microplate reader
was used to detect the luminescence of samples immediately and ATP
levels were calculated as follows: ATP cell viability
(%)=(experimental group luminescence/control group luminescence)
×100.
Annexin V/PI double staining
BC cell lines, MCF-7 and 4T1, were seeded into
12-well plates overnight at a density of 1×105
cells/well and treated with different concentrations of SNS-032 for
24 h in the presence or absence of DAC, Z-VAD-FMK (20 µmol/l),
Necrostatin-1 (10 µmol/l) or Z-DEVD-FMK (20 µmol/l) at 37°C. Cells
were collected, centrifuged at 300 × g for 5 min at 4°C and washed
twice with cold PBS. The following staining procedure was performed
according to the manufacturer's protocol. Resuspended cells were
mixed with 200 µl 1× Binding Buffer and incubated with 5 µl Annexin
V-FITC and 5 µl PI at room temperature for 10 min. SNS-032-induced
cell death was monitored with Annexin V-FITC/PI and detected with
CytoFLEX flow cytometer (Beckman Coulter, Inc.). All data were
analyzed using FlowJo V10.8.1 (BD Biosciences).
Western blotting analysis
Total cell protein was extracted from BC cells after
treatment with SNS-032 (10 µmol/l in MCF-7 and 15 µmol/l in 4T1
cells) for 24 h with or without DAC (5 µmol/l) pretreatment for 24
h or Z-DEVE-FMK (20 µmol/l) pretreatment for 3 h at 37°C using
radioimmunoprecipitation lysis buffer (cat. no G2002; Wuhan
Servicebio Technology Co., Ltd.) for western blotting. The buffer
contained a 1% protease inhibitor cocktail (cat. no G2006; Wuhan
Servicebio Technology Co., Ltd.). The cell samples were incubated
in cold lysis buffer for 20 min, centrifuged at 13,600 × g for 20
min at 4°C. Protein concentration was measured using the
bicinchoninic acid assay. Equal amounts of protein sample (30 µg)
were loaded and separated by SDS-polyacrylamide gel electrophoresis
(Dakewe Biotech Co., Ltd.) and transferred to polyvinylidene
difluoride (PVDF) membranes (MilliporeSigma). The PVDF membranes
were blocked with 5% bovine serum albumin (cat. no GC305006; Wuhan
Servicebio Technology Co., Ltd.) for 1 h at room temperature and
incubated with the primary antibody at 4°C overnight. The following
antibodies were used: Anti-GSDME (1:1,000; cat. no ab215191;
Abcam), anti-caspase-3 (1:1,000; cat. no #9662; Cell Signaling
Technology, Inc.), anti-Bcl-2-associated X protein (BAX; 1:2,000;
cat. no ab32503; Abcam), anti-B-cell lymphoma-2 (BCL-2; 1:2,000;
cat. no ab182858; Abcam), anti-mouse cleaved N-terminal GSDMD
(Asp275; 1:1,000; cat. no #36425; Cell Signaling Technology, Inc.),
anti-cleaved N-terminal GSDMD (1:1,000; cat. no ab215203; Abcam)
and anti-GAPDH (1:5,000; cat. no A19056; ABclonal Biotech Co.,
Ltd.). The PVDF membrane was exposed to anti-rabbit secondary
antibody (1:20,000; cat. no ANT020; Wuhan Antejie Biotechnology
Co., Ltd.) at room temperature for 1 h after washing three times
with TBST (0.1% Tween-20 in Tris-HCl buffer). Protein bands were
visualized using a multimode chemiluminescence system (Bio-Rad
Laboratories, Inc.) and ImageJ software (version 1.5) for
densitometry analysis.
Statistical analysis
All data presented were obtained from ≥3 independent
experiments and are expressed as mean ± standard deviation. Data
were analyzed using GraphPad Prism 8 (Dotmatics). The significance
of the differences was assessed using one-way analysis of variance
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference in all
comparisons.
Results
SNS-032 can affect the cell viability
of BC cells
To evaluate the anticancer effect of SNS-032 on BC
cells, a CCK-8 assay was used to assess the effects of SNS-032 on
the activity of human and mouse BC cell lines, MCF-7 and 4T1.
Results from CCK-8 experiments (Fig.
1B) demonstrated that SNS-032 caused significant decreases in
cell viability in a dose-dependent manner in BC cells compared with
the control. The half-maximal inhibitory concentrations of the two
BC cell lines were calculated to be 9 µmol/l (MCF-7) and 13.5
µmol/l (4T1). Based on these results, the following concentrations
were used in the subsequent time-course investigations: 10 µmol/l
for MCF-7 and 15 µmol/l for 4T1. SNS-032 significantly decreased
the viability of BC cells in a time-dependent manner as shown by
CCK-8 assays (Fig. 1C). These
results indicate that the inhibitory effect of SNS-032 on BC cells
was dose- and time-dependent, and the results were significantly
different compared with the untreated control or 0 h timepoint
(P<0.001), with the exceptions being the 0.5 µmol/l
concentration in 4T1 cells, and the 6 and 12 h timepoints in both
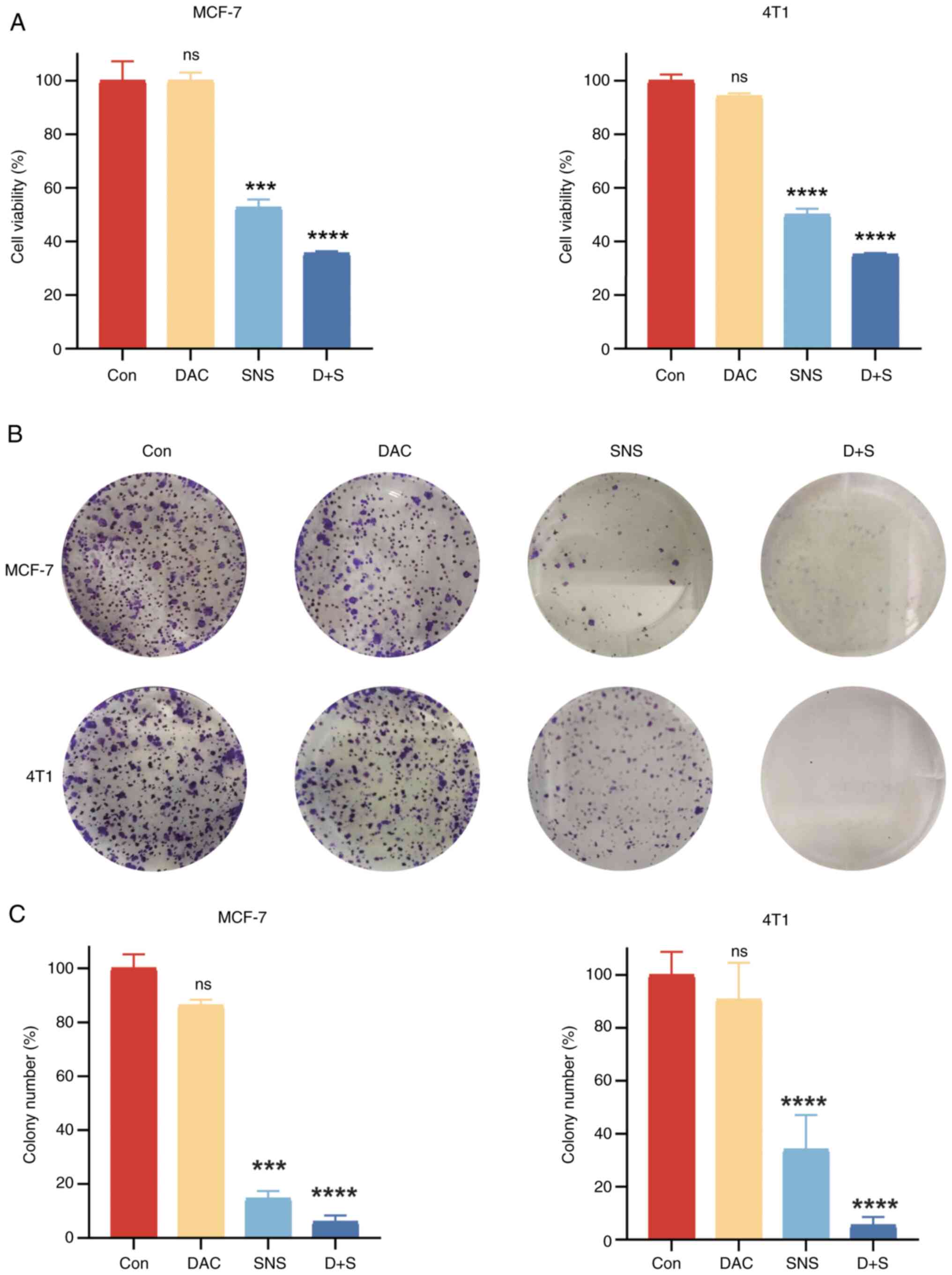
MCF-7 and 4T1 cells. To further support these results, colony
formation experiments were performed on MCF-7 and 4T1 cells, as
shown in Fig. 2B and C, which
demonstrated that SNS-032 significantly inhibited the cloning of BC
cells (P<0.001). In summary, these data show that SNS-032
treatment reduces the viability of BC cells.
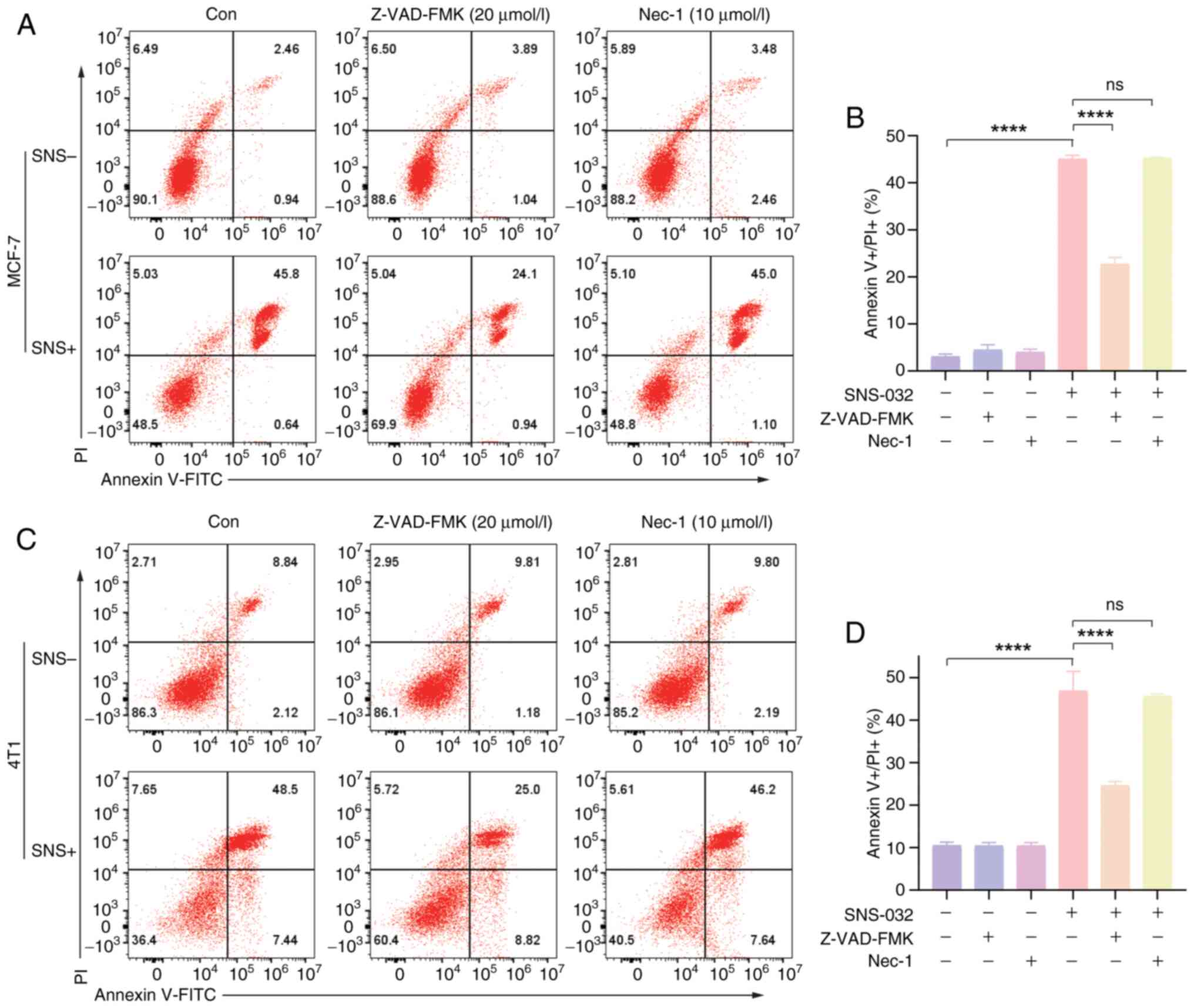
Evaluation of the cell death pathways
of SNS-032 in BC cells
To explore the cell death pathway of SNS-032 in BC
cells, cells were pretreated for 3 h with pan-caspase inhibitor,
Z-VAD-FMK (20 µmol/l), necroptosis inhibitor Necrostatin-1 (10
µmol/l) or specific cell caspase-3 inhibitor Z-DEVD-FMK (20
µmol/l). MCF-7 and 4T1 cells were treated with SNS-032 for 24 h to
observe cellular changes. Annexin V-FITC and PI were used to stain
the cells for apoptosis studies, and the efficacy of the three
inhibitors in reducing cell death caused by SNS-032 was assessed,
as shown in Figs. 3 and 4B. Based on the results of flow cytometry,
Necrostatin-1 had little effect on inhibiting SNS-032 induced-BC
cell death, Z-VAD-FMK could partially inhibit the cell death of BC
cells induced by SNS-032, while Z-DEVD-FMK was able to more
markedly inhibit the SNS-032-induced cell death of BC cells,
indicating the effect of the caspase-3 specific inhibitor was
superior. Therefore, the possibility that cell death induced by
SNS-032 is due to necroptosis can be excluded. Additionally, the
results suggest that the cell death triggers may be related to the
caspase family and caspase-3.
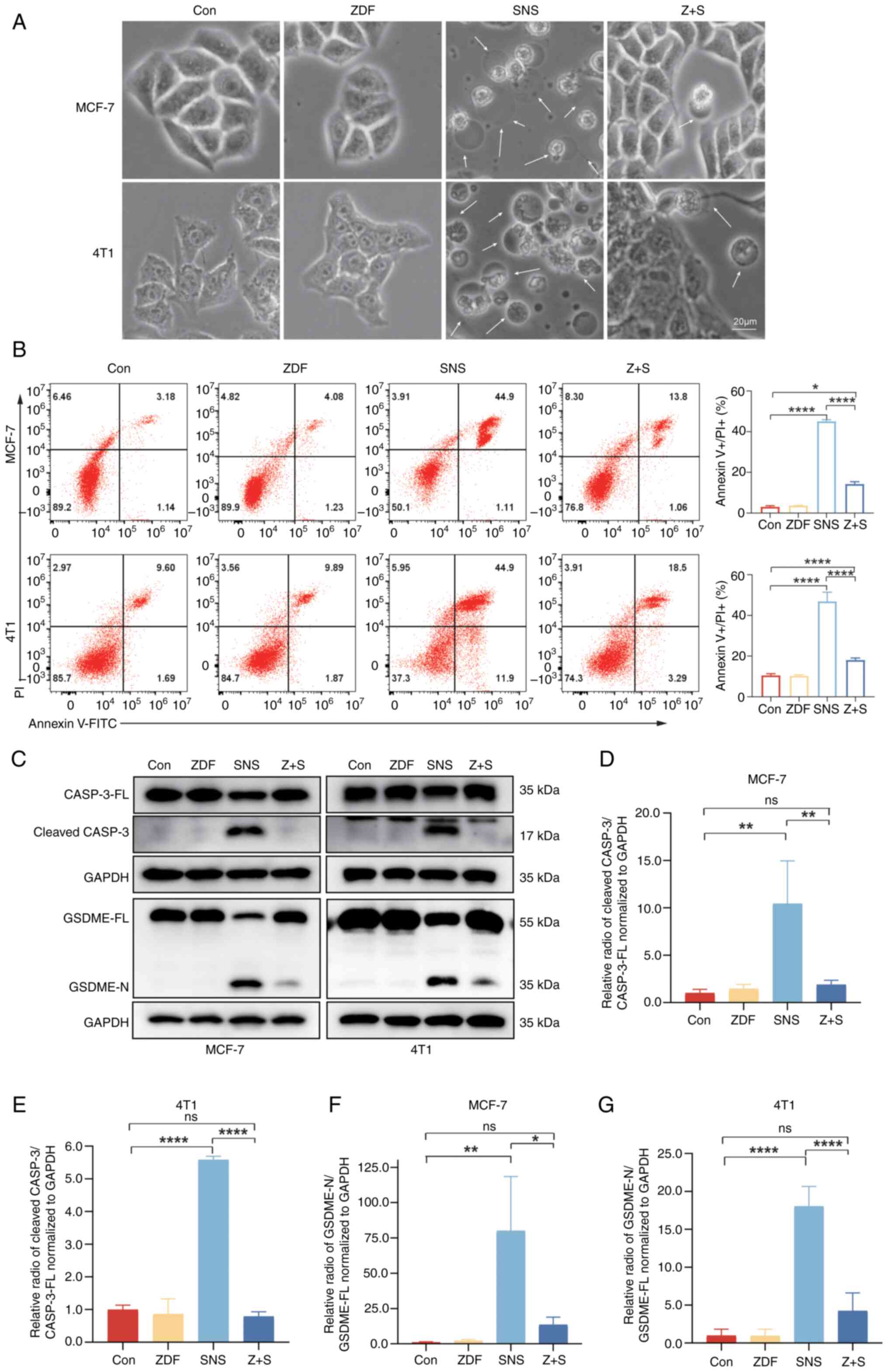 | Figure 4.Caspase-3 is required for
SNS-032-dependent pyroptosis. (A) Representative microscopic images
of MCF-7 and 4T1 cells pretreated with SNS-032 (10 and 15 µmol/l,
respectively) with or without Z-DEVD-FMK (20 µmol/l) for 3 h. The
white arrows indicate pyroptotic cells. Scale bar, 20 µm. (B)
Annexin V-FITC/PI assay was performed to analyze pyroptosis and
apoptosis of cells after pretreatment with SNS-032 for 24 h with or
without Z-DEVD-FMK (20 µmol/l) for 3 h. The percentage of
late-stage cell apoptosis in cells was quantified. Representative
images are shown. (C) Cleaved-caspase-3 and GSDME proteins were
detected after SNS-032 pretreatment with or without Z-DEVD-FMK (20
µmol/l) for 3 h by western blotting in MCF-7 and 4T1 cells.
Semi-quantitative western blot analysis of cleaved caspase-3 in (D)
MCF-7 and (E) 4T1 cells and GSDME-N in (F) MCF-7 and (G) 4T1 cells.
The cells were exposed to 0.1% dimethyl sulfoxide as a control.
Data are presented as mean ± standard deviation (n=3). *P<0.05,
**P<0.01 and ****P<0.0001 vs. control. ns, not significant;
FITC, fluorescein isothiocyanate; Con, control; ZDF, Z-DEVD-FMK;
SNS, SNS-032; Z + S, Z-DEVD-FMK + SNS-032; GSDME, gasdermin E;
CASP-3, caspase-3; FL, full-length; N, N-terminus. |
SNS-032-induced pyroptosis is
dependent on activation of caspase-3
MCF-7 and 4T1 cells were treated with four different
treatments. During 24 h SNS-032 treatment, swelling, plasma
membrane fragmentation and morphological alterations were observed
(Fig. 4A). There were no
discernible morphological changes in the control group, indicating
that SNS-032 acts as an anticancer agent by inducing pyroptosis in
BC cells. These morphological changes were consistent with the
typical morphological changes of pyroptosis rather than with the
classical changes observed during apoptosis, including shrinkage,
rounding, volume reduction, deformation and maintenance of membrane
integrity. As pyroptosis results in the destruction of the cell
membrane, which is comparable to that of late-stage apoptotic
cells, the potential of SNS-032 treatment to promote pyroptosis was
evaluated by detecting the percentage of Annexin V-FITC+/PI+ cells
using flow cytometry in BC cells. The results demonstrated that
following exposure to SNS-032, the populations of Annexin V+/PI+
cells in MCF-7 and 4T1 cells significantly increased, resulting in
a notably higher rate of late-stage apoptosis (Figs. 3A, C, 4B). These results demonstrate that SNS-032
is associated with BC cell death.
Previous studies have reported that pyroptosis is a
caspase-dependent PCD pathway in which GSDME is cleaved by active
caspase-3, releasing its N-terminal domain to penetrate the cell
membrane, leading to cell swelling, rupture and ultimately cell
death (11,12). To assess this, BC cells were treated
with the caspase-3 inhibitor Z-DEVD-FMK. Subsequently, western
blotting and semi-quantitative analysis were performed to evaluate
the changes of GSDME full-length (GSDME-FL) and N-terminus domain
(GSDME-N) expression levels in BC cells treated using different
approaches (Fig. 4C). Western
blotting revealed that the N-terminus of GSDME could barely be
detected after Z-DEVD-FMK inhibited caspase-3 activation (Fig. 4C-E). SNS-032 treatment alone
significantly increased cleavage and activation of caspase-3, with
a corresponding significant increase in GSDME-N compared with those
in the control and Z-DEVD-FMK treatment groups. However, after
SNS-032 treatment of BC cells pretreated with Z-DEVD-FMK, activated
caspase-3-induced GSDME-N production was significantly reduced
compared with SNS-032 treatment alone, providing further evidence
that activated caspase-3 is required for GSDME cleavage (Fig. 4C-G). BC cells pretreated with
Z-DEVD-FMK also showed significantly fewer FITC/PI double-positive
cells compared with SNS-032 treatment alone (Fig. 4B). In addition to pyroptosis, the
caspase family serves an important role in cell apoptosis (34–36).
Z-VAD-FMK alone had no effect on cell death compared with the
control in either of the cells and could inhibit BC cell death
induced by SNS-032 in the present study (Fig. 3A and C). These results suggest that
SNS-032 induces pyroptosis by activating caspase-3 cleavage and
GSDME in BC cells. This indicates that caspase-3 serves a crucial
role in the process of BC cell death induced by SNS-032 and
provides an important basis for further exploration of the
mechanism of cell death and the development of associated
therapeutic targets.
DAC can enhance the pyroptosis induced
by SNS-032 in BC cells
DAC, a DNA methyltransferase inhibitor that exhibits
anticancer activity in various types of cancer (37), can demethylate BC cells and increase
GSDME expression (38,39). In the present study, in combination
with the CDK inhibitor SNS-032, DAC was used as a pretreatment
agent in BC cells. According to the results shown in Fig. S1A, the expression of GSDME-FL was
assessed in two BC cell lines following a 48 h treatment with DAC
at concentrations of 2.5, 5 and 7.5 µmol/l. GSDME-FL expression was
significantly increased when the cells were treated with 5 µmol/l
DAC. Therefore, pretreatment with DAC at 5 µmol/l in the dark for
24 h was chosen before adding SNS-032. To evaluate the effects of
selected concentrations of DAC on BC cells, cell viability was
assessed using CCK-8 and colony formation assays. The cell
viability of BC cells was not significantly affected with DAC
treatment compared with the control group, as shown in Fig. 2A-C. Compared with SNS-032
monotherapy, the combination of DAC and SNS-032 demonstrated
significantly reduced viability of BC cells (P<0.001). Marked
pyroptotic morphological changes, such as large vacuoles and
membrane balloon-like changes, occurred more frequently in BC cells
following combination treatment with DAC and SNS-032 (Fig. 5A) compared with morphological
changes in cells treated with SNS-032. Compared with the control
group and single-drug treatment groups, BC cells treated with DAC
and SNS-032 exhibited a significantly greater proportion of
late-stage apoptotic cells (>60%), as illustrated in Fig. 5C.
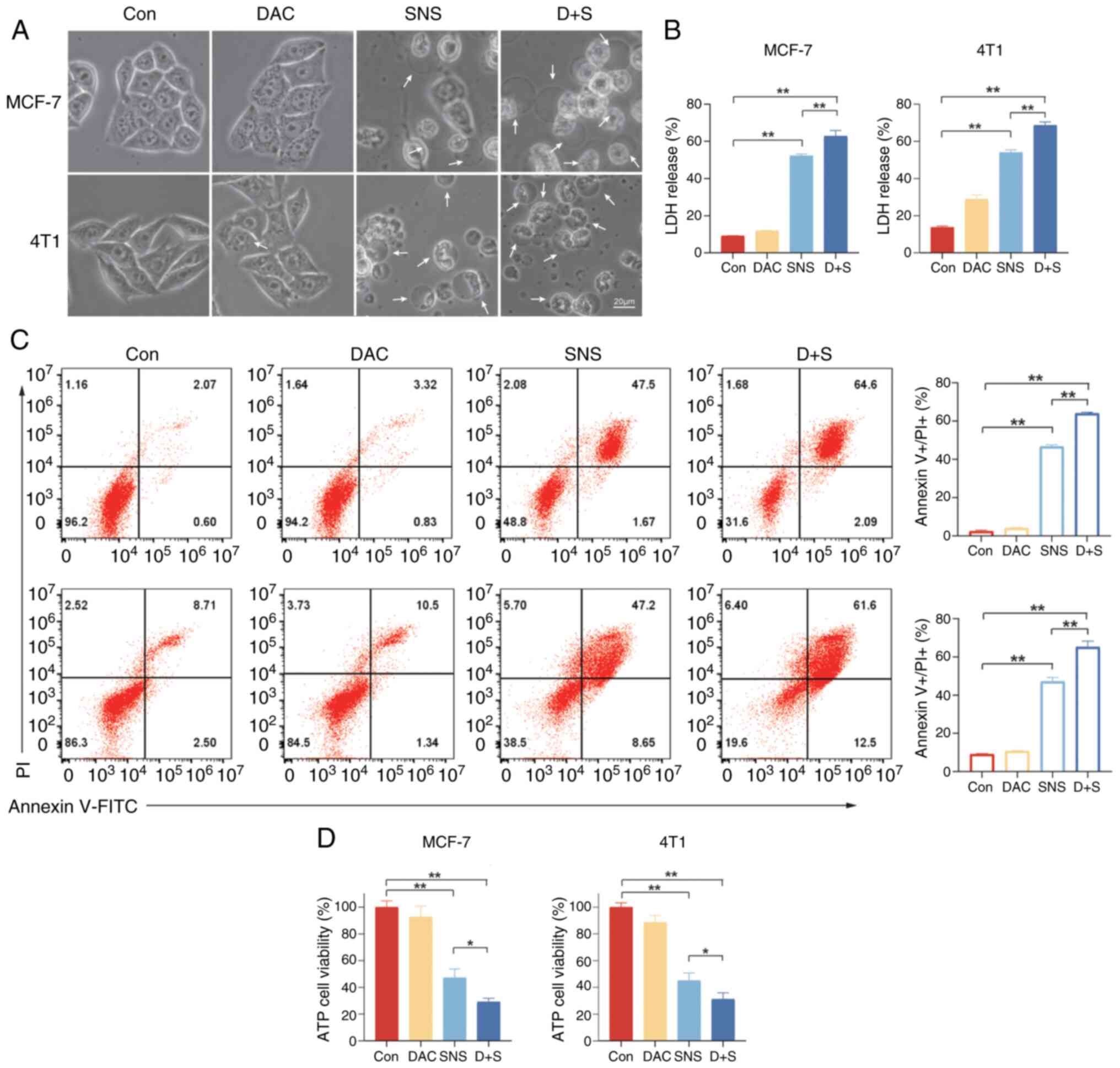 | Figure 5.SNS-032 can cause the release of
breast cancer cell contents and DAC can enhance the effect. (A)
Representative microscopic images of MCF-7 (10 µmol/l) and 4T1 (15
µmol/l) cells treated with SNS-032 for 24 h with or without
pretreatment with DAC (5 µmol/l). White arrowheads indicate the
large vacuoles, cell membrane ruptures and organelle edema. Scale
bar, 20 µm. (B) Quantification of the release of LDH after
treatment with SNS-032 for 24 h with or without DAC (5 µmol/l)
pretreatment for 24 h. (C) An Annexin V-FITC/PI assay was performed
to analyze pyroptosis and apoptosis of cells after treatment with
SNS-032 for 24 h with or without pretreatment with DAC (5 µmol/l)
for 24 h in MCF-7 (10 µmol/l) and 4T1 (15 µmol/l) cells.
Representative images are shown. (D) Quantification of ATP levels
to assess cell viability after treatment with SNS-032 with or
without DAC (5 µmol/l) pretreatment for 24 h. Data are presented as
mean ± standard deviation (n=3). The cells were exposed to 0.1%
dimethyl sulfoxide as a control. *P<0.05 and **P<0.01 vs.
control. DAC, decitabine; SNS, SNS-032, D + S, decitabine +
SNS-032; Con, control; ATP, adenosine 5’-triphosphate; LDH, lactate
dehydrogenase; FITC, fluorescein isothiocyanate. |
During pyroptosis, cells disintegrate and lose
membrane integrity, releasing large quantities of ATP and LDH. As
illustrated in Fig. 5B and D, upon
exposure to SNS-032, intracellular ATP levels were significantly
decreased and LDH release levels were significantly increased
(P<0.01) in BC cells compared with those in the control group.
Furthermore, Fig. 5B and D
demonstrate that there was a significant decrease in intracellular
ATP levels and a significant increase in LDH release levels in
MCF-7 and 4T1 cells with DAC and SNS-032 treatment compared with
the control and SNS-032 treatment alone. By contrast, the DAC group
demonstrated little effect compared with that of the control group,
corroborating the suggestion that combined DAC treatment could
accelerate SNS-032-triggered cell pyroptosis. A similar pattern was
observed in subsequent experiments.
Previously, the process of pyroptosis has been
divided into GSDMD-mediated classical and non-classical pathways,
and other GSDME-mediated pathways (7). As shown in Fig. S1C, the expression of the N-terminus
domain of GSDMD was evaluated in BC cells after exposure to SNS-032
treatment to determine whether SNS-032-triggered pyroptosis. The
results demonstrated that no GSDMD N-terminus domain expression was
observed in SNS-032-treated BC cells. Western blotting was
performed on MCF-7 and 4T1 BC cells to assess the expression of
specific proteins triggered by pyroptosis. As shown in Fig. 6A, GSDME-FL was visibly increased in
the DAC treatment group compared with the control group. The levels
of cleaved GSDME-N were significantly increased in the drug
combination group compared with SNS-032 alone (Fig. 6E). Consequently, the expression of
apoptosis-related proteins in MCF-7 and 4T1 cells was assessed
(Fig. 6B). As shown in Fig. 6D and F, following SNS-032 treatment,
the expression of pro-apoptotic protein, BAX, was significantly
increased whereas the expression of the anti-apoptotic protein,
BCL-2, was significantly decreased. This suggested that SNS-032
also induces apoptosis in MCF-7 and 4T1 BC cells. There were no
significant changes in the expression levels of cleaved caspase-3,
BAX or BCL-2 between the DAC and control groups. The pro-apoptotic
proteins, BAX and cleaved caspase-3, were significantly increased
in SNS-032 treated groups and the anti-apoptotic protein BCL-2 was
significantly decreased. Treatment with DAC and SNS-032 increased
the expression of cleaved caspase-3 further and significantly
decreased the expression of BCL-2 compared with SNS-032 alone
(Fig. 6C and F). Based on the
aforementioned results, it can be concluded that BC cells exposed
to DAC demonstrate increased susceptibility to pyroptosis induced
by SNS-032, due to the upregulated expression of GSDME. These
results indicated that SNS-032 has anticancer effects on BC cells
by inducing pyroptosis and that GSDME, but not GSDMD, is involved
in pyroptosis induced by SNS-032 through caspase-3 activation.
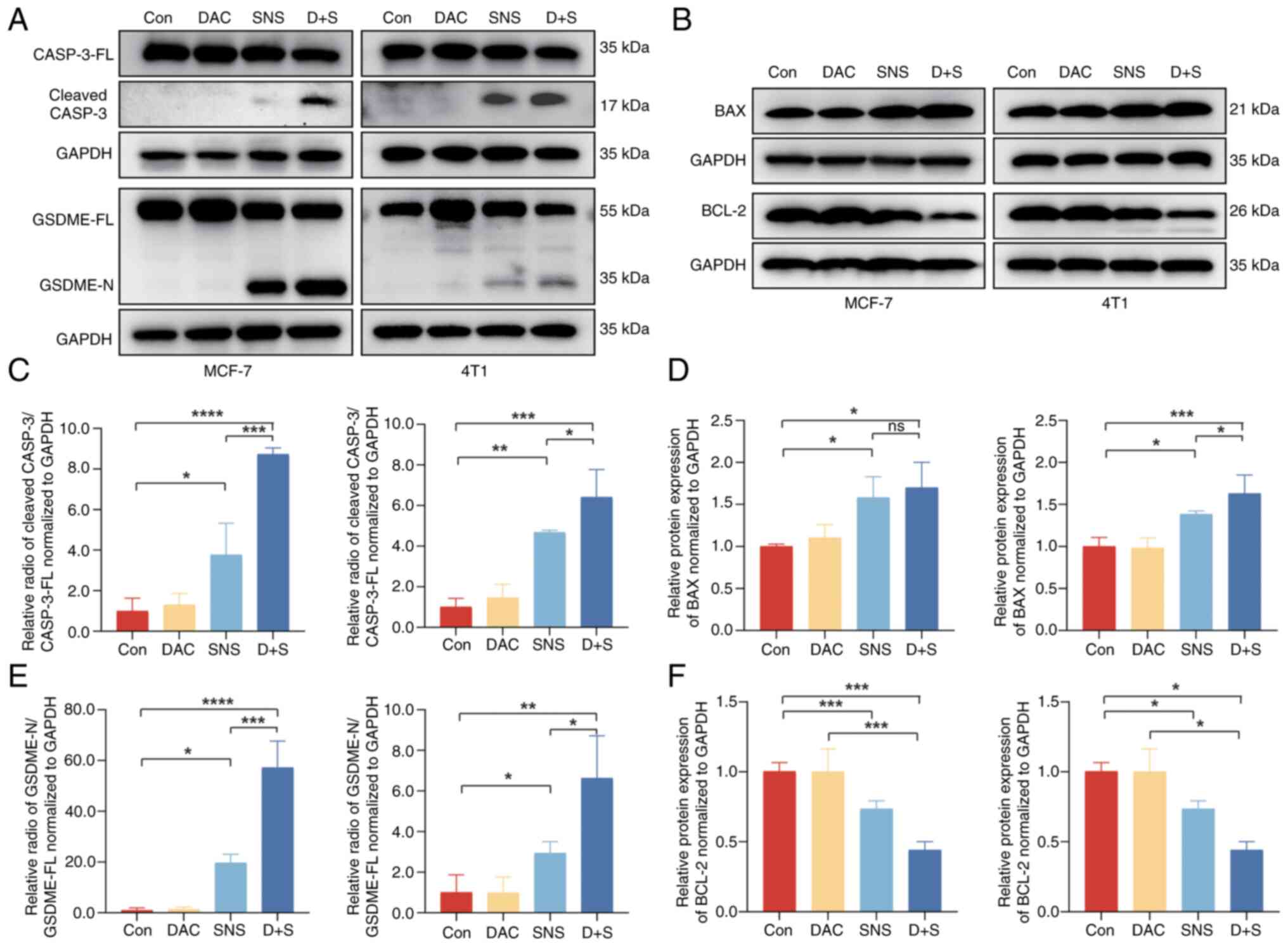 | Figure 6.DAC enhances the protein expression
changes of SNS-032-treated breast cancer cells. (A) Western
blotting of cleaved-caspase-3 and GSDME proteins after SNS-032
treatment in MCF-7 (10 µmol/l) and 4T1 (15 µmol/l) cells with or
without DAC (5 µmol/l) pretreatment for 24 h. (B) BAX and BCL-2
proteins were detected after SNS-032 treatment with or without DAC
(5 µmol/l) pretreatment for 24 h by western blotting in MCF-7 (10
µmol/l) and 4T1 (15 µmol/l) cells. (C) Semi-quantitative western
blot analysis of cleaved caspase-3 in MCF-7 and 4T1 cells. (D)
Semi-quantitative western blot analysis of BAX in MCF-7 and 4T1
cells. (E) Semi-quantitative western blot analysis of GSDME-N in
MCF-7 and 4T1 cells. (F) Semi-quantitative western blot analysis of
BCL-2 in MCF-7 and 4T1 cells. The cells were exposed to 0.1%
dimethyl sulfoxide as a control. Representative images are shown.
Data are presented as mean ± standard deviation (n=3). *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001. ns, not
significant; DAC, decitabine; SNS, SNS-032; D + S, decitabine +
SNS-032; BCL-2, B-cell lymphoma-2; BAX, Bcl-2-associated X protein;
CASP-3, caspase-3; GSDME, gasdermin E; FL, full-length; N,
N-terminus; Con, control. |
Discussion
Over the past several decades, progress has been
achieved in the prevention, detection and treatment of cancer. In
women, BC is the most commonly diagnosed cancer and the leading
cause of cancer-associated mortalities (1,3).
Despite the development of several targeted drugs, effective BC
therapeutic drugs are still limited (2,4). As
reported in previous studies, more CDK-specific inhibitors are
being developed with encouraging results and potential to become
available therapeutic targets for malignant tumors (40,41).
The present study reported the effects of the CDK
inhibitor, SNS-032, on BC cells. SNS-032 is a specific and potent
anticancer drug that inhibits CDKs 2, 7 and 9 (24,25),
blocks RNA polymerase II phosphorylation and inhibits RNA synthesis
(26,27). SNS-032 is a promising anticancer
drug for a number of cancers (25–29),
nevertheless its anticancer mechanisms remain unclear. In the
present study, it was experimentally validated that SNS-032 may
induce GSDME-mediated pyroptosis, which is activated via caspase-3
pathway, in BC cell lines.
Previously, pyroptosis has been implicated in
inflammatory PCD (8,42). Pyroptosis has emerged as a promising
research topic in the context of traditional chemotherapy
resistance. In cancers that develop resistance to conventional
chemotherapy, pyroptosis represents a novel cell death pathway.
Conventional chemotherapy relies predominantly on inducing
apoptosis; however, when tumor cells acquire resistance to
apoptosis, the pyroptotic pathway may still be effective. For
instance, multiple drug-resistant tumor cells are sensitive to
drugs that induce pyroptosis (43),
thereby offering new insights for overcoming resistance. Moreover,
studying different drug-induced pyroptosis mechanisms can further
optimize the design of drugs and enhance their specificity and
efficacy. Pyroptosis-inducing drugs can be used in combination with
existing therapies such as chemotherapy drugs, to overcome tumor
cell resistance (44), and
immunotherapy drugs, to strengthen the immune response (45). In addition, the tumor cells of
different patients vary in their gene expression and signaling
pathways, leading to different sensitivities to pyroptosis-inducing
drugs (46). Analyzing patient
tumor samples to determine the expression of pyroptosis-related
proteins and potential targets is beneficial for improving the
accuracy of treatments and reducing drug side effects.
In eukaryotic cells, pyroptosis is defined as the
creation of 10–20 nm pores in the cell membrane, followed by
nuclear concentration, cell enlargement and the release of
cytoplasmic contents into the extracellular environment. The
formation of pores in the cell membrane (47,48)
promotes the release of several components, including the
inflammatory cytokine IL-18 (49).
Pyroptosis enhances the immune response against cancer cells. When
cancer cells undergo pyroptosis, they release certain
damage-associated molecular patterns (DAMPs) (10). These DAMPs can activate
antigen-presenting cells in the immune system, such as dendritic
cells, which subsequently trigger the activation and recruitment of
immune cells, such as T cells. This process strengthens the immune
surveillance and killing ability of the body towards cancer cells.
This property is crucial in the current context of the rapid
development of immunotherapy, particularly in cancer types such as
BC, and in the application of stereotactic body radiotherapy in
metastatic disease. Inducing pyroptosis in combination with
immunotherapy could improve the therapeutic effect in clinical
trials.
Pyroptosis occurs through four signaling pathways:
Typical and atypical inflammasome pathways, GZM-based systems and
caspase-mediated pathways of apoptosis (13). The GSDM family is the final enforcer
of these signaling cascades, which require upstream caspases or
GZMs to cleave them (9). Most
studies to date have focused on the roles of GSDMD and GSDME in
pyroptosis. A previous study demonstrated caspase-3 can cleave
GSDME during chemotherapy to generate GSDME-N, which then
penetrates the plasma membrane and leads to pyroptosis (14). Pyroptosis stimulation is challenging
in BC cells because of the markedly decreased expression of GSDME
compared with expression in normal cells (14,15,22).
Endogenous GSDME expression was profiled in 57 cancer cells from
the National Cancer Institute-60 cell lines screen, which includes
numerous BC cell lines. And MCF-7 cells exhibited the highest level
of endogenous GSDME expression, indicating a higher probability of
inducing GSDME-mediated pyroptosis (14). Therefore, this cell line was chosen
as the human BC cell line for the present study.
It has been reported that DAC is capable of
augmenting the expression of GSDME protein in 4T1 cells (50). Pyroptosis has the capacity to
activate the body's immune system, which potentially responds to
the tumor-killing effect of the drugs. To optimize the anticancer
effect of SNS-032-induced pyroptosis in BC, the BC cell line 4T1
was chosen, which is derived from BALB/c mice. The 4T1 cells can be
utilized to construct immunocompetent BC mouse models in BALB/c
mice, thereby paving the way for further investigations into the
anticancer efficacy of SNS-032 in vivo. In addition, the DNA
methyltransferase inhibitor, DAC, was utilized to induce functional
re-expression of the abnormally silenced GSDME gene in cancer
(22,23), and elevate the expression of GSDME
in BC cells with low GSDME expression, thus promoting the
anticancer ability through cell pyroptosis. Based on previous
research (39,51) and the results of the present study,
the optimal DAC concentration was determined to be 5 µmol/l. The
optimal concentration of SNS-032 was determined to be 10 µmol/l in
MCF-7 cells and 15 µmol/l in 4T1 cells for the subsequent
experiments in the present study.
Initially, SNS-032 was shown to inhibit in the
viability of BC cells using CCK-8 and colony formation assays, and
the combination of DAC and SNS-032 further enhanced this effect.
Furthermore, the morphology of MCF-7 and 4T1 cells following
SNS-032 treatment revealed swelling and generation of large
vacuoles on the cell membrane, which is a characteristic
morphological manifestation of pyroptosis.
Previous studies have shown that pyroptosis and
apoptosis can be differentiated by the positivity of Annexin V/PI
staining. Generally, pyroptosis occurs more rapidly than apoptosis.
For double-staining with Annexin V and PI, in the early stage of
pyroptosis, the integrity of the cell membrane is compromised,
leading to the externalization of phosphatidylserine, to which
Annexin V can specifically bind. Concurrently, due to the increased
membrane permeability, PI can enter the cell through pores formed
on the cell membrane and stain the nucleus (34). In flow cytometric analysis, a cell
population that is positive for both Annexin V and PI will be
detected. In the early stage of apoptosis, although
phosphatidylserine also externalizes on the cell membrane, the
integrity of the cell membrane remains intact initially. At this
point, cells exhibit Annexin V-positive and PI-negative staining.
In the present study, BC cells were pretreated with Z-VAD-FMK,
Necrostatin-1 or Z-DEVD-FMK, and the proportion of cell death was
detected using Annexin V/PI staining. The findings of present study
suggested that cell death induced by SNS-032 is not due to
necroptosis. However, the pan-caspase inhibitor, Z-VAD-FMK,
significantly inhibited late-stage apoptosis induced by SNS-032,
while Z-DEVD-FMK almost completely inhibited its anticancer effect.
Meanwhile, SNS-032 combined with DAC could further increase the
proportion of late-stage apoptotic cells. In addition, in BC cells,
SNS-032 significantly increased LDH release and decreased
intracellular ATP, and these effects were markedly enhanced in
combination with DAC. Z-DEVD-FMK was used to inhibit the activation
of caspase-3 in BC cells treated with SNS-032. Consequently, the
characteristic morphological features of pyroptosis in MCF-7 and
4T1 cells were notably alleviated and the protein expression levels
of cleaved caspase-3 and GSDME-N were markedly decreased compared
with SNS-032 treatment alone. Therefore, pretreatment with
Z-DEVD-FMK effectively reduced pyroptosis in BC cells treated with
SNS-032.
Western blotting was used to evaluate caspase-3 and
GSDME protein expression in BC cells during treatment with SNS-032.
The results demonstrated that caspase-3 was activated, GSDME was
cleaved and GSDME N-terminus protein was significantly increased
compared with the negative control group. Furthermore, DAC
treatment enhanced GSDME-FL expression and when combined with
SNS-032, the expression of GSDME-N was higher compared with the
SNS-032 group. These findings suggest that SNS-032 triggers
pyroptosis in MCF-7 and 4T1 cells via the caspase-3/GSDME signaling
pathway.
The study indicated that SNS-032 decreased the
expression of BCL-2, and increased the expression of BAX, and those
changes became more apparent when combined with DAC. These data
demonstrate that SNS-032 can trigger the pyroptosis in MCF-7 and
4T1 cells through the caspase-3/GSDME pathway, and DAC can boost
the expression of GSDME protein by methylation, thus enhancing
pyroptosis induced by SNS-032 in BC cells. In conclusion, the
present article demonstrates that the CDK2/7/9 inhibitor SNS-032
can induce pyroptosis and apoptosis of human BC MCF-7 cells and
mouse BC 4T1 cells through the caspase-3/GSDME pathway in
vitro, thereby reducing the viability of cancer cells,
providing a potential new strategy for the treatment of BC.
SNS-032 as a CDK inhibitor, may have potential
off-target effects such as the inhibition of other kinases with
similar structures or functions. This might lead to unintended
consequences in cellular processes which are not associated with
the expected target. Another effect of SNS-032 could be its impact
on transcriptional regulation which might affect the expression of
genes not directly associated with its primary mechanism of action.
In addition, it might potentially alter the overall cellular gene
expression profile and lead to unforeseen physiological changes or
cytotoxicity in cells that are not the target of treatment.
Furthermore, there are considerable clinical limitations regarding
CDK inhibitors. As low-dose CDK inhibitors are ineffective, highly
selective CDK inhibitors require higher doses, making the side
effects of CDK inhibitors in clinical trials a limiting factor
(52).
CDK inhibitors are tyrosine kinase inhibitors, with
a main concern being potential acquired resistance during treatment
(53). Future research should
utilize a number of strategies to address these potential
off-target impacts. Firstly, high-throughput screening techniques,
such as proteomics and genomics assays, can be utilized to
comprehensively map the interactome of SNS-032. By identifying
proteins and genes with which it interacts, on-target and
off-target effects can be differentiated more precisely. In order
to improve clinical treatment efficacy and prevent needless drug
exposure that leads to drug resistance, biomarkers associated with
CDK inhibitor resistance should be assessed in patient blood or
tumor tissues. These could then be used to screen patient groups
that are less likely to develop resistance and are most likely to
benefit from SNS-032 treatment. Secondly, structure-activity
relationship studies should be conducted. By implementing chemical
modifications to SNS-032, analogs with improved selectivity for the
intended target may be developed, thereby minimizing interactions
with off-target molecules and delaying the emergence of resistance.
In addition, SNS-032 can be combined with drugs that have different
mechanisms of action, such as targeting tumor angiogenesis, to
treat the tumor from multiple directions to diminish the
possibility of developing drug resistance (54). Immunomodulatory drugs, for instance
immune checkpoint inhibitors, can be used to stimulate the body's
immune system, synergizing with the inflammatory response induced
by SNS-032-induced pyroptosis, to attack tumor cells that are
resistant to treatment. Thirdly, it is imperative to utilize more
physiologically relevant in vitro and in vivo models.
For instance, patient-derived organoids or genetically engineered
mouse models can offer a more precise evaluation of off-target
effects, particularly those that are tissue-specific or
disease-specific. Finally, long-term follow-up studies in both
preclinical and clinical settings are crucial. Monitoring treated
subjects over an extended duration can facilitate the detection of
any delayed or cumulative off-target effects that may not be
immediately evident. This approach allows for the optimization of
dosage and treatment regimens aimed at reducing off-target toxicity
while maximizing therapeutic efficacy.
The present study established that SNS-032 can cause
apoptosis and pyroptosis of BC cells through caspase-3. Further
research is required to establish the detailed mechanism underlying
SNS-032-induced pyroptosis. Additionally, whether the antitumor
activity of DAC in combination with SNS-032 could be utilized in
in vivo experiments should be studied in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by the National Natural Science
Foundation of China (grant no 82172717).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The present study was proposed and designed by YC
and LX. YC and DZ performed the experiments. Data collection and
analysis were performed by JL, JW and YS. The manuscript was
drafted by YC and checked by LX. YC and DZ confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATP
|
adenosine 5′-triphosphate
|
|
BAX
|
Bcl-2-associated X protein
|
|
BC
|
breast cancer
|
|
BCL-2
|
B-cell lymphoma-2
|
|
CLL
|
chronic lymphocytic leukemia
|
|
DAC
|
decitabine
|
|
DMSO
|
dimethyl sulfoxide
|
|
FITC
|
fluorescein isothiocyanate
|
|
GSDM
|
gasdermin
|
|
GSDME
|
gasdermin E
|
|
GZM
|
granzyme
|
|
LDH
|
lactate dehydrogenase
|
|
ns
|
not significant
|
|
PBS
|
phosphate-buffered saline
|
|
PCD
|
programmed cell death
|
|
PI
|
propidium iodide
|
|
PVDF
|
polyvinylidene difluoride
|
References
|
1
|
World Health Organization, . Global breast
cancer initiative implementation framework: assessing,
strengthening and scaling up of services for the early detection
and management of breast cancer: executive summary. World Health
Organization. 2023.
|
|
2
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Britt KL, Cuzick J and Phillips KA: Key
steps for effective breast cancer prevention. Nat Rev Cancer.
20:417–436. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000-14 (CONCORD-3): Analysis of individual records for 37 513 025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kerr AJ, Dodwell D, McGale P, Holt F,
Duane F, Mannu G, Darby SC and Taylor CW: Adjuvant and neoadjuvant
breast cancer treatments: A systematic review of their effects on
mortality. Cancer Treat Rev. 105:1023752022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anampa J, Makower D and Sparano JA:
Progress in adjuvant chemotherapy for breast cancer: An overview.
BMC Med. 13:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frank D and Vince JE: Pyroptosis versus
necroptosis: Similarities, differences, and crosstalk. Cell Death
Differ. 26:99–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kovacs SB and Miao EA: Gasdermins:
Effectors of pyroptosis. Trends Cell Biol. 27:673–684. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du T, Gao J, Li P, Wang Y, Qi Q, Liu X, Li
J, Wang C and Du L: Pyroptosis, metabolism, and tumor immune
microenvironment. Clin Transl Med. 11:e4922021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Xia S, Zhang Z, Wu H and Lieberman
J: Channelling inflammation: Gasdermins in physiology and disease.
Nat Rev Drug Discov. 20:384–405. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding J, Wang K, Liu W, She Y, Sun Q, Shi
J, Sun H, Wang DC and Shao F: Pore-forming activity and structural
autoinhibition of the gasdermin family. Nature. 535:111–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao
C, Liu W, Deng H, Li J, Ning P and Wang Z: Pyroptosis in
inflammatory diseases and cancer. Theranostics. 12:4310–4329. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rogers C, Fernandes-Alnemri T, Mayes L,
Alnemri D, Cingolani G and Alnemri ES: Cleavage of DFNA5 by
caspase-3 during apoptosis mediates progression to secondary
necrotic/pyroptotic cell death. Nat Commun. 8:141282017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia X, Wang X, Cheng Z, Qin W, Lei L,
Jiang J and Hu J: The role of pyroptosis in cancer: Pro-cancer or
pro-‘host’? Cell Death Dis. 10:6502019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen L, Weng B, Li H, Wang H, Li Q, Wei X,
Deng H, Wang S, Jiang C, Lin R and Wu J: A thiopyran derivative
with low murine toxicity with therapeutic potential on lung cancer
acting through a NF-kappaB mediated apoptosis-to-pyroptosis switch.
Apoptosis. 24:74–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou CB and Fang JY: The role of
pyroptosis in gastrointestinal cancer and immune responses to
intestinal microbial infection. Biochim Biophys Acta Rev Cancer.
1872:1–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng H, Yang H, Song Y, Fang D, Chen L,
Zhao Z, Wang C and Xie S: Transcriptional inhibition by CDK7/9
inhibitor SNS-032 suppresses tumor growth and metastasis in
esophageal squamous cell carcinoma. Cell Death Dis. 12:10482021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu
X, Junqueira C, Meza-Sosa KF, Mok TMY, Ansara J, et al: Gasdermin E
suppresses tumour growth by activating anti-tumour immunity.
Nature. 579:415–420. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thompson DA and Weigel RJ:
Characterization of a gene that is inversely correlated with
estrogen receptor expression (ICERE-1) in breast carcinomas. Eur J
Biochem. 252:169–177. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akino K, Toyota M, Suzuki H, Imai T,
Maruyama R, Kusano M, Nishikawa N, Watanabe Y, Sasaki Y, Abe T, et
al: Identification of DFNA5 as a target of epigenetic inactivation
in gastric cancer. Cancer Sci. 98:88–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim MS, Chang X, Yamashita K, Nagpal JK,
Baek JH, Wu G, Trink B, Ratovitski EA, Mori M and Sidransky D:
Aberrant promoter methylation and tumor suppressive activity of the
DFNA5 gene in colorectal carcinoma. Oncogene. 27:3624–3634. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen R, Wierda WG, Chubb S, Hawtin RE, Fox
JA, Keating MJ, Gandhi V and Plunkett W: Mechanism of action of
SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic
lymphocytic leukemia. Blood. 113:4637–4645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Liu S, Ye Q and Pan J:
Transcriptional inhibition by CDK7/9 inhibitor SNS-032 abrogates
oncogene addiction and reduces liver metastasis in uveal melanoma.
Mol Cancer. 18:1402019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Y, Chen C, Sun X, Shi X, Jin B, Ding K,
Yeung SC and Pan J: Cyclin-dependent kinase 7/9 inhibitor SNS-032
abrogates FIP1-like-1 platelet-derived growth factor receptor alpha
and bcr-abl oncogene addiction in malignant hematologic cells. Clin
Cancer Res. 18:1966–1978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng H, Jin Y, Liu H, You L, Yang C, Yang
X and Qian W: SNS-032 inhibits mTORC1/mTORC2 activity in acute
myeloid leukemia cells and has synergistic activity with perifosine
against Akt. J Hematol Oncol. 6:182013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang MA, Kim W, Jo HR, Shin YJ, Kim MH and
Jeong JH: Anticancer and radiosensitizing effects of the
cyclin-dependent kinase inhibitors, AT7519 and SNS-032, on cervical
cancer. Int J Oncol. 53:703–712. 2018.PubMed/NCBI
|
|
29
|
Kodym E, Kodym R, Reis AE, Habib AA, Story
MD and Saha D: The small-molecule CDK inhibitor, SNS-032, enhances
cellular radiosensitivity in quiescent and hypoxic non-small cell
lung cancer cells. Lung Cancer. 66:37–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heath EI, Bible K, Martell RE, Adelman DC
and Lorusso PM: A phase 1 study of SNS-032 (formerly BMS-387032), a
potent inhibitor of cyclin-dependent kinases 2, 7 and 9
administered as a single oral dose and weekly infusion in patients
with metastatic refractory solid tumors. Invest New Drugs.
26:59–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tong WG, Chen R, Plunkett W, Siegel D,
Sinha R, Harvey RD, Badros AZ, Popplewell L, Coutre S and Fox JA:
Phase I and pharmacologic study of SNS-032, a potent and selective
Cdk2, 7, and 9 inhibitor, in patients with advanced chronic
lymphocytic leukemia and multiple myeloma. J Clin Oncol.
28:3015–3022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie G, Tang H, Wu S, Chen J, Liu J and
Liao C: The cyclin-dependent kinase inhibitor SNS-032 induces
apoptosis in breast cancer cells via depletion of Mcl-1 and
X-linked inhibitor of apoptosis protein and displays antitumor
activity in vivo. Int J Oncol. 45:804–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeng H, Yang H, Song Y, Fang D, Chen L,
Zhao Z, Wang C and Xie S: Transcriptional inhibition by CDK7/9
inhibitor SNS-032 suppresses tumor growth and metastasis in
esophageal squamous cell carcinoma. Cell Death Dis. 12:10482021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miao EA, Rajan JV and Aderem A:
Caspase-1-induced pyroptotic cell death. Immunol Rev. 243:206–214.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ai Y, Meng Y, Yan B, Zhou Q and Wang X:
The biochemical pathways of apoptotic, necroptotic, pyroptotic, and
ferroptotic cell death. Mol Cell. 84:170–179. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kesavardhana S, Malireddi RKS and
Kanneganti TD: Caspases in cell death, inflammation, and
pyroptosis. Annu Rev Immunol. 38:567–595. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marino G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang B, Li H, Yang R, Zhou S and Zou S:
Decitabine inhibits the cell growth of cholangiocarcinoma in
cultured cell lines and mouse xenografts. Oncol Lett. 8:1919–1924.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mi D, Li J, Wang R, Li Y, Zou L, Sun C,
Yan S, Yang H, Zhao M and Shi S: Postsurgical wound management and
prevention of triple-negative breast cancer recurrence with a
pryoptosis-inducing, photopolymerizable hydrogel. J Control
Release. 356:205–218. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Parua PK and Fisher RP: Dissecting the Pol
II transcription cycle and derailing cancer with CDK inhibitors.
Nat Chem Biol. 16:716–724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Swaffer MP, Jones AW, Flynn HR, Snijders
AP and Nurse P: CDK substrate phosphorylation and ordering the cell
cycle. Cell. 167:1750–1761. e17162016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang F, Bettadapura SN, Smeltzer MS, Zhu H
and Wang S: Pyroptosis and pyroptosis-inducing cancer drugs. Acta
Pharmacol Sin. 43:2462–2473. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen W, Yang KB, Zhang YZ, Lin ZS, Chen
JW, Qi SF, Wu CF, Feng GK, Yang DJ, Chen M, et al: Synthetic
lethality of combined ULK1 defection and p53 restoration induce
pyroptosis by directly upregulating GSDME transcription and
cleavage activation through ROS/NLRP3 signaling. J Exp Clin Cancer
Res. 43:2482024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Su L, Chen Y, Huang C, Wu S, Wang X, Zhao
X, Xu Q, Sun R, Kong X, Jiang X, et al: Targeting Src reactivates
pyroptosis to reverse chemoresistance in lung and pancreatic cancer
models. Sci Transl Med. 15:eabl78952023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang S, Zhang Y, Feng Y, Wu J, Hu Y, Lin
L, Xu C, Chen J, Tang Z, Tian H and Chen X: Biomineralized
two-enzyme nanoparticles regulate tumor glycometabolism inducing
tumor cell pyroptosis and robust antitumor immunotherapy. Adv
Mater. 34:e22068512022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pan J, Li Y, Gao W, Jiang Q, Geng L, Ding
J, Li S and Li J: Transcription factor Sp1 transcriptionally
enhances GSDME expression for pyroptosis. Cell Death Dis.
15:662024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Broz P, Pelegrin P and Shao F: The
gasdermins, a protein family executing cell death and inflammation.
Nat Rev Immunol. 20:143–157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Y, Chen X, Gueydan C and Han J:
Plasma membrane changes during programmed cell deaths. Cell Res.
28:9–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Man SM, Karki R and Kanneganti TD:
Molecular mechanisms and functions of pyroptosis, inflammatory
caspases and inflammasomes in infectious diseases. Immunol Rev.
277:61–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xie B, Liu T, Chen S, Zhang Y, He D, Shao
Q, Zhang Z and Wang C: Combination of DNA demethylation and
chemotherapy to trigger cell pyroptosis for inhalation treatment of
lung cancer. Nanoscale. 13:18608–18615. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gong W, Fang P, Leng M and Shi Y:
Promoting GSDME expression through DNA demethylation to increase
chemosensitivity of breast cancer MCF-7/Taxol cells. PLoS One.
18:e02822442023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bose P, Simmons GL and Grant S:
Cyclin-dependent kinase inhibitor therapy for hematologic
malignancies. Expert Opin Investig Drugs. 22:723–738. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bixby D and Talpaz M: Seeking the causes
and solutions to imatinib-resistance in chronic myeloid leukemia.
Leukemia. 25:7–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Uzhachenko RV, Bharti V, Ouyang Z, Blevins
A, Mont S, Saleh N, Lawrence HA, Shen C, Chen SC, Ayers GD, et al:
Metabolic modulation by CDK4/6 inhibitor promotes
chemokine-mediated recruitment of T cells into mammary tumors. Cell
Rep. 35:1089442021. View Article : Google Scholar : PubMed/NCBI
|