Introduction
Lung cancer remains the leading cause of
cancer-related deaths worldwide, with an estimated 2 million new
cases diagnosed each year (1). Of
patients with lung cancer, ~85% are attributed to non-small cell
lung cancer (NSCLC) (2), which is
further classified into lung adenocarcinoma (LUAD) and lung
squamous cell carcinoma. Notably, 30% patients with NSCLC are
diagnosed at advanced stages (3),
reducing the likelihood of effective intervention and leading to
poor treatment outcomes. With a 5-year survival rate of only 15%,
the prognosis for patients with NSCLC remains poor and recurrence
is common (2,4). Tobacco smoking remains the primary
etiological factor for all lung cancer subtypes, with research
indicating that smoking contributes to >80% of lung cancer cases
in numerous nations (5).
Consequently, smoking cessation is considered to be crucial in the
prevention of lung cancer, necessitating ongoing enhancements in
treatment strategies.
In this context, cisplatin, a chemotherapy agent
used for >30 years, has shown varying efficacy across different
cancer types. Particularly, cisplatin has emerged as the
established treatment standard for advanced NSCLC (6). Cisplatin has the ability to undergo
hydration within cancer cells, acquiring a positive charge that
enables its integration into DNA to create cross-links and disrupt
the DNA structure, leading to apoptosis of cancer cells (7,8). Lung
cancer comprises cells or clonal populations with unique molecular
profiles, resulting in intratumoral diversity and vulnerability to
genetic alterations, which markedly impede the therapeutic efficacy
of cisplatin (9). Therefore,
developing novel treatment approaches is essential to enhance the
therapeutic potential of cisplatin and improve the prognosis of
patients with NSCLC.
Among various drug candidates, Chinese herbal
medicine has a history of >50 years in the treatment of lung
cancer (10). A number of approved
cancer therapies are modified natural products or semi-synthetic
derivatives, including compounds such as camptothecin, paclitaxel
and vincristine (11). In addition,
numerous monomers derived from Traditional Chinese Medicine possess
individual anticancer properties. For instance, flavonoids such as
luteolin and baicalein (12),
alkaloids such as berberine and oxymatrine (13) and terpenoids, such as triptolide and
andrographolide (14), all
demonstrate therapeutic potential, along with other compounds such
polyphenols, anthraquinones and polysaccharides (15). Compared with conventional treatments
for NSCLC, Chinese herbal medicines offer a distinct and innovative
pharmacological mechanism, accompanied by lower toxicity (16,17).
Notably, integrative medicine is effective in the management of
NSCLC, improving the efficacy of chemotherapy and radiotherapy
while reducing side effects such as bone marrow suppression, nausea
and vomiting (18).
Glycyrrhizin is a triterpenoid compound that is
extracted from the roots of the licorice plant, which initially
gained attention for its antiviral and anti-inflammatory effects
(19). In previous years, studies
(20–22) have reported that glycyrrhizin also
possesses notable anticancer properties, demonstrating antitumor
effects across several cancer types. Its primary mechanism of
action involves increasing reactive oxygen species levels,
activating caspase-3 and reducing the mitochondrial membrane
potential, leading to cell cycle arrest and DNA damage in cancer
cells, thereby inhibiting cell proliferation. For instance, in
leukemia, glycyrrhizin inhibits the AKT/mTOR/STAT3 signaling
pathway, leading to a reduction in the expression of cyclin D1 and
survivin, thereby promoting apoptosis (23). In gastric cancer, glycyrrhizin
inhibits the phosphorylation of the PI3K/AKT pathway and reduces
the expression of cyclin D1, survivin and p65. In addition,
glycyrrhizin enhances the activity of B-cell lymphoma 2-associated
X protein (Bax) and poly(ADP-ribose) polymerase and subsequently
inhibits cancer cell proliferation and induces apoptosis (24).
Glycyrrhizin has also demonstrated potential in the
treatment of NSCLC. Research has indicated that glycyrrhizin can
inhibit the growth of LUAD cells (25). Notably, Deng et al (26) demonstrated that glycyrrhizin not
only enhances the antitumor effect of cisplatin in LUAD xenograft
mice but also reduces liver and kidney damage caused by cisplatin
treatment. These studies suggest that glycyrrhizin may serve as an
adjuvant to cisplatin, improving the therapeutic efficacy of NSCLC
while minimizing the side effects of cisplatin. However, the
molecular mechanisms underlying the combined use of glycyrrhizin
and cisplatin remain unclear and require further investigation.
Materials and methods
Cell culture and grouping
A549 cells, a NSCLC cell line (American Type Culture
Collection no. #CCL-185), were purchased from the Cell Resource
Center of Peking Union Medical College (Beijing, China). Prior to
experiments, the cells were subjected to polymerase chain reaction
analysis to ensure that they were free from mycoplasma
contamination. Subsequently, A549 cells were maintained in Roswell
Park Memorial Institute 1640 medium (cat. no R8758; Sigma-Aldrich;
Merck KGaA) supplemented with 10% fetal bovine serum (FBS; cat. no
12103C; Sigma-Aldrich; Merck KGaA), 100 U/ml penicillin (cat. no
P4458; Sigma-Aldrich; Merck KGaA) and 100 µg/ml streptomycin (cat.
no S9137; Sigma-Aldrich; Merck KGaA). Cell culture was performed in
a 37°C cell culture incubator (cat. no COI-160-ST; Beijing Labgic
Technology Co., Ltd.) with 5% CO2. Upon reaching 80–90%
confluence, the cells were subcultured and those that had undergone
three consecutive passages were utilized for experimental purposes.
To evaluate the effect of glycyrrhizin on the sensitivity of A549
cells to cisplatin, cells in each group were randomly assigned to
different treatment conditions. The control group consisted of
untreated A549 cells. The treatment groups received cisplatin (20
µM) (21), glycyrrhizin (2 mM)
(19) or a combination of both
drugs at the same concentration. The cells were incubated at 37°C
for 48 h before harvest for further analysis. Cisplatin and
glycyrrhizin were purchased from Sigma-Aldrich (Merck KGaA).
MTT assay
The MTT assay kit (cat. no E-CK-A341; Wuhan
Elabscience Biotechnology Co., Ltd.) was employed to assess the
viability of the normal human lung epithelial cell line BEAS-2B
(American Type Culture Collection) and A549 cells under different
treatment conditions. BEAS-2B cells were used as a normal control
to evaluate the cytotoxicity of glycyrrhizin and cisplatin. BEAS-2B
cells and A549 cells were seeded into 96-well plates at a density
of 2×104 cells/ml, with a volume of 200 µl per well. The
plates were randomly assigned to experimental groups and placed in
an incubator until the cells were fully adhered. Experimental
groups were then treated with several concentrations of
glycyrrhizin (0, 0.25, 0.5, 1, 2, 4 and 8 mM) and cisplatin (0, 10,
20, 40 and 160 µM), either alone or in combination. After 48 h
incubation, 20 µl MTT solution (5 mg/ml) was added to each well.
Following 4 h incubation, 100 µl dimethyl sulfoxide was added to
the wells and the optical density at 490 nm was measured using an
iMark microplate reader (Bio-Rad Laboratories, Inc.) to determine
cell viability. The viability of cells in each experimental group
was calculated relative to the control group, which exhibited 100%
viability for A549 cells, while BEAS-2B cells served as a
comparative indicator for the cytotoxicity of treatments.
Cell colony formation assay
A cell colony formation assay was utilized to
evaluate the effect of cisplatin and glycyrrhizin, both separately
and in combination, on the colony-forming ability of A549 cells.
Briefly, A549 cells were seeded into 12-well plates at a density of
500 cells/ml, with 1 ml per well. After 24 h incubation in a cell
culture incubator, the cells were randomly assigned to different
treatment groups and exposed to cisplatin (20 µM), glycyrrhizin (2
mM) or a combination of both. Following 48 h incubation, the cells
were washed with sterile phosphate-buffered saline (PBS) buffer
(cat no. ST447-1L; Beyotime Institute of Biotechnology),
replenished with fresh complete medium and further incubated in a
cell culture incubator at 37°C for 14 days to allow colony
formation. The cells were subsequently fixed with a fixative
solution (1:7 mixture of 100% acetic acid and pure methanol) at
room temperature for 20 min. After washing the cells three times
with sterile PBS, they were stained with 0.5% crystal violet
solution (cat. no C805211; Shanghai Macklin Biochemical Co., Ltd.)
at room temperature for 1 h, rinsed with distilled water and
observed under a light microscope (XSP; Zhejiang Lichen Instrument
Technology Co., Ltd.). Finally, the images were captured and the
colonies were quantified using ImageJ software (National Institutes
of Health; version 1.53). A colony was defined as >50 cells that
formed after 14 days of incubation.
Apoptosis assay
The effects of cisplatin, glycyrrhizin and their
combination on apoptosis levels in A549 cells were assessed using
the Annexin V Alexa Fluor™ 647/Propidium Iodide (PI)
Apoptosis Assay kit (cat. no AC10862; Shanghai Acmec Biochemical
Co., Ltd.) to detect early and late apoptosis. Firstly, A549 cells
were cultured in 6-well plates at a density of 8×105
cells/ml in 1 ml of medium and incubated in a cell culture
incubator for 24 h. Following incubation, cells were treated with
cisplatin (20 µM), glycyrrhizin (2 mM) or both for 48 h. After
treatment, cells were harvested, centrifuged at 300 × g for 5 min
at 37°C, washed twice with PBS and adjusted to a concentration of
1×106 cells/ml using culture medium. Subsequently, the
treated cells were resuspended in 100 µl PBS, stained with 5 µl
annexin V/Alexa Fluor™ 647 and 10 µl of 20 µg/ml PI
solution and incubated for 15 min at room temperature in the
absence of light. Controls included unstained, single-stained and
double-stained samples to ensure proper gating and compensation.
Subsequently, the cells were analyzed using a CytoFLEX S flow
cytometer (Beckman Coulter, Inc.) and the data were processed with
CytExpert software (version 2.3; Beckman Coulter, Inc.).
Fluorescence intensities were measured for FITC (annexin V) and PE
(PI) signals at an excitation wavelength of 488 nm and an emission
wavelength of 530 nm in 400 µl PBS.
Cell cycle assay
The effects of cisplatin and glycyrrhizin
administered individually or in combination on the cell cycle of
A549 cells were evaluated using Cell Cycle Staining Kit (cat. no
E-CK-A351; Wuhan Elabscience Biotechnology Co., Ltd.). A549 cells
were seeded in 6-well culture plates in a volume of 1 ml and a
density of 8×105 cells/ml for 24 h. They were randomly
assigned to different treatment groups and treated with cisplatin
(20 µM), glycyrrhizin (2 mM) or a combination of cisplatin (20 µM)
and glycyrrhizin (2 mM). After 48 h incubation, the cells were
collected in centrifuge tubes and centrifuged at 300 × g for 5 min
at 37°C. Subsequently, the cell pellet was washed twice with PBS,
adjusted to a concentration of 5×105 cells/ml using PBS
and resuspended in 300 µl PBS. To fix the cells, 1.2 ml ice-cold
ethanol (−20°C) was added, mixed thoroughly and the suspension was
stored at −20°C overnight. The fixed cells were centrifuged at 300
× g for 5 min at 4°C, resuspended in 1 ml PBS, and left to stand
for 15 min. After a second centrifugation at 300 × g for 5 min at
4°C, the pellet was resuspended in 100 µl RNase A reagent and
incubated in a 37°C water bath for 30 min. Subsequently, 400 µl PI
reagent (50 µg/ml) was added, mixed thoroughly and incubated at 4°C
in the dark for 30 min. Lastly, the intensity of PE signals
(excitation, 488 mm; emission, 530 nm) was measured using flow
cytometry (CytoFLEX S; Beckman Coulter, Inc.). The data were
analyzed using FlowJo software (version 10.6.2; BD
Biosciences).
Comet assay
Comet Assay kits (cat. no C2041S, Beyotime Institute
of Biotechnology) were used to assess the impact of cisplatin and
glycyrrhizin, both individually and in combination, on DNA damage
in A549 cells. A549 cells were initially seeded in 6-well culture
plates at a density of 8×105 cells/ml with a seeding
volume of 1 ml and incubated for 24 h. Cells were then randomly
assigned to different treatment groups and treated with cisplatin
(20 µM), glycyrrhizin (2 mM) or a combination of both for 48 h.
Subsequently, the cells were harvested, centrifuged at 300 × g for
5 min at 37°C, washed twice with PBS and adjusted to a
concentration of 1×106 cells/ml. The cells were then
lysed using lysis buffer (Beyotime Institute of Biotechnology), DNA
was unwound under alkaline conditions and gel electrophoresis was
conducted at 4°C using a 1% agarose gel at low voltage (25 V) for
30 min. Subsequently, neutral buffer (Beyotime Biotechnology) was
added, followed by staining with 20 µl PI solution for 20 min at
room temperature in the absence of light. Finally, DNA damage was
assessed by analyzing DNA levels and comet tail length using a
fluorescence microscope (excitation, 535 nm; emission, 617 nm; IX73
microscope; Olympus Corporation). The images were analyzed using
ImageJ software (version 1.53u; National Institutes of Health).
Western blotting
A549 cells were cultured with cisplatin (20 µM)
and/or glycyrrhizin (2 mM), after which the cell culture medium was
removed and the cells were washed twice with pre-chilled PBS. The
cells were then collected into sterile centrifuge tubes, lysed in 1
ml radioimmunoprecipitation assay buffer (cat. no P0013C; Beyotime
Institute of Biotechnology) and subjected to sonication for 10 min
at 20 kHz and on ice. Lysates were centrifuged at 12,000 × g for 10
min at 4°C and the supernatant was collected and kept on ice. Total
protein concentration was measured using the Bicinchoninic Acid
Protein Assay kit (cat. no P0010S; Beyotime Institute of
Biotechnology). For analysis, 20 µg total protein was mixed with
sodium dodecyl sulfate loading buffer (Beyotime Institute of
Biotechnology) and denatured at 95°C for 10 min. Proteins were
separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (cat. no E302-01; Vazyme Biotech Co., Ltd.) at 110
V for 1 h, with 40 µg protein loaded in each lane, and transferred
onto a polyvinylidene fluoride membrane (cat. no IPVH00010;
MilliporeSigma) at 90 V. The membrane was then blocked with 5%
skimmed milk at room temperature for 1 h and incubated with primary
antibodies overnight at 4°C. The primary antibodies (all BIOSS)
used included Bax (1:1,000; cat. no bsm-60772R), B-cell lymphoma 2
(Bcl-2; 1:1,000; cat. no bsm-33411M), cleaved-caspase-3 (1:1,000;
cat. no bsm-33199M), caspase-3 (1:1,000; cat. no bsm-61071R),
cyclin D1 (1:1,000; cat. no bs-20596R), cyclin-dependent kinase
(CDK) 2 (1:1,000; cat. no bs-0757R), CDK4 (1:1,000; cat. no
bs-0633R), γH2AX (1:1,000; cat. no bs-2560R), checkpoint kinase 1
(Chk1; 1:1,000; cat. no bs-1681R), phosphorylated-Chk1 (p-Chk1;
1:1,000; cat. no bs-13906R), p53 (1:1,000; cat. no bs-4181R),
phosphorylated p53 (p-p53; 1:1,000; cat. no bs-3710R) and β-actin
(1:1,000; cat. no bs-0061R). The membrane was incubated at room
temperature for 1 h with goat anti-rabbit (1:5,000; cat. no
bs-0295G-HRP) or anti-mouse (1:5,000; cat. no bs-0368G-HRP)
secondary antibodies (BIOSS). Protein bands were visualized using
enhanced chemiluminescence (SuperSignal ECL; cat. no 34580; Thermo
Fisher Scientific, Inc.). In addition, β-actin served as the
internal control to calculate the relative expression of the target
protein using Image J software (version 1.53u; National Institutes
of Health).
Statistical analysis
The study findings were derived from ≥3 independent
experiments and analyzed using SPSS software (version 23.0; IBM
Corp.). For comparisons among multiple groups, one-way analysis of
variance followed by Tukey's post hoc test was used. The normality
was tested using the Shapiro-Wilk test, and P<0.05 was
considered to indicate a statistically significant difference.
Graphical outputs were generated using GraphPad Prism 9.2.0
(Dotmatics).
Results
Effects of glycyrrhizin and
combination with cisplatin to A549 cells
Glycyrrhizin (Fig.
1A) was initially recognized for its antiviral properties, with
a subsequent study reporting its anti-inflammatory and antitumor
activities (27). More recently,
glycyrrhizin has been demonstrated to effectively inhibit the
growth of LUAD cells (25). In
addition, glycyrrhizin has been identified as a compound capable of
overcoming cisplatin resistance in hepatocellular carcinoma when
combined with lamivudine (28).
These findings suggest that glycyrrhizin not only inhibits tumor
progression but also reduces cancer cell drug resistance. In the
present study, the effect of glycyrrhizin combined with cisplatin
on NSCLC cells was evaluated.
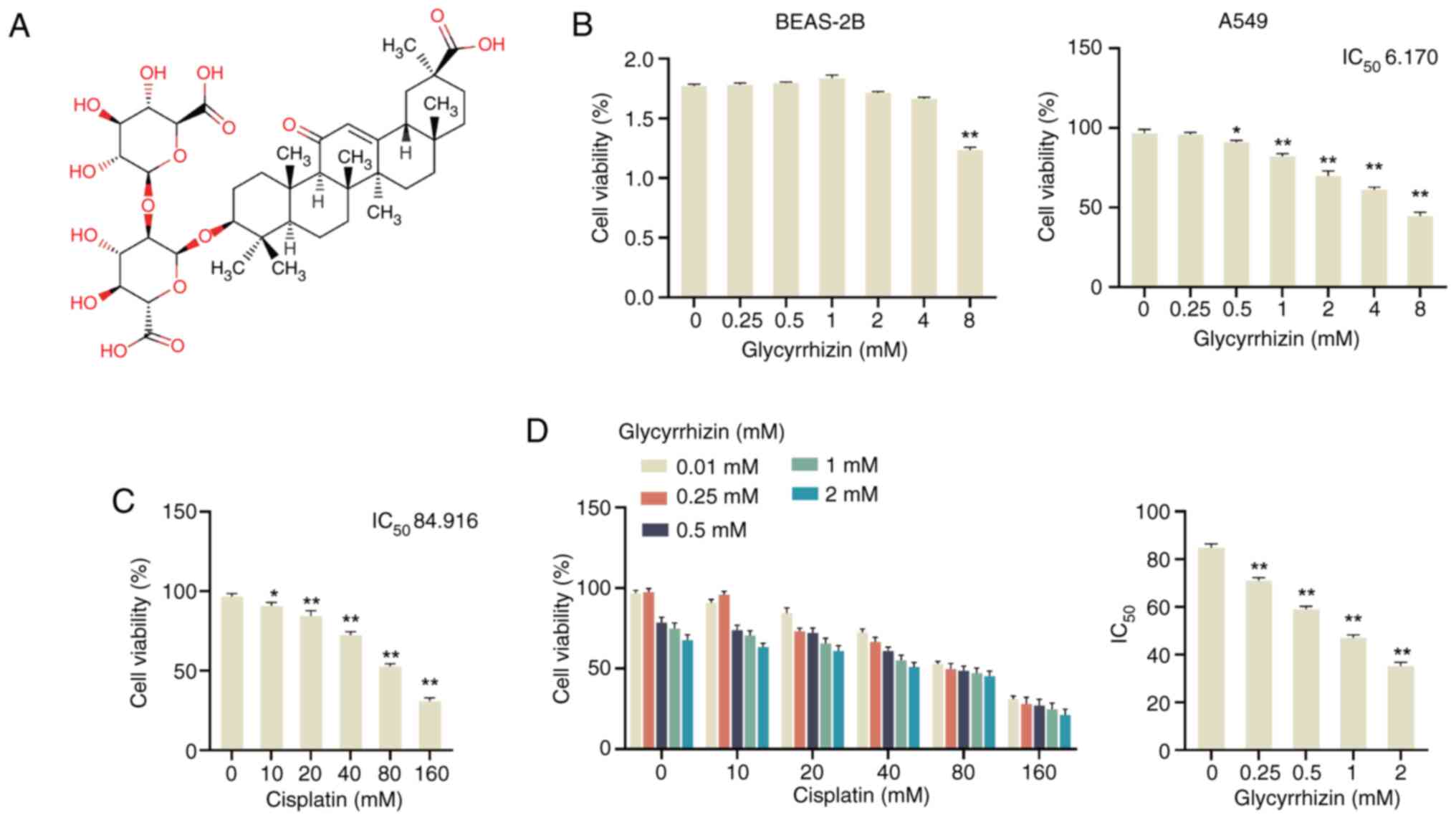 | Figure 1.Inhibition of glycyrrhizin and its
combination with cisplatin in A549 cells. (A) Chemical structure of
glycyrrhizin. MTT was used to detect the cell viability of (B)
BEAS-2B and (C) A549 cells after treatment with different
concentrations of glycyrrhizin (0, 0.25, 0.5, 1, 2, 4 and 8 mM) for
48 h, and the IC50 was calculated for A549 cells. (D)
MTT was used to detect the cell viability of A549 cells after
treatment with different concentrations of cisplatin (0, 10, 20,
40, 80 and 160 µM) for 48 h, and the IC50 was
calculated. (E) MTT was used to assess the cell viability of A549
cells after treatment with 0, 0.25, 0.5, 1 and 2 mM glycyrrhizin
and 0, 10, 20, 40, 80 and 160 µM cisplatin, respectively, and the
IC50 of cisplatin was calculated. Data are presented as
mean ± SD (n=3). *P<0.05, **P<0.01 vs. 0 mM. IC50,
half-maximal inhibitory concentration. |
Initially, to assess cytotoxicity, BEAS-2B and A549
cells were treated with glycyrrhizin (0–8 mM) and analyzed using
the MTT assay. Glycyrrhizin had minimal effect on BEAS-2B cell
viability, with significant changes observed only at higher
concentrations (8 mM) (Fig. 1B). In
contrast, glycyrrhizin treatment significantly reduced the
viability of A549 cells in a dose-dependent manner, with a
calculated half-maximal inhibitory concentration (IC50) value of
6.17 mM (Fig. 1C). Concurrently,
the impact of several concentrations of cisplatin on A549 cell
viability was evaluated, revealing a significant decrease in cell
viability when treated with 10, 20, 40, 80 and 160 µM of cisplatin
compared with 0 mM, with the calculated IC50 of
cisplatin for A549 cells being 84.916 mM (Fig. 1D). Subsequent investigations aimed
to explore the combined effect of both compounds on A549 cell
viability. The findings demonstrated a decrease in A549 cell
viability with increasing concentrations of both glycyrrhizin and
cisplatin. Notably, the IC50 of cisplatin significantly
decreased with increasing glycyrrhizin concentration compared with
0 mM, with the IC50 of cisplatin ~35 µM at 2 mM
glycyrrhizin (Fig. 1E). These
results indicate that glycyrrhizin significantly diminishes A546
cell viability and enhances the cytotoxic effect of cisplatin on
A546 cells.
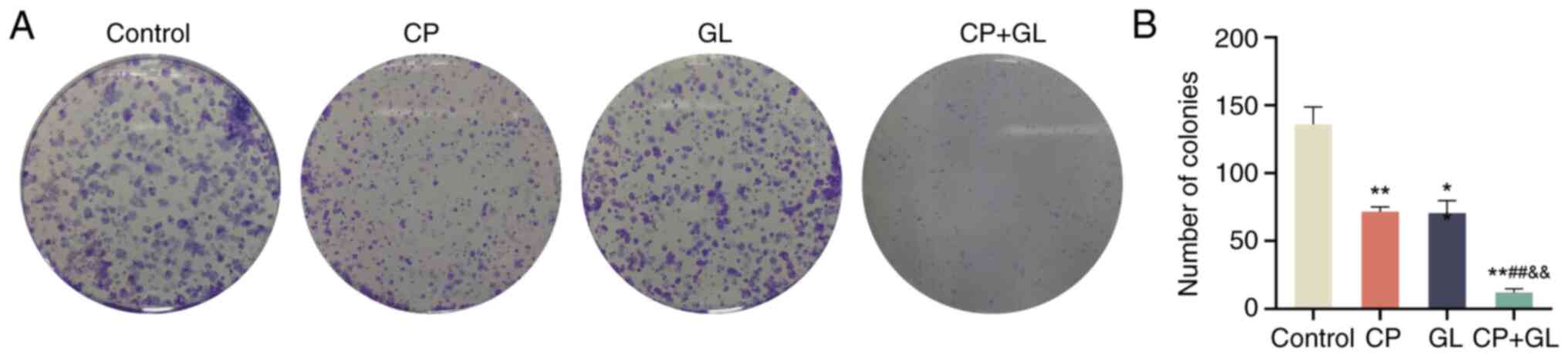
Glycyrrhizin enhances the inhibitory
effect of cisplatin on colony formation in A549 cells
In order to comprehensively assess the potential
synergistic impact of glycyrrhizin in enhancing the efficacy of
cisplatin against NSCLC, a colony formation assay was conducted on
A549 cells treated with glycyrrhizin, cisplatin or their
combination. The results of the cell colony formation assay
demonstrated a significant reduction in the colony-forming capacity
of A549 cells treated with glycyrrhizin alone, cisplatin alone or
the combination of glycyrrhizin and cisplatin when compared with
the control group (Fig. 2).
Notably, the colony-forming ability of A549 cells treated with
cisplatin and glycyrrhizin in combination was significantly lower
compared with that of cells treated with cisplatin or glycyrrhizin
alone (Fig. 2). These findings
indicate that glycyrrhizin may potentiate the inhibitory effects of
cisplatin on colony formation in A549 cells.
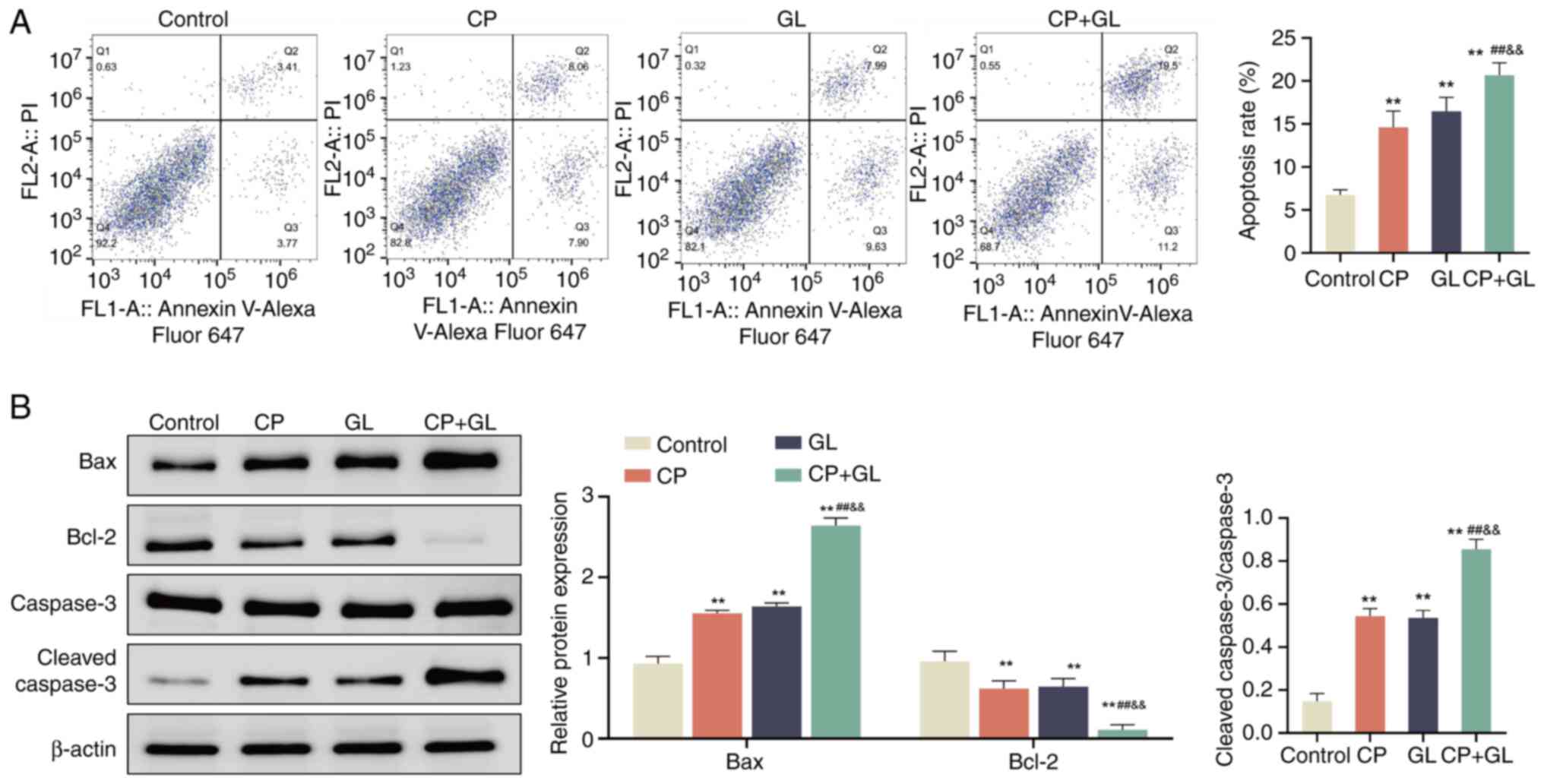
Glycyrrhizin enhances the effect of
cisplatin on apoptosis of A549 cells
The aforementioned findings indicate that
glycyrrhizin may serve as an adjuvant in NSCLC therapy. To explore
the mechanism by which glycyrrhizin enhances the tumor inhibitory
effects of cisplatin, further investigations were undertaken.
Previous studies have demonstrated that glycyrrhizin inhibits
monocytes (29), cervical cancer
cells (30) and prostate cancer
cells (31). Therefore, we
hypothesized that glycyrrhizin may potentiate the anticancer
properties of cisplatin by facilitating apoptosis in A549 cells.
Initially, annexin V Alexa Fluor™ 647/PI staining
analysis demonstrated that there was a significant increase in A549
cells treated with cisplatin, glycyrrhizin or their combination in
comparison with the control group. Notably, the combined therapy
induced a significantly higher apoptotic rate than either treatment
alone (Fig. 3A). Subsequent
analysis of apoptosis-related proteins indicated a significant
increase in the protein levels of Bax and
cleaved-caspase-3/caspase-3, along with a decrease in Bcl-2 levels,
in A549 cells treated with cisplatin, glycyrrhizin or their
combination compared with the control group. Furthermore, there was
a significant increase in Bax and cleaved-caspase-3/caspase-3 and a
decrease in Bcl-2 levels in the combined treatment group compared
with cisplatin and glycyrrhizin alone (Fig. 3B). These results suggest that
glycyrrhizin enhances apoptosis of A549 cells when combined with
cisplatin.
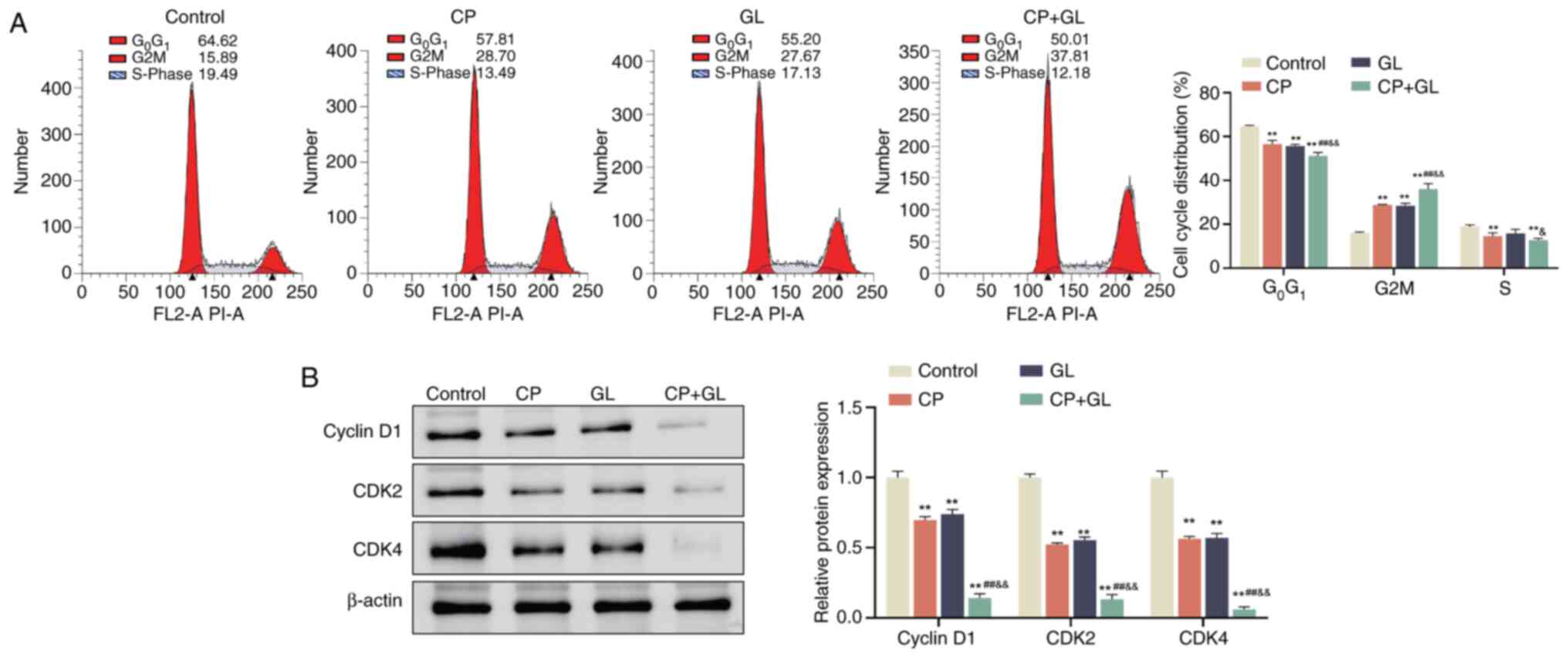
Glycyrrhizin enhances the effect of
cisplatin on the cell cycle of A549 cells
According to a previous study, there is a close link
between the cell cycle and apoptosis, and the level of apoptosis is
regulated by the cell cycle (32).
Therefore, the impact of the drug treatments on the cell cycle was
investigated using PI staining of A549 cells. The results
demonstrated alteration in the cell cycle distribution of A549
cells (Fig. 4A). Specifically, the
percentage of cells in the G0/G1 phase
decreased significantly and the proportion of cells in the
G2/M phase increased significantly upon treatment with
cisplatin and glycyrrhizin, either individually or in combination,
compared with the control group. Additionally, the S phase
population significantly decreased when A549 cells were treated
with cisplatin alone or in combination with glycyrrhizin compared
with the control group. Notably, treatment with the combination of
cisplatin and glycyrrhizin further significantly reduced the
percentage of cells in the G0/G1 phase while
increasing the G2/M phase population when compared with
either treatment alone (Fig.
4A).
Subsequent analysis using western blotting was
conducted to assess the expression levels of cell cycle-related
proteins. The analysis demonstrated a significant decrease in the
protein levels of cyclin D1, CDK2 and CDK4 in A549 cells treated
with cisplatin and glycyrrhizin individually or in combination
compared with the control group. Furthermore, the protein levels of
cyclin D1, CDK2 and CDK4 were significantly lower in A549 cells
exposed to cisplatin and glycyrrhizin together compared with those
treated with cisplatin or glycyrrhizin alone (Fig. 4B). These observations suggest that
glycyrrhizin augments the impact of cisplatin on the cell cycle of
A549 cells.
Glycyrrhizin enhances the effect of
cisplatin on DNA damage of A549 cells
DNA damage has been identified in prior studies as a
key factor contributing to genome instability, leading to either
cell cycle arrest or apoptosis via a cascade of molecular responses
(33). In the present study,
treatment with cisplatin or glycyrrhizin individually significantly
increased DNA damage in A549 cells compared with the control group,
as demonstrated by elevated comet tail DNA and comet tail distance.
Furthermore, the combination of cisplatin and glycyrrhizin resulted
in a significant increase in DNA damage, comet tail DNA and comet
tail distance compared with individual treatments (Fig. 5A). Subsequent western blotting
analysis revealed upregulation of DNA damage-associated proteins,
including γH2AX, p-Chk1/Chk1 and p-p53/p53, in cells treated with
either cisplatin or glycyrrhizin alone compared with the control
group, with further significant increases observed in the
combination treatment group compared with the treatments alone
(Fig. 5B). These results
demonstrate that glycyrrhizin potentiates the DNA damage in A549
cells induced by cisplatin.
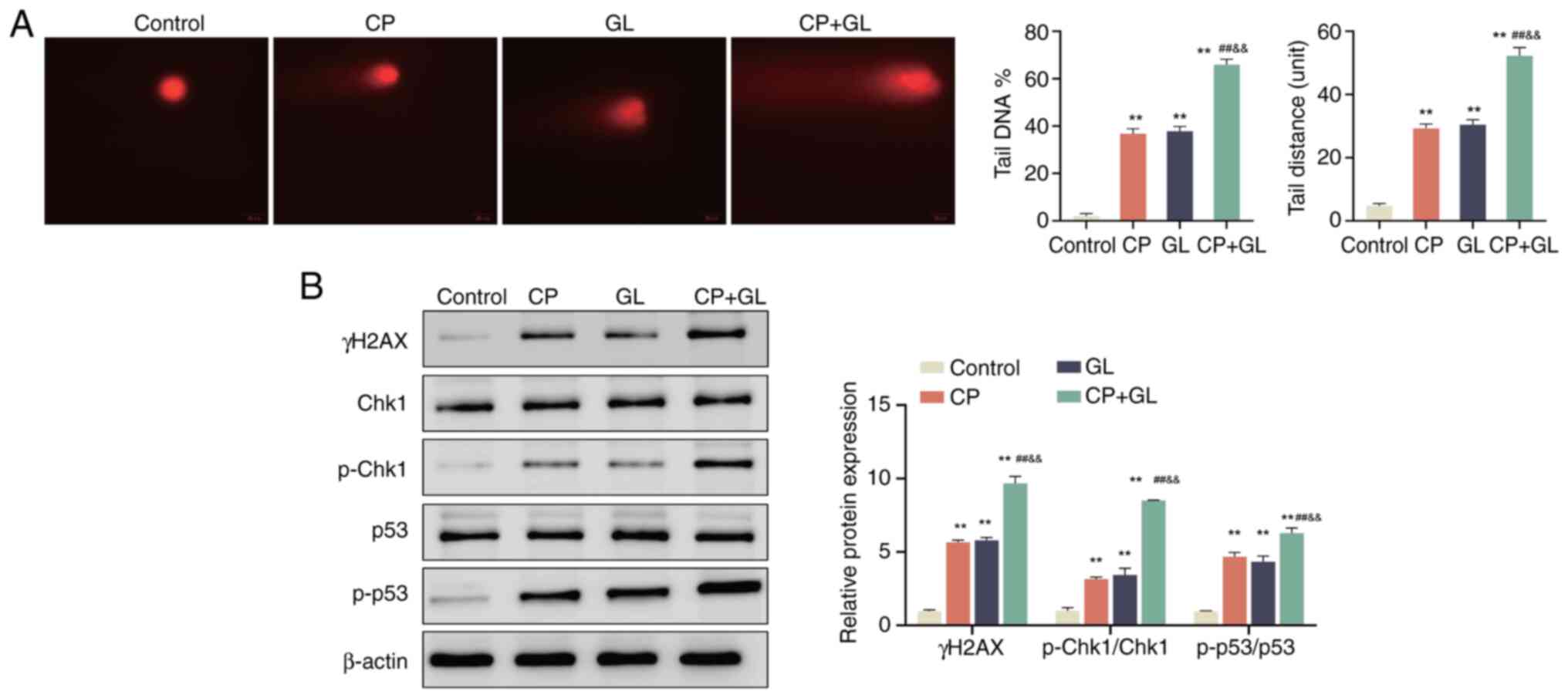 | Figure 5.Glycyrrhizin enhances the effect of
cisplatin on DNA damage of A549 cells. (A) DNA damage level of A549
cells in the control, CP, GL and CP + GL groups was assessed using
a comet assay, and the DNA level and tail distance of the comet
tail were analyzed. (B) Western blotting was used to evaluate the
protein levels of γH2AX, Chk1, p-Chk1/Chk1 and p-p53/p53 in A549
cells in the control, CP, GL and CP + GL groups. **P<0.01 vs.
control; ##P<0.01 vs. CP;
&&P<0.01 vs. GL. Data are presented as mean ±
SD (n=3). CP, cisplatin; GL, glycyrrhizin; Chk1, checkpoint kinase
1; p-Chk1, phosphorylated-Chk1; p-p53, phosphorylated p53. Scale
bar, 20 µm. |
Discussion
Lung cancer is a growing threat to human health,
accounting for 18% of all cancer-associated mortalities, which is
markedly higher than other cancer types (34). Several treatment approaches are
required for distinct subcategories of patients with lung cancer.
Surgery, radiotherapy, systemic chemotherapy, interventional
therapy and targeted therapy are frequently employed techniques for
individuals with NSCLC (35).
Chemotherapy is considered the primary choice for patients with
advanced metastases or NSCLC who are unsuitable for surgical
intervention. Nevertheless, resistance to chemotherapy is
progressively emerging in non-small cell carcinoma, prompting
concern regarding the adverse effects and toxicity associated with
chemotherapy medications (36).
Consequently, the exploration of potent anticancer compounds
derived from natural medicinal sources is gaining importance.
Terpenoids are a category of plant secondary
metabolites known for their diverse biological activities, such as
anti-inflammatory, antibacterial, antiviral and anticancer
properties. Glycyrrhizin, comprising two glucuronic acid molecules
and one glycyrrhetinic acid molecule, is recognized for its dual
role in suppressing cancer cell migration and invasion while
enhancing immune system function (37). In the present study, A549 cells
exhibited a glycyrrhizin IC50 value of 6.17 mM.
Conversely, cisplatin remains the conventional first-line
chemotherapy for patients with NSCLC (38). The present study indicated that the
IC50 of A549 cells for cisplatin was as high as 84.9 mM.
While cisplatin can hinder cancer cell division by DNA binding, it
may also form glutathione adducts with thiol-containing compounds
such as glutathione within the cell, thereby reducing its cancer
cell-killing efficacy (39).
Notably, research suggests that glycyrrhizin can enhance drug
absorption by cancer cells, impede drug resistance development
(40) and inhibit the activity of
the ATP-binding cassette transport system in cancer cells to
prevent chemotherapy drug efflux (41). Consequently, the present study
investigated the cytotoxic impact of glycyrrhizin on A549 cells
when combined with cisplatin. The results demonstrated a
significant reduction in the IC50 of cisplatin in A549
cells following glycyrrhizin supplementation. Furthermore, the
combined treatment notably diminished the aggregation capacity of
A549 cells, indicating the potential of glycyrrhizin as an adjunct
in anticancer chemotherapy.
Cisplatin primarily exerts its anticancer effects by
inducing apoptosis in cancer cells (42). Previous research has reported that
glycyrrhizin serves a complex role in regulating apoptosis,
depending on specific cellular and pathological conditions. For
example, glycyrrhizin has been demonstrated to enhance apoptosis in
hepatocellular carcinoma and tacrolimus-induced proximal tubular
epithelial cell injury by activating pro-apoptotic signals
(43,44). Conversely, in lung tissue and carbon
tetrachloride-induced hepatocyte injury models, glycyrrhizin
exhibits anti-apoptotic effects (45,46).
These findings suggest that the regulatory function of glycyrrhizin
in apoptosis may be influenced by variations in cellular
transcription and protein levels, as well as multiple potential
targets (47–49). Future research should focus on the
potential of glycyrrhizin in various cell death pathways, such as
the cuproptosis pathway, and explore how the combination of
glycyrrhizin with nano-delivery systems can synergistically
overcome chemotherapy resistance, aiming to improve the treatment
outcomes of NSCLC (50).
In addition to its dual regulatory role in
apoptosis, glycyrrhizin and cisplatin may exert synergistic
anticancer effects by targeting multiple signaling pathways,
demonstrating potential therapeutic advantages when used in
combination. Cisplatin is the standard treatment for NSCLC, but its
clinical application is often limited by the issue of resistance.
Cisplatin resistance is primarily achieved through several
mechanisms, including enhanced DNA repair capabilities,
overexpression of drug efflux pumps, inhibition of apoptosis and
activation of key signaling pathways such as PI3K/AKT and MAPK
(7,51,52).
Glycyrrhizin, as a natural extract, has been shown to modulate
these mechanisms, thereby alleviating cisplatin resistance.
Specifically, glycyrrhizin inhibits the PI3K/AKT pathway, reducing
AKT phosphorylation and downstream survival signals, leading to
G1/S phase arrest and enhancing cisplatin-induced
apoptosis (53,54). This effect may enhance the
anticancer efficacy of cisplatin by reducing resistance-related
anti-apoptotic signals (23,55).
Additionally, glycyrrhizin and cisplatin may exhibit synergistic
potential in modulating the MAPK pathway, where glycyrrhizin
activates pro-apoptotic p38 signaling and cisplatin induces
mitochondrial dysfunction and apoptosis through enhanced oxidative
stress (55). Previous research has
reported that glycyrrhizin disrupts cancer stem cell maintenance by
downregulating the Notch-Hes family BHLH transcription factor 1
pathway, which helps overcome cisplatin resistance (30). The synergistic role of cisplatin in
this pathway warrants further investigation, particularly in
overcoming cancer stem cell-mediated resistance (30). The combination of glycyrrhizin and
cisplatin not only enhances anticancer effects but may also help
optimize treatment strategies by alleviating resistance (56). Future studies could explore the
combined effects of glycyrrhizin and cisplatin on these pathways
using advanced molecular techniques, such as pathway-specific
inhibitors and gene-editing tools, to validate their synergistic
mechanisms and optimize therapeutic strategies.
To investigate the mechanism through which
glycyrrhizin enhances the biological function of cisplatin, the
present study initially examined the impact of glycyrrhizin
combined with cisplatin on apoptosis levels. The individual
administration of glycyrrhizin or cisplatin led to an increase in
apoptosis levels in A549 cells. Notably, the apoptotic induction in
A549 cells was increased when cisplatin was combined with
glycyrrhizin. The results also demonstrated a significant reduction
in the G1 phase cell population, coupled with a
concurrent increase in the G2 phase population following
glycyrrhizin treatment, reflecting alterations in the cell cycle
dynamics. Glycyrrhizin has been reported to arrest gastric cancer
cells at the G2 phase, while it has also been observed
to halt leukemia cells in the G1 phase (24,57).
Notably, glycyrrhizin has been found to enhance cell cycle
disruption induced by cisplatin (28). Cisplatin is recognized for its
capacity to trigger apoptosis and cell cycle arrest through DNA
damage induction. Despite previous research demonstrating the
ability of glycyrrhizin to induce DNA damage in cancer cells over a
decade ago, the specific mechanisms through which glycyrrhizin and
cisplatin induce DNA damage (31),
as well as any potential antagonistic effects between them, remain
unclear. Comet assay indicated that glycyrrhizin and cisplatin
induce DNA damage, and their combined treatment may further enhance
this effect. The results of the present study revealed that
glycyrrhizin and cisplatin had a synergistic anticancer effect in
A549 cells, marked by increased levels of DNA damage and
apoptosis-related proteins (γH2AX, p-Chk1/Chk1 and p-p53/p53).
Although the total levels of Chk1 and p53 remained unchanged, the
upregulation of p-Chk1 and p-p53 suggested that their
phosphorylation served a critical role in the DNA damage response,
indicating that activation states may be more important than
overall expression. To explore the synergistic anticancer mechanism
of glycyrrhizin and cisplatin, future studies should adjust dosage,
test other cell lines and focus on the phosphorylation response of
the Chk1 and p53 pathways. Furthermore, preclinical model testing
will also provide support for clinical application.
In the present study, a marked impact of the
combination treatment of glycyrrhizin and cisplatin was observed on
the cell cycle of A549 cells, specifically indicated by the
transition of cells from the G0/G1 and S
phases to the G2 phase. This finding suggests that the
synergistic effect of glycyrrhizin and cisplatin may serve an
important role in the regulation of cell proliferation and
apoptosis. To enhance the generalizability and applicability of the
present research, future studies will focus on similar experiments
in additional cell lines, particularly in the G2 or S
phase. Additionally, by integrating Gene Ontology and Kyoto
Encyclopedia of Genes and Genomes analyses, future studies should
delve deeper into the clinical importance of these cell cycle
changes. Understanding how alterations in the cell cycle influence
cancer treatment outcomes and prognosis will potentially provide
novel insights for optimizing the combined therapy of glycyrrhizin
and cisplatin, ultimately offering more effective treatment options
for patients with cancer.
Although glycyrrhizin is known for its broad
therapeutic applications and established safety, the potential side
effects of its co-administration with cisplatin have yet to be
thoroughly investigated. Furthermore, differences between
experimental conditions and clinical practice, such as dosing
regimens and patient heterogeneity, may impact the translatability
of findings. Additionally, the long-term risk of resistance to the
combination therapy remains unclear. Therefore, well-designed
clinical trials are crucial. Future clinical studies should adopt
multicenter, randomized, double-blind designs to comprehensively
evaluate the efficacy, safety and tolerability of glycyrrhizin and
cisplatin combination therapy in patients with NSCLC. Moreover,
optimizing dosage and administration methods will be essential for
ensuring successful clinical translation. These efforts will
contribute to a deeper understanding of the combined mechanisms of
glycyrrhizin and cisplatin, providing stronger scientific support
for their application in clinical practice.
To conclude, combining glycyrrhizin with cisplatin
enhances cytotoxicity in A549 cells by inducing DNA damage,
apoptosis and cell cycle arrest. This synergistic effect improves
the efficacy of cisplatin in NSCLC and offers a potential strategy
for overcoming chemotherapy resistance and minimizing side effects.
Further research is needed to validate these findings and explore
the underlying mechanisms.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZT and ZW designed the research study. JJ, WF and SH
performed the research. ZW, JJ and WF provided advice on
experiments. ZT and SH analyzed the data. ZT and ZW confirm the
authenticity of all the raw data. All authors contributed to
editorial changes in the manuscript. All authors read and approved
the final version of the manuscript. All authors have participated
sufficiently in the work and agreed to be accountable for all
aspects of the work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen P, Liu Y, Wen Y and Zhou C: Non-small
cell lung cancer in China. Cancer Commun (Lond). 42:937–970. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miao D, Zhao J, Han Y, Zhou J, Li X, Zhang
T, Li W and Xia Y: Management of locally advanced non-small cell
lung cancer: State of the art and future directions. Cancer Commun
(Lond). 44:23–46. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hendriks LEL, Remon J, Faivre-Finn C,
Garassino MC, Heymach JV, Kerr KM, Tan DSW, Veronesi G and Reck M:
Non-small-cell lung cancer. Nat Rev Dis Primers. 10:712024.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu J and Gewirtz DA: Is autophagy always a
barrier to cisplatin therapy? Biomolecules. 12:4632022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dasari S, Njiki S, Mbemi A, Yedjou CG and
Tchounwou PB: Pharmacological effects of cisplatin combination with
natural products in cancer chemotherapy. Int J Mol Sci.
23:15322022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi L, Luo Q, Zhang Y, Jia F, Zhao Y and
Wang F: Advances in toxicological research of the anticancer drug
cisplatin. Chem Res Toxicol. 32:1469–1486. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun C, Gao W, Liu J, Cheng H and Hao J:
FGL1 regulates acquired resistance to Gefitinib by inhibiting
apoptosis in non-small cell lung cancer. Respir Res. 21:2102020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gui YR, Zhang Y, Wang XQ, Fan BJ, Li JL,
Zhang LX, Fan F, Cao KD, Zhang XG and Hou W: Treatment of lung
cancer with orally administered Chinese herbal medicine: An
evidence map between 1970–2020. Chin J Integr Med. 28:930–938.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh M, Sharma P, Singh PK, Singh TG and
Saini B: Medicinal potential of heterocyclic compounds from diverse
natural sources for the management of cancer. Mini Rev Med Chem.
20:942–957. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Masraksa W, Tanasawet S, Hutamekalin P,
Wongtawatchai T and Sukketsiri W: Luteolin attenuates migration and
invasion of lung cancer cells via suppressing focal adhesion kinase
and non-receptor tyrosine kinase signaling pathway. Nutr Res Pract.
14:127–133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalaiarasi A, Anusha C, Sankar R,
Rajasekaran S, John Marshal J, Muthusamy K and Ravikumar V: Plant
isoquinoline alkaloid berberine exhibits chromatin remodeling by
modulation of histone deacetylase to induce growth arrest and
apoptosis in the A549 cell line. J Agric Food Chem. 64:9542–9550.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng QD, Lei XP, Zhong YH, Chen MS, Ke YY,
Li Z, Chen J, Huang LJ, Zhang Y, Liang L, et al: Triptolide
suppresses the growth and metastasis of non-small cell lung cancer
by inhibiting β-catenin-mediated epithelial-mesenchymal transition.
Acta Pharmacol Sin. 42:1486–1497. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei Z, Chen J, Zuo F, Guo J, Sun X, Liu D
and Liu C: Traditional Chinese medicine has great potential as
candidate drugs for lung cancer: A review. J Ethnopharmacol.
300:1157482023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Yan S, Li Y, Zhang J, Luo Y, Li
P, Yang Y, Li Y, Huang Y and Wang E: Inhibin βA is an independent
prognostic factor that promotes invasion via Hippo signaling in
non-small cell lung cancer. Mol Med Rep. 24:7892021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Li C, Zhang L, Heng Y, Xu T,
Zhang Y, Chen X, Hoffman RM and Jia L: Andrographolide induces
noxa-dependent apoptosis by transactivating ATF4 in human lung
adenocarcinoma cells. Front Pharmacol. 12:6805892021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Q, Jiao L, Wang S, Chen P, Bi L, Zhou
D, Yao J, Li J, Wang L, Chen Z, et al: Adjuvant chemotherapy with
Chinese herbal medicine formulas versus placebo in patients with
lung adenocarcinoma after radical surgery: A multicenter,
randomized, double-blind, placebo-controlled trial. Biol Proced
Online. 22:52020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chrzanowski J, Chrzanowska A and Graboń W:
Glycyrrhizin: An old weapon against a novel coronavirus. Phytother
Res. 35:629–636. 2021. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bravo V, Serrano M, Duque A, Ferragud J
and Coronado PJ: Glycyrrhizinic acid as an antiviral and anticancer
agent in the treatment of human papillomavirus. J Pers Med.
13:16392023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han Y, Sheng W, Liu X, Liu H, Jia X, Li H,
Wang C, Wang B, Hu T and Ma Y: Glycyrrhizin ameliorates colorectal
cancer progression by regulating NHEJ pathway through inhibiting
HMGB1-induced DNA damage response. Sci Rep. 14:249482024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jain R, Hussein MA, Pierce S, Martens C,
Shahagadkar P and Munirathinam G: Oncopreventive and
oncotherapeutic potential of licorice triterpenoid compound
glycyrrhizin and its derivatives: Molecular insights. Pharmacol
Res. 178:1061382022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Sheng Z, Xiao J, Li Y, Huang J,
Jia J, Zeng X and Li L: Advances in the roles of glycyrrhizic acid
in cancer therapy. Front Pharmacol. 14:12651722023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Ge X, Qu H, Wang N, Zhou J, Xu W,
Xie J, Zhou Y, Shi L, Qin Z, et al: Glycyrrhizic acid inhibits
proliferation of gastric cancer cells by inducing cell cycle arrest
and apoptosis. Cancer Manag Res. 12:2853–2861. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang RY, Chu YL, Jiang ZB, Chen XM, Zhang
X and Zeng X: Glycyrrhizin suppresses lung adenocarcinoma cell
growth through inhibition of thromboxane synthase. Cell Physiol
Biochem. 33:375–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng QP, Wang MJ, Zeng X, Chen GG and
Huang RY: Effects of glycyrrhizin in a mouse model of lung
adenocarcinoma. Cell Physiol Biochem. 41:1383–1392. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huan C, Xu Y, Zhang W, Guo T, Pan H and
Gao S: Research progress on the antiviral activity of glycyrrhizin
and its derivatives in liquorice. Front Pharmacol. 12:6806742021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wakamatsu T, Nakahashi Y, Hachimine D,
Seki T and Okazaki K: The combination of glycyrrhizin and
lamivudine can reverse the cisplatin resistance in hepatocellular
carcinoma cells through inhibition of multidrug
resistance-associated proteins. Int J Oncol. 31:1465–1472.
2007.PubMed/NCBI
|
|
29
|
Tan JY, Zhao F, Deng SX, Zhu HC, Gong Y
and Wang W: Glycyrrhizin affects monocyte migration and apoptosis
by blocking HMGB1 signaling. Mol Med Rep. 17:5970–5975.
2018.PubMed/NCBI
|
|
30
|
Ahmad A, Tiwari RK, Saeed M, Ahmad I and
Ansari IA: Glycyrrhizin mediates downregulation of notch pathway
resulting in initiation of apoptosis and disruption in the cell
cycle progression in cervical cancer cells. Nutr Cancer.
74:622–639. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thirugnanam S, Xu L, Ramaswamy K and
Gnanasekar M: Glycyrrhizin induces apoptosis in prostate cancer
cell lines DU-145 and LNCaP. Oncol Rep. 20:1387–1392.
2008.PubMed/NCBI
|
|
32
|
Sun Y, Liu Y, Ma X and Hu H: The influence
of cell cycle regulation on chemotherapy. Int J Mol Sci.
22:69232021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. Apr 5–2021.(Epub ahead of
print). View Article : Google Scholar
|
|
35
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 2.2021. J Natl Compr Canc Netw. 19:254–266. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan H, Jiang M, Yang F, Tang X, Lin M,
Zhou C, Tan Y and Liu D: Ajuforrestin A, an abietane diterpenoid
from Ajuga ovalifolia var. calanthe, induces A549 cell apoptosis by
targeting SHP2. Molecules. 27:54692022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roohbakhsh A, Iranshahy M and Iranshahi M:
Glycyrrhetinic acid and its derivatives: Anti-cancer and cancer
chemopreventive properties, mechanisms of action and
structure-cytotoxic activity relationship. Curr Med Chem.
23:498–517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bi YY, Chen Q, Yang MY, Xing L and Jiang
HL: Nanoparticles targeting mutant p53 overcome chemoresistance and
tumor recurrence in non-small cell lung cancer. Nat Commun.
15:27592024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li F, Zheng Z, Chen W, Li D, Zhang H, Zhu
Y, Mo Q, Zhao X, Fan Q, Deng F, et al: Regulation of cisplatin
resistance in bladder cancer by epigenetic mechanisms. Drug Resist
Updat. 68:1009382023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Su X, Wu L, Hu M, Dong W, Xu M and Zhang
P: Glycyrrhizic acid: A promising carrier material for anticancer
therapy. Biomed Pharmacother. 95:670–678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen L, Yang J, Davey AK, Chen YX, Wang JP
and Liu XQ: Effects of diammonium glycyrrhizinate on the
pharmacokinetics of aconitine in rats and the potential mechanism.
Xenobiotica. 39:955–963. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Romani AMP: Cisplatin in cancer treatment.
Biochem Pharmacol. 206:1153232022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsai JJ, Pan PJ, Hsu FT, Chung JG and
Chiang IT: Glycyrrhizic acid modulates apoptosis through
extrinsic/intrinsic pathways and inhibits protein kinase B- and
extracellular signal-regulated kinase-mediated metastatic potential
in hepatocellular carcinoma in vitro and in vivo. Am J Chin Med.
48:223–244. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao R, Li Y, Hu X, Qiu Y, Li S, Xie Y, Xu
C, Lu C, Chen G and Yang J: Glycyrrhizic acid improves
tacrolimus-induced renal injury by regulating autophagy. FASEB J.
37:e227492023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Y, Wang L, Luo R, Sun Y, Zou M, Wang
T, Guo Q and Peng X: Glycyrrhizic acid against mycoplasma
gallisepticum-induced inflammation and apoptosis through
suppressing the MAPK pathway in chickens. J Agric Food Chem.
70:1996–2009. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liang B, Guo XL, Jin J, Ma YC and Feng ZQ:
Glycyrrhizic acid inhibits apoptosis and fibrosis in
carbon-tetrachloride-induced rat liver injury. World J
Gastroenterol. 21:5271–5280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Azzahra SNA, Hanif N and Hermawan A: MDM2
is a potential target gene of glycyrrhizic acid for circumventing
breast cancer resistance to tamoxifen: Integrative bioinformatics
analysis. Asian Pac J Cancer Prev. 23:2341–2350. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bentz GL, Lowrey AJ, Horne DC, Nguyen V,
Satterfield AR, Ross TD, Harrod AE, Uchakina ON and McKallip RJ:
Using glycyrrhizic acid to target sumoylation processes during
Epstein-Barr virus latency. PLoS One. 14:e02175782019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Slovin S, Carissimo A, Panariello F,
Grimaldi A, Bouché V, Gambardella G and Cacchiarelli D: Single-cell
RNA sequencing analysis: A step-by-step overview. Methods Mol Biol.
2284:343–365. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wei C and Fu Q: Cell death mediated by
nanotechnology via the cuproptosis pathway: A novel horizon for
cancer therapy. View. 4:202300012023. View Article : Google Scholar
|
|
51
|
Zhou J, Kang Y, Chen L, Wang H, Liu J,
Zeng S and Yu L: The drug-resistance mechanisms of five
platinum-based antitumor agents. Front Pharmacol. 11:3432020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang L, Liu YN, Gu Y and Guo Q: Deltonin
enhances gastric carcinoma cell apoptosis and chemosensitivity to
cisplatin via inhibiting PI3K/AKT/mTOR and MAPK signaling. World J
Gastrointest Oncol. 15:1739–1755. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang J, Zhou Q, Xie K, Cheng L, Peng S,
Xie R, Liu L, Zhang Y, Dong W, Han J, et al: Targeting WD repeat
domain 5 enhances chemosensitivity and inhibits proliferation and
programmed death-ligand 1 expression in bladder cancer. J Exp Clin
Cancer Res. 40:2032021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang Y, Yang Z, Zhang R, Jia C, Mao R,
Mahati S, Zhang Y, Wu G, Sun YN, Jia XY, et al: MiR-27a-3p enhances
the cisplatin sensitivity in hepatocellular carcinoma cells through
inhibiting PI3K/Akt pathway. Biosci Rep. 41:BSR201920072021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li H, Wen X, Ren Y, Fan Z, Zhang J, He G
and Fu L: Targeting PI3K family with small-molecule inhibitors in
cancer therapy: Current clinical status and future directions. Mol
Cancer. 23:1642024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Omidi F, Shahbazi S, Reiisi S, Azhdari S
and Karimzadeh MR: Glycyrrhizic acid enhances the anticancer
activity of cisplatin in the human ovarian cancer cell line.
Toxicol In Vitro. 93:1056872023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chueh FS, Hsiao YT, Chang SJ, Wu PP, Yang
JS, Lin JJ, Chung JG and Lai TY: Glycyrrhizic acid induces
apoptosis in WEHI-3 mouse leukemia cells through the caspase- and
mitochondria-dependent pathways. Oncol Rep. 28:2069–2076. 2012.
View Article : Google Scholar : PubMed/NCBI
|