Introduction
Gastric cancer is a prevalent malignancy associated
with a high mortality rate, which poses a significant threat to
human health. Despite a declining trend in incidence over recent
decades, gastric cancer remains the fifth most common cancer and
the fourth leading cause of cancer-related death worldwide
(1). In China, gastric cancer ranks
third in both incidence and mortality among all types of cancer,
with an estimated global age-standardized incidence rate of 11.1
per 100,000 (2). Systemic
treatments, including radical surgery, endoscopic resection,
chemotherapy, targeted therapy and immunotherapy, have notably
evolved, allowing clinicians to tailor therapeutic strategies based
on individual disease conditions (1,3).
Primary liver cancer, encompassing hepatocellular
carcinoma and cholangiocarcinoma, presents a substantial global
mortality burden; it ranks sixth in incidence among all cancer
types and is the third leading cause of cancer-related death
(4). The global age-standardized
incidence of primary liver cancer was 8.657 per 100,000 in 2017
(5). China, where the prevalence of
hepatitis B virus is high, accounts for ~50% of the global liver
cancer burden (6). Hepatectomy
remains the cornerstone of treatment strategies for liver cancer,
complemented by systemic therapies, such as chemotherapy and
immunotherapy (7).
Synchronous tumors, defined as independent primary
tumors that arise simultaneously, have become increasingly
recognized with advancements in diagnostic and therapeutic methods.
Multiple primary malignancies are not uncommon in clinical
practice; however, cases involving synchronous primary gastric and
liver cancer are rarely reported (8,9). The
present study aimed to present the diagnosis and treatment progress
of a patient with synchronous primary gastric and liver cancer,
providing insights into the clinical management of similar cases
(10).
Case report
Case presentation
A 60-year-old man presented to Xiangyang Central
Hospital (Xiangyang, China) in September 2020 with a hepatic
space-occupying lesion identified via B-ultrasonography during
routine physical examinations. Subsequent upper abdominal enhanced
magnetic resonance imaging (MRI) indicated a high probability of
liver cancer in the right lobe, as well as a gastric
space-occupying lesion. The family history was unremarkable, with
no reported malignancies among relatives. The patient had a
personal history of long-term heavy alcohol consumption (~250 ml of
50% ABV spirits every day for 40 years) but was a non-smoker.
Additionally, the patient had a 20-year history of hypertension and
had been diagnosed with a fatty liver 1 year prior. For the
management of hypertension, the patient was taking nimodipine (20
mg, three times/day) and captopril (25 mg, three times/day)
orally.
Physical examination revealed a generally good
condition, with no signs of liver palms or spider angiomas.
Respiratory and cardiovascular systems were normal, and there were
no abdominal symptoms. The blood chemistry tests revealed the
following results: Hepatitis B surface antigen, 0 IU/ml; antibody
to hepatitis B surface antigen, 36.62 mIU/ml; hepatitis B e
antigen, 0.304 s/co; antibody to hepatitis B e antigen, 1.04 s/co;
antibody to hepatitis B core antigen, 7.38 s/co; and anti-hepatitis
C virus, 0.04 s/co (negative). Additionally, tumor markers
including α-fetoprotein, carcinoembryonic antigen, cancer antigen
(CA)125, CA19-9, squamous cell carcinoma antigen, total
prostate-specific antigen and free prostate-specific antigen were
all within normal limits. However, prothrombin induced by vitamin K
absence or antagonist II was elevated at 46.75 mAU/ml (normal
range, <40 mAU/ml). The patient had been exposed to
Helicobacter pylori, as indicated by the results of a H.
pylori antibody typing test. while levels of alanine
aminotransferase (ALT; 48 U/l; normal range, 9–50 U/l) and
aspartate aminotransferase (AST; 32 U/l; normal range, 15–30 U/l)
remained normal.
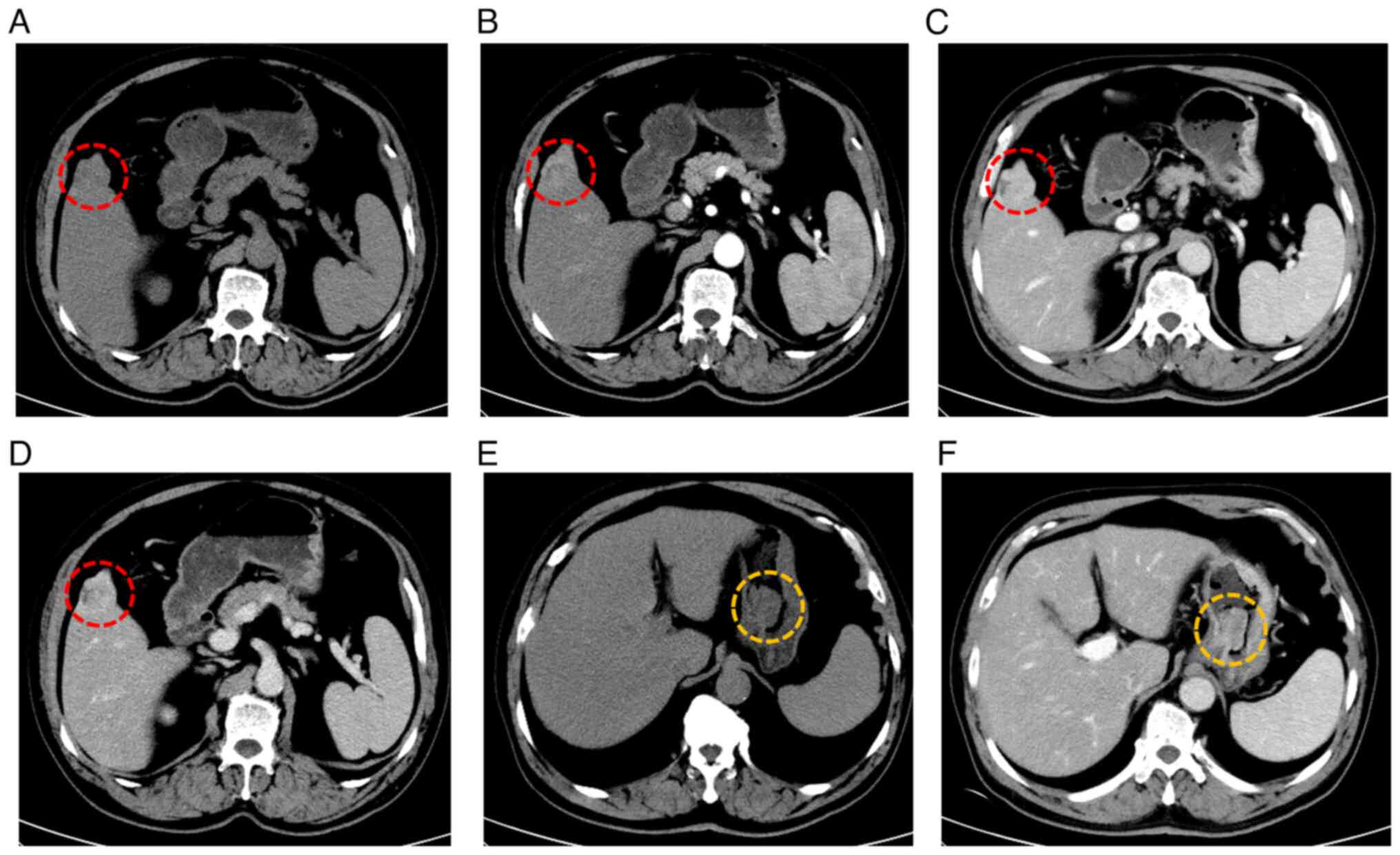
A total abdominal enhanced computed tomography (CT)
scan suggested a high likelihood of primary liver cancer and
identified a stromal tumor on the lesser curvature of the stomach.
Yellow circles highlight a lesion in the gastric fundus (Fig. 1). To further evaluate the gastric
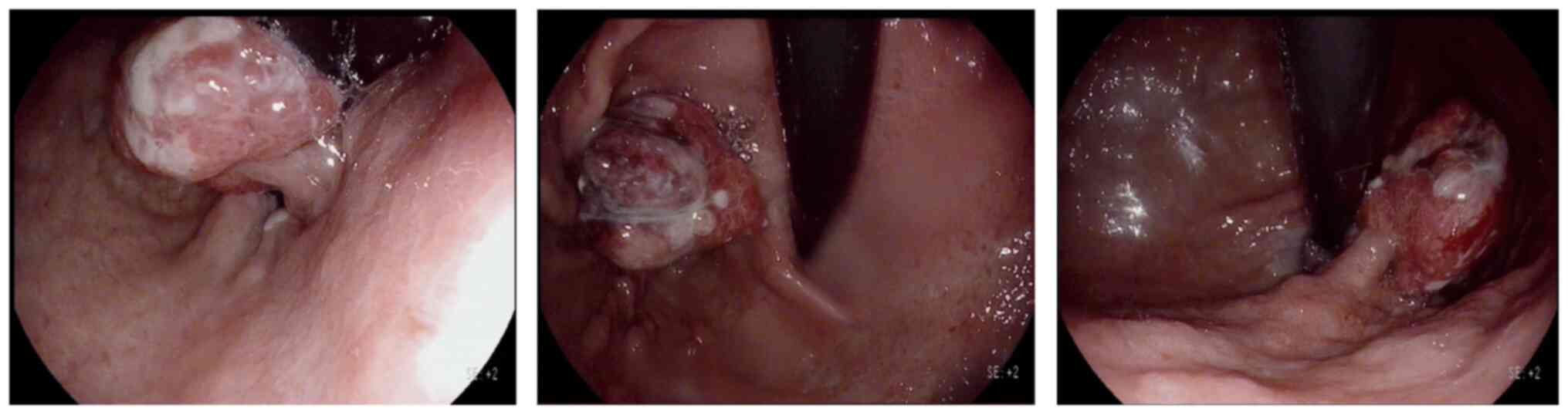
soft tissue lesion, gastroscopy was performed, revealing a neoplasm
measuring 2.5 cm in diameter with a pedicle, alongside necrosis and
ulceration on its surface (Fig. 2).
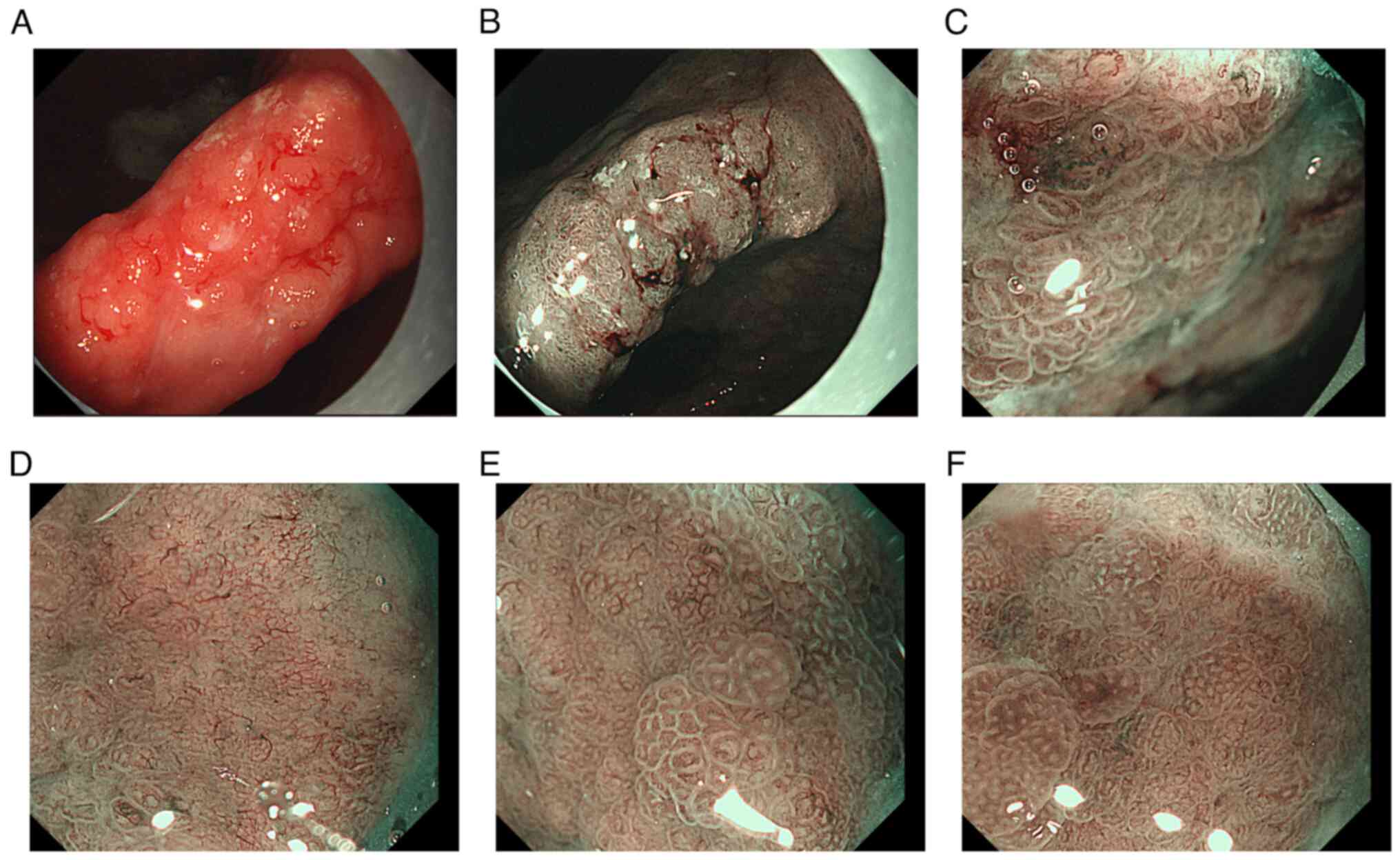
A patchy, rough mucous membrane exhibiting a granular appearance
was observed in the gastric angle but not seen under CT images,
classified as type IIa + IIc according to the Japanese Research
Society for Gastric Cancer (Fig. 3)
(11). Narrow band imaging revealed
local irregularities in glandular ducts and neovascularization.
Pathological examination indicated high-grade dysplasia of the
mucosal glandular epithelium, accompanied by chronic active
inflammation, surface necrosis and erosion. Given the presence of
tumors in both the liver and gastric angle, it remained uncertain
whether the patient had synchronous primary tumors or a gastric
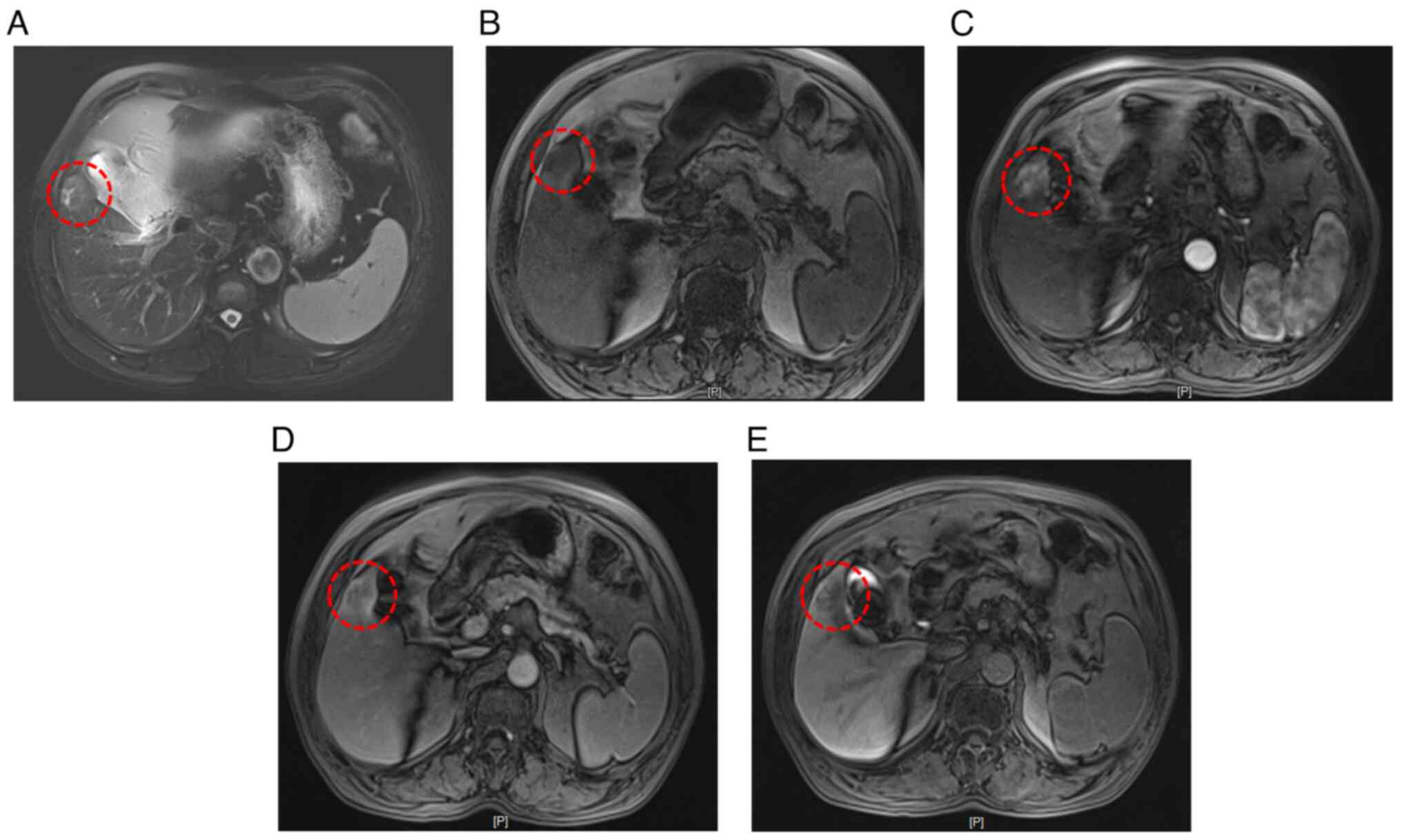
tumor with liver metastasis. To clarify the diagnosis, an enhanced
MRI with a liver-specific contrast agent (disodium gadoxelate) was
conducted, confirming primary liver cancer in the S5 segment and
early gastric cancer in the gastric angle (Fig. 4).
A multidisciplinary discussion led to the
formulation of a clinical therapeutic strategy. The patient
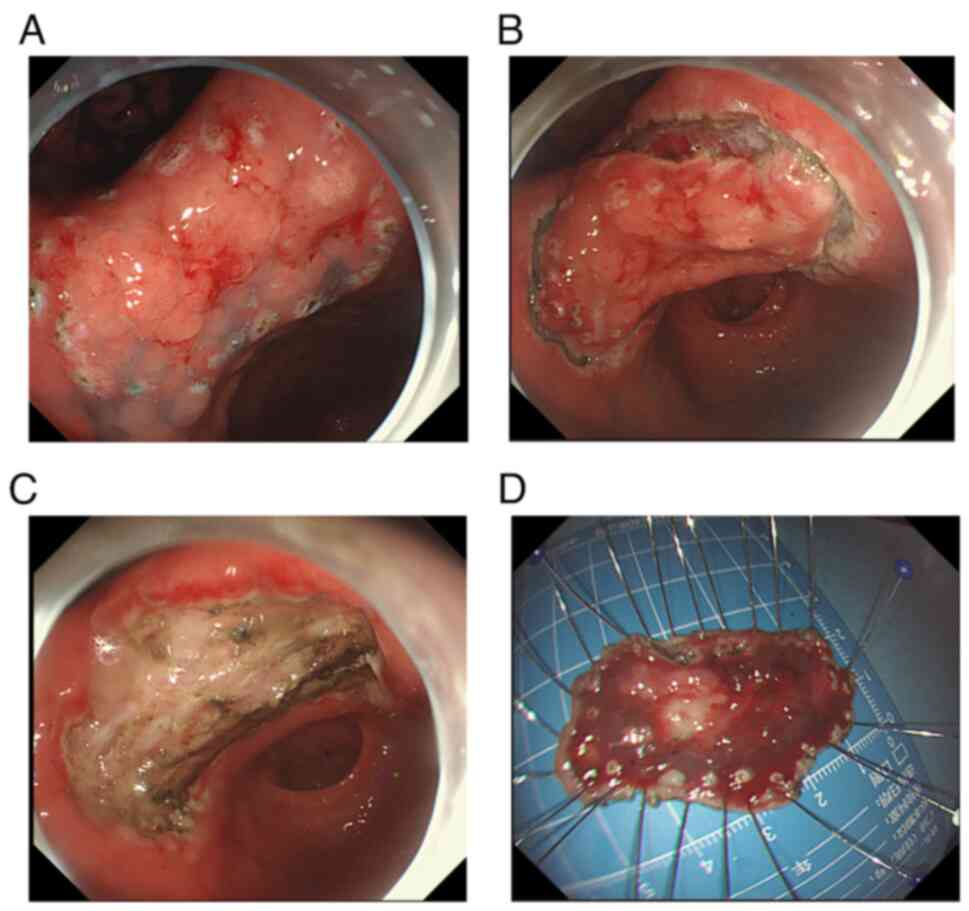
underwent endoscopic submucosal dissection (ESD) for the gastric
angle lesion, followed by laparoscopic resection of the small liver
cancer 2 weeks later. A hook knife was used to mark the lesion edge
(Fig. 5A) and a dye-saline solution
was injected to enhance visibility (Fig. 5B). The procedure involved gradually
dissecting the lesion to ensure a complete resection (Fig. 5C); the resected specimen measured
~5×3 cm (Fig. 5D). The patient did
not receive chemotherapy or radiotherapy before or after surgery
based on their clinical condition.
Pathological findings
Microscopic findings
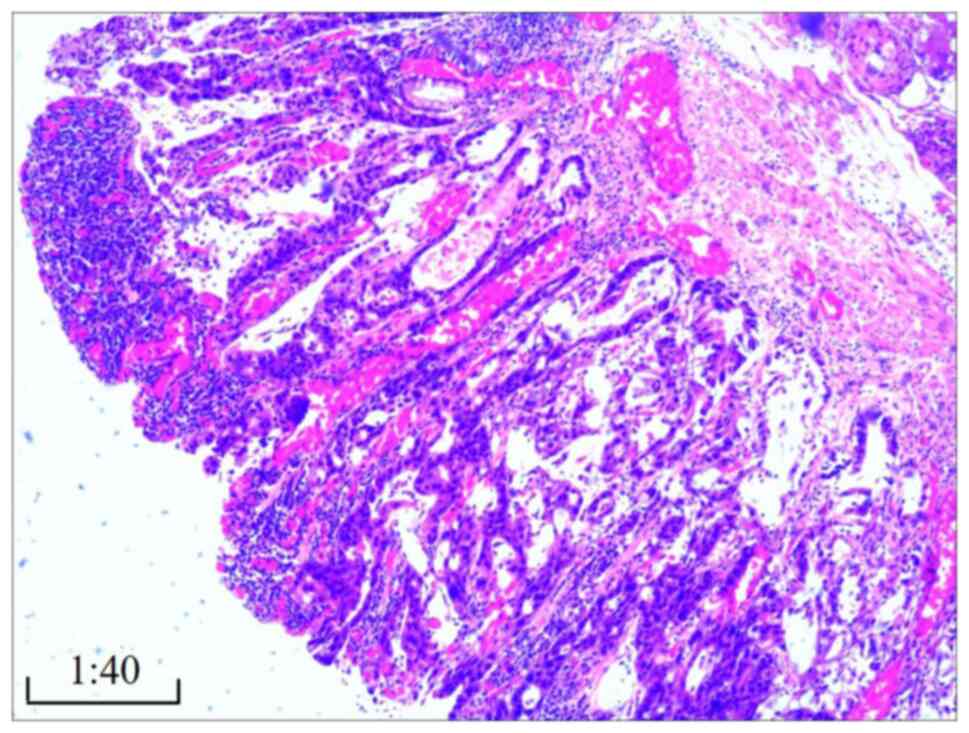
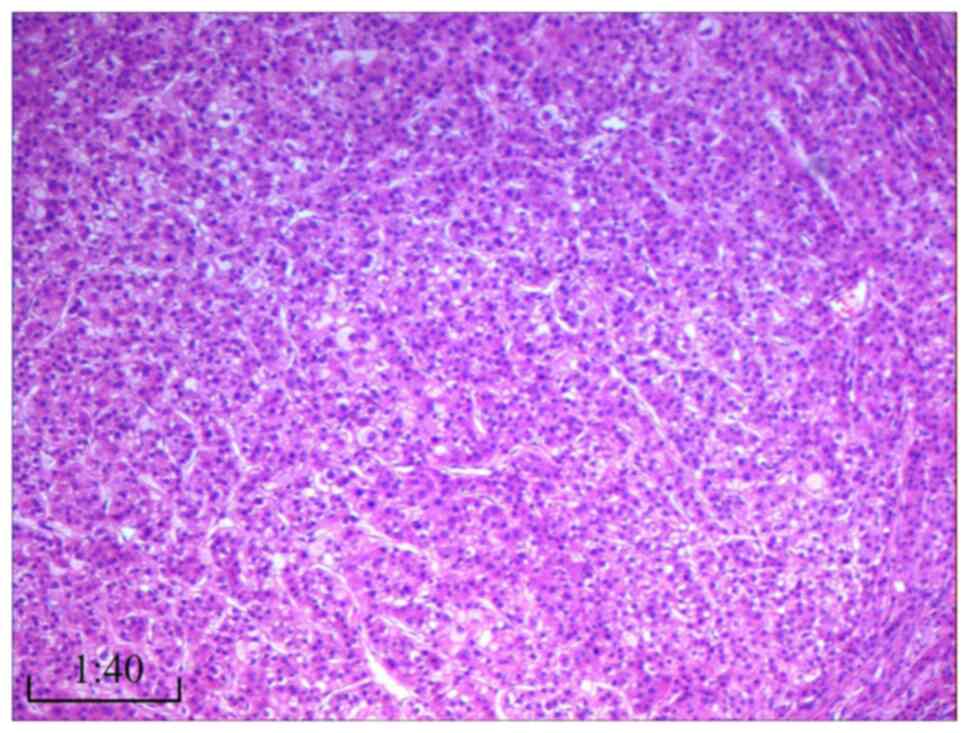
The gastric cancer displayed features of moderately
differentiated adenocarcinoma (Fig.
6), as determined by hematoxylin and eosin staining. Staining
was performed as follows: Tissues were fixed in 10% neutral
formalin solution at room temperature (20–25°C) for 24 h.
Subsequently, the fixed tissue samples were dehydrated by
sequentially placing them in different concentrations of ethanol
(70, 80, 90, 95 and 100%, each for 1–2 h), followed by immersion in
xylene for 10–30 min. The sections were then immersed in paraffin
wax at 58–60°C for 2–4 h, poured into embedding molds, and allowed
to cool and solidify at room temperature. A microtome was used to
cut the embedded tissue into thin sections (4–6 µm) and the
sections were dried in an oven at 70°C for 30 min. Hematoxylin and
eosin staining was then performed at 25–30°C. The sections were
deparaffinized and rehydrated, then stained in 0.5% hematoxylin
solution for 5 min. The excess dye was removed, the sections were
differentiated in a differentiation solution (1% hydrochloric acid
+ 75% ethanol) for 2–5 sec and then soaked in 0.5% ammonia solution
for 30 sec. Finally, the sections were stained in 1% eosin for 3–5
min, and rinsed in running water before dehydrating and mounting
the slides. The sections were observed under a Nikon ECLIPSE Ci
optical microscope (Nikon Corporation). No cancerous tissue was
detected in the submucosa or peripheral surgical margins. The liver
cancer exhibited characteristics of highly differentiated
hepatocellular carcinoma without significant capsule invasion
(Fig. 7). Surrounding liver tissue
showed no signs of cirrhosis; liver cell arrangement was regular,
with localized hydropic degeneration of hepatocytes and infiltrates
of chronic inflammatory cells, such as lymphocytes, in the portal
area. No metastatic cancer was found in level 3 (0/3), 5 (0/3) or 7
(0/1) lymph nodes.
Immunohistochemistry
Immunohistochemistry was performed as described
previously (12). Liver cancer
cells were revealed to be positive for cytokeratin (CK)18 (1:100;
cat. no. ab668; Abcam), CD34 (1:50; cat. no. M7165; Dako; Agilent
Technologies, Inc.), Glypican-3 (1:200; cat. no. 758102; BioLegend,
Inc.) and Ki-67 (5%) (1:100; cat. no. M7240; Dako; Agilent
Technologies, Inc.), whereas they were negative for CK19 (1:100;
cat. no. ab52625; Abcam) and CD10 (1:50; cat. no. 555373; BD
Biosciences) (Fig. S1). Based on
microscopic and immunohistochemical findings, the diagnosis was
established as synchronous intramucosal adenocarcinoma in the
gastric angle and hepatocellular carcinoma. Positive markers:
Diagnosis
A gastroscopy and biopsy was performed first to
determine high-grade dysplasia of mucosal glandular epithelium with
chronic active inflammation, surface necrosis and erosion.
Subsequently, enhanced MRI indicated primary gastric and liver
cancer. Nodular long T1 and long T2 signals were seen in the S5
segment of the hepatic parenchyma, and the signals were slightly
higher on diffused weighted imaging. The signal was markedly
increased during the arterial phase following contrast
administration, then slightly decreased in the portal and delayed
phases, with intensity lower than that of the surrounding hepatic
parenchyma. No marked enhancement was observed in the hepatobiliary
phase, and the cross-section size of the neoplasm was ~2.0×2.4 cm,
which was considered hepatocellular carcinoma. The final diagnosis
depended on the postoperative pathology.
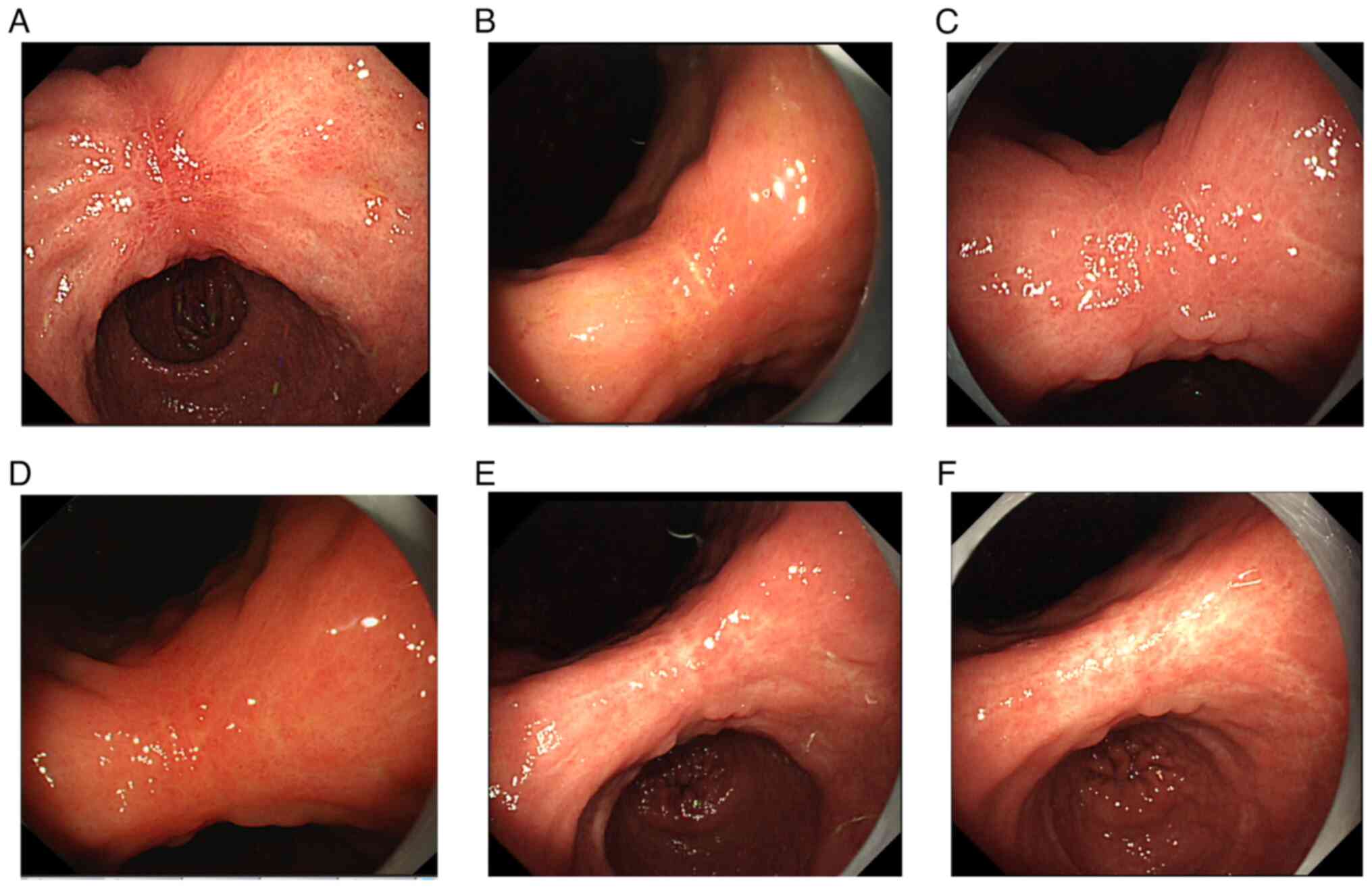
Follow-up
The patient was monitored at 6-month intervals for 3
years. As of November 2023, the patient remains asymptomatic.
Repeated gastroscopy with histopathological examination and
abdominal enhanced MRI revealed no recurrence of gastric or liver
cancer (Fig. 8).
Discussion
Multiple primary cancers are not uncommon in
clinical practice, especially with the advancement of diagnostic
techniques. A previous study reported that one-third of patients
with synchronous primary cancer had gastric cancer (10). However, although there has been a
report regarding synchronous liver metastases from gastric cancer
(13), the simultaneous occurrence
of these two primary malignancies is rare. The present study
described the case of a patient with primary gastric and liver
cancer, which, to the best of our knowledge, has rarely been
reported.
The term ‘multiple cancers’ refers to the
synchronous or metachronous appearance of primary cancers in the
same patient (14). The
Surveillance, Epidemiology, and End Results program recommends that
second primary cancers occurring within 2 months of the first
primary tumor should be defined as synchronous multiple primary
cancers (15,16). For patients with multiple types of
cancer, it is crucial to determine whether the cancers are all
primary or if one is a primary cancer and the other a metastatic
cancer (17).
In the present case, the patient visited the
hospital due to the presence of space-occupying lesions in the
stomach and liver. In this case, the patchy, rough mucous membrane
with a granular appearance in the gastric angle, classified as type
IIa + IIc, indicated that the lesion displayed both elevated and
depressed features, suggesting a complex lesion that could
potentially have implications for diagnosis and treatment,
including the possibility of malignancy. Pathological examination
showed high-grade dysplasia of the mucosal glandular epithelium,
making a definitive diagnosis of early gastric cancer difficult.
However, the small early gastric carcinoma (gastric angle lesion)
cannot easily be recognized on CT/MRI. Gastroscopy is the most
effective way to detect early gastric cancer. Additionally, it was
unclear whether the lesion in the S5 segment of the liver was a
primary cancer or a metastatic lesion. Therefore, narrow-band
imaging was performed, which showed irregular glandular ducts and
neovascularization in the local area. Collectively, the lesion in
the gastric angle was considered an early-stage lesion.
Liver-specific contrast agent-enhanced MRI suggested that the liver
lesion was highly likely to be primary liver cancer (18,19).
The S5 space occupying lesion exhibited a ‘fast in and fast out’
appearance in the enhanced MRI; that is, the primary hepatocellular
carcinomatosis was enhanced in the arterial stage, the liver tissue
was strengthened in the venous stage suggesting primary
hepatocellular carcinoma. The postoperative pathology test showed
that the liver cancer exhibited characteristics of highly
differentiated hepatocellular carcinoma without significant capsule
invasion. Surrounding liver tissue showed no signs of cirrhosis;
liver cell arrangement was regular, with localized hydropic
degeneration of hepatocytes and infiltrates of chronic inflammatory
cells, such as lymphocytes, in the portal area. The patient had a
personal history of long-term heavy drinking and hypertension for
20 years, and fatty liver for 1 year. Therefore, there may be
multiple possibilities for the degeneration. Regarding the levels
of ALT and AST in the serum, these were normal. The most common
explanation for why AST and ALT levels were normal is that a number
of hepatitis B virus carriers have normal transaminase levels
during annual physical examinations, but they can develop cirrhosis
after a number of years. Based on the comprehensive examinations,
the patient was diagnosed with synchronous primary liver cancer and
early gastric cancer.
While studies on the treatment of primary gastric
cancer with metastatic liver cancer are widely reported (20,21),
treatment strategies for patients with synchronous primary liver
and gastric cancer have not been well-documented. According to
guidelines, ESD is suitable for the treatment of early gastric
cancer (22), and local treatment
or surgical resection is appropriate for small liver cancer
(23,24). However, the optimal management of
patients with synchronous primary small liver cancer and early
gastric cancer remains a challenge, as no specific guidelines are
available.
After multidisciplinary discussions involving the
gastroenterology, hepatobiliary surgery, gastrointestinal surgery
and imaging departments, and after thorough communication and
consultation with the patient, the final treatment plan was
decided: ESD for the gastric angle cancer and laparoscopic
resection for the small liver cancer. Postoperative pathological
examination confirmed the diagnosis of primary hepatocellular
carcinoma and gastric angle adenocarcinoma, both at an early stage,
validating the appropriateness of the chosen therapeutic strategy.
After 3 years of follow-up, the patient had a good prognosis with
no tumor recurrence.
Tanjak et al (14) analyzed 109,054 patients with a
primary solid cancer and revealed that 1,785 patients (1.63%) had
multiple primary cancers. In patients with multiple cancers, the
second most common primary cancer type was liver cancer. Therefore,
it is of great clinical importance to conduct comprehensive
examinations for patients with suspected liver cancer during their
initial visit and during follow-up after treatment.
In conclusion, in clinical practice, caution should
be exercised when dealing with patients with definite lesions to
avoid overlooking subtle lesions. Comprehensive examinations should
be performed for patients with cancer to check for the presence of
other primary cancers. For patients with multiple cancers,
determining whether the cancers are primary or metastatic is
crucial, and a personalized therapeutic strategy based on
multidisciplinary discussion is of utmost clinical value.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XZ and XH drafted and edited the manuscript. XH
treated the patient, and provided insights into the work-up and
treatment of the patient. XZ also participated in the follow-up
management of the patient, and was involved in the
conceptualization of the article, data analysis, drafting the
manuscript and interpretation of the findings. XZ and XH confirm
the authenticity of all the raw data. Both authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the case report and relevant images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet (London, England).
396:635–648. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang WJ, Zhao HP, Yu Y, Wang JH, Guo L,
Liu JY, Liu JY, Pu J and Lv J: Updates on global epidemiology, risk
and prognostic factors of gastric cancer. World J Gastroenterol.
29:2452–2468. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guan WL, He Y and Xu RH: Gastric cancer
treatment: Recent progress and future perspectives. J Hematol
Oncol. 16:572023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anwanwan D, Singh SK, Singh S, Saikam V
and Singh R: Challenges in liver cancer and possible treatment
approaches. Biochim Biophys Acta Rev Cancer. 1873:1883142020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gravitz L: Liver cancer. Nature.
516:S12014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao Z, Dai C, Yang J, Xu M, Meng H, Hu X
and Lin N: Time-trends in liver cancer incidence and mortality
rates in the U.S. from 1975 to 2017: A study based on the
surveillance, epidemiology, and end results database. J
Gastrointest Oncol. 14:312–324. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen CH, Wu MS, Yang YW, Liu YT, Chiu YF,
Hsu CC, Chuang SC, Chung TC, Tsai TL, Huang WH, et al: Longitudinal
changes in physical and mental health of older adults with chronic
hepatitis B infection: Trajectories and predictors. Prev Med Rep.
23:1014322021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Demir T, Lee SS and Kaseb AO: Systemic
therapy of liver cancer. Adv Cancer Res. 149:257–294. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan H, Lu P, Xu L, Qin Y and Li J:
Synchronous occurrence of hereditary gastric adenocarcinoma,
gastrointestinal stromal tumor, and esophageal small cell and
squamous carcinoma in situ: An extremely rare case report. BMC
Cancer. 17:7202017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gastric Cancer Association, . Japanese
gastric cancer treatment guidelines 2018 (5th edition). Gastric
Cancer. 24:1–21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Magaki S, Hojat SA, Wei B, So A and Yong
WH: An Introduction to the performance of immunohistochemistry.
Methods Mol Biol. 1897:289–298. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu P, Zhang Y, Ye Z, Chen X, Huang L, Du Y
and Cheng X: Treatment of synchronous liver metastases from gastric
cancer: A single-center study. Cancer Manag Res. 12:7905–7911.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanjak P, Suktitipat B, Vorasan N,
Juengwiwattanakitti P, Thiengtrong B, Songjang C, Therasakvichya S,
Laiteerapong S and Chinswangwatanakul V: Risks and cancer
associations of metachronous and synchronous multiple primary
cancers: A 25-year retrospective study. BMC Cancer. 21:10452021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan H, Wen R, Zhou L, Gao X, Lou Z, Hao L,
Meng R, Gong H, Yu G and Zhang W: Clinicopathological features and
prognosis of synchronous and metachronous colorectal cancer: A
retrospective cohort study. Int J Surg. 109:4073–4090.
2023.PubMed/NCBI
|
|
16
|
Xiong J, Su Y, Bing Z and Zhao B: Survival
between synchronous and non-synchronous multiple primary cutaneous
melanomas-a SEER database analysis. Peer J. 8:e83162020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vogt A, Schmid S, Heinimann K, Frick H,
Herrmann C, Cerny T and Omlin A: Multiple primary tumours:
Challenges and approaches, a review. ESMO Open. 2:e0001722017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ringe KI, Fischbach F, Grenacher L,
Juchems MS, Kukuk G, Lauenstein T, Wessling J and Schreyer AG:
Application of liver-specific contrast agents for evaluation of
focal liver lesions-Expert recommendations from the
Gastrointestinal and Abdominal Imaging Workgroup of the German
Roentgen Society. Rofo. 196:690–698. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thian YL, Riddell AM and Koh DM:
Liver-specific agents for contrast-enhanced MRI: Role in
oncological imaging. Cancer imaging. 13:567–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jagric T and Horvat M: Surgical resection
of synchronous liver metastases in gastric cancer patients. A
propensity score-matched study. Radiol Oncol. 55:57–65. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martella L, Bertozzi S, Londero AP,
Steffan A, De Paoli P and Bertola G: Surgery for liver metastases
from gastric cancer: A meta-analysis of observational studies.
Medicine (Baltimore). 94:e11132015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ono H, Yao K, Fujishiro M, Oda I, Uedo N,
Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y and Fujimoto K:
Guidelines for endoscopic submucosal dissection and endoscopic
mucosal resection for early gastric cancer (second edition). Dig
Endosc. 33:4–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshida H, Yoshida H, Shiina S and Omata
M: Early liver cancer: Concepts, diagnosis, and management. Int J
Clin Oncol. 10:384–390. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vogel A, Cervantes A, Chau I, Daniele B,
Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, et al:
Hepatocellular carcinoma: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 29
(Suppl):iv238–iv55. 2018. View Article : Google Scholar : PubMed/NCBI
|