Introduction
According to the American Cancer Society, Renal cell
carcinoma (RCC) is the most common malignant kidney tumor type in
the United States, ranking sixth among cancer types in men and
tenth in women, accounting for 5 and 3% of all tumor diagnoses,
respectively; its incidence is on the rise (1,2).
Despite advances in diagnostic and therapeutic techniques in recent
years, a significant proportion of patients are still diagnosed
with locally advanced disease and 17% of patients present with
distant metastases at diagnosis, leading to a poor prognosis
(3). Although radical or partial
nephrectomy is the standard surgical treatment for patients with
non-metastatic RCC, the prognosis for patients remains poor,
especially for those with regional or distant advanced disease
(2,4). In addition, immune checkpoint
inhibitors (ICIs) and combination therapies have notably changed
the treatment landscape of advanced renal cancer, but there are
still some limitations in their application, such as heterogeneity
in efficacy, immune-related adverse events and drug resistance
(5). Therefore, identifying a
reliable prognostic marker is crucial for individualized risk
assessment and adjustment of treatment strategies. In previous
years, increasing evidence has suggested that blood-based
inflammatory markers, particularly the neutrophil-to-lymphocyte
ratio (NLR), can predict the prognosis of patients with RCC
(6–9).
The relationship between inflammatory responses and
cancer has garnered significant attention in recent years (10,11).
Due to their low cost and easy accessibility, hematological
inflammatory markers, such as the NLR, have been widely tested
(12). NLR, a simple and readily
available inflammatory marker, is closely related to systemic
inflammation (13), and has been
widely used in prognostic studies of various cancer types, such as
colorectal (14), prostate
(15), uroepithelial (16), penile (17), lung (18) and breast (19) cancer. NLR is linked to poorer
outcomes in multiple cancer types, including penile, colorectal,
bladder, lung, breast, throat and ovarian cancer (17,20–25),
and high NLR levels often predict poorer survival. Several
meta-analyses have already explored the prognostic value of NLR in
patients with RCC, with existing research indicating that a high
NLR is associated with a poor prognosis (8,26).
Despite this, most studies suffer from several problems, such as
small sample sizes or single-center studies, or being limited to a
single hospital or region, limiting their external validity and
wide applicability. There are also inconsistent conclusions, with
some studies finding a significant correlation between NLR and RCC
prognosis (27,28), while others failed to reach a clear
conclusion (29–31).
The present study included the most recent articles,
thus ensuring the timeliness and reliability of the conclusions.
The role of NLR in the prognosis of RCC, especially its predictive
value for patient survival, recurrence and disease progression, was
further clarified in the present study to provide a more up-to-date
and accurate prognostic assessment.
Materials and methods
Search strategy
Multiple databases, including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase
(https://www.embase.com/), Cochrane Library
(https://www.cochranelibrary.com/) and
Web of Science (http://webofscience.com) were searched for all
relevant studies published from July 2021 to August 2024. The main
terms used in the search strategy included the following: (‘renal’
or ‘kidney’) and (‘carcinoma’ or ‘neoplasms’ or ‘cancer’ or
‘tumor’) and (‘NLR’ or ‘neutrophil-lymphocyte ratio’ or
‘neutrophil-to-lymphocyte ratio’). No language restrictions were
applied in the literature search to ensure the comprehensiveness of
the included studies. Some studies [such as Asif et al
(32)] have multiple sets of data;
therefore, in some analyses, certain studies are included >1 in
the analysis.
Inclusion and exclusion criteria
Studies were selected based on the following
inclusion criteria: i) Prospective or retrospective cohort studies
that evaluated the relationship between NLR and overall survival
(OS), recurrence-free survival (RFS), progression-free survival
(PFS) and cancer-specific survival (CSS) in patients with RCC. The
NLR values were taken before, during and after treatment. Most
studies collected NLR within the first 30 days of treatment, while
the study by Asif et al (32) included preoperative, perioperative
and postoperative NLR; ii) the included patients had not received
any treatment other than tumor-specific therapy prior to sample
collection; and iii) studies that directly provided hazard ratios
(HRs) with 95% confidence intervals (CIs) or had sufficient data to
calculate these statistics. If study data were duplicated, only the
data from the most recent study were used. The following exclusion
criteria were applied: i) Studies that did not provide sufficient
survival data for further analysis; ii) duplicate studies or
publications; and iii) expert opinions, conference abstracts,
editorials, case reports, letters, reviews or meta-analyses.
Date extraction
For each eligible study, two authors independently
extracted the following items: Study characteristics (first
author's name, recruitment region, publication year, study type and
sample size), patient information (sex, age and ethnicity),
pathological characteristics [TNM stage and histological subtype
(33)], disease type (localized or
metastatic), NLR cut-off values (number and/or percentage of
patients with high vs. low NLR), clinical characteristics
(treatment strategy, patient survival outcomes and follow-up
duration), and OS, RFS, PFS and CSS outcomes. In cases of
disagreement, consensus was reached through discussion with a third
researcher.
Quality assessment
The quality of each included study was assessed
using the Newcastle-Ottawa Scale (NOS), which comprises three
factors: Selection, comparability and exposure (34). The highest possible NOS score is 9,
with studies scoring ≥7, 4–6 and <4 being considered to have a
low, medium and high risk of bias, respectively. Disputes regarding
the quality assessment were resolved through discussion with a
third reviewer.
Statistical analysis
The primary endpoints of the present meta-analysis
were OS, RFS, PFS and CSS for all patients with RCC. If the
included studies directly reported survival analyses, HRs and 95%
CIs were extracted to calculate the pooled HR; otherwise, these
data were calculated and estimated from Kaplan-Meier survival
curves using Engauge Digitizer software (version 4.1; http://engauge-digitizer.updatestar.com/en) (35,36).
Cochran's Q test and the I2 statistic were used to
assess heterogeneity among the included studies (37). The present systematic review
followed the Cochrane Handbook for Evaluation of Intervention
Systems, and all analyses used only the random-effects model.
Sensitivity analysis was conducted by omitting each study
one-by-one to evaluate the stability of the results. Subgroup
analysis was also performed to explore the potential sources of
heterogeneity. Additionally, funnel plots and Egger's test were
used to assess the risk of publication bias. Egger's test and the
trim-and-fill method were conducted using Stata 12.0 software
(StataCorp LP). Other statistical analyses were performed using
Review Manager 5.3 software (Cochrane Collaboration). All P-values
were two-sided, and P<0.05 was considered to indicate a
statistically significant difference.
Quality of evidence
The quality of evidence regarding the prognostic
value of pre-treatment NLR for patients with RCC was evaluated
using the Grading of Recommendations Assessment, Development and
Evaluation system (38).
Results
Included literature
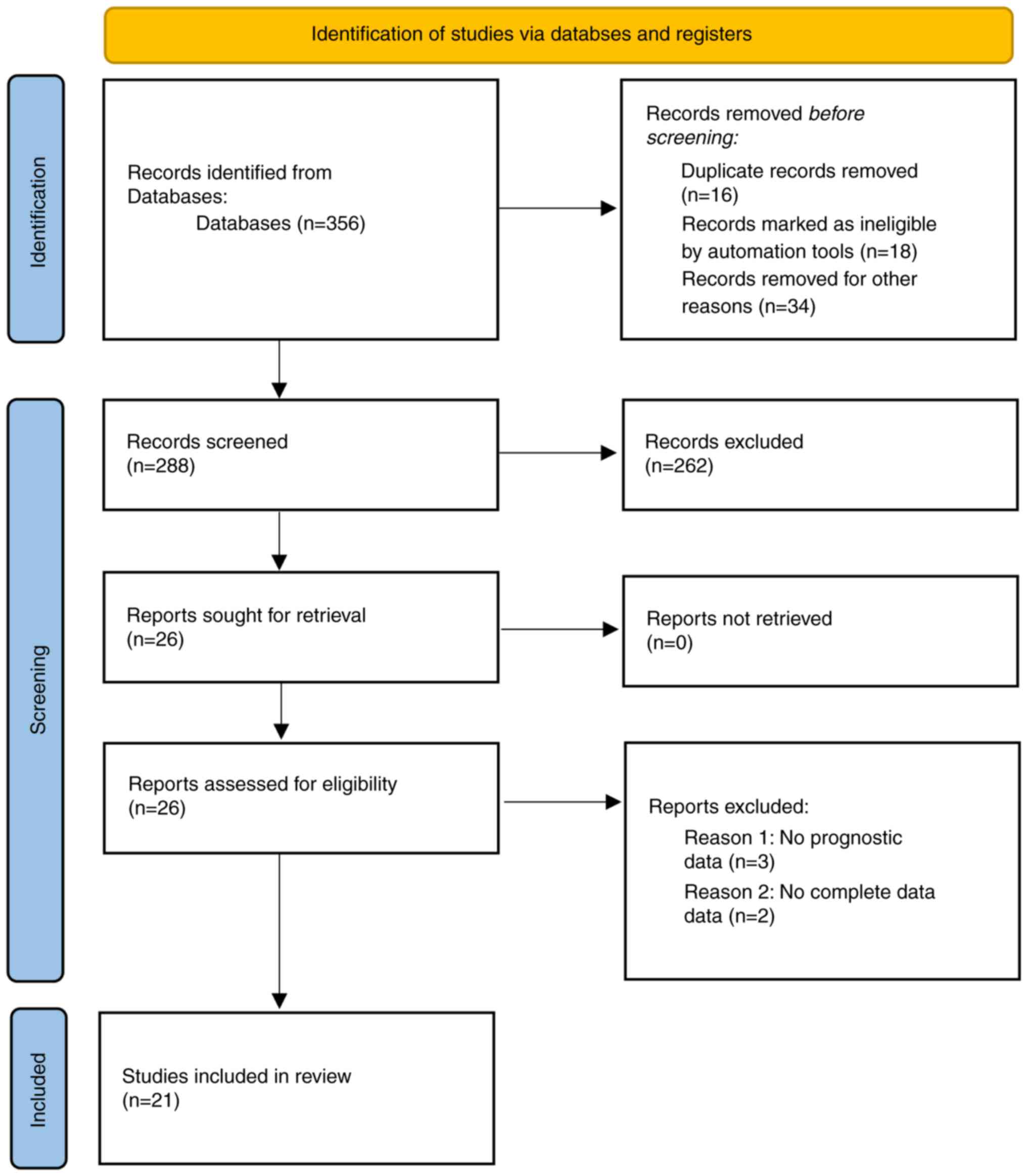
Based on the search strategy, 356 potentially
relevant records were identified. After removing duplicates, the
titles and abstracts of the remaining 288 records were reviewed.
The full texts of 68 records that met the inclusion criteria were
then assessed. Ultimately, 21 studies were included in the present
meta-analysis (7,27–32,39–52).
The study selection process is illustrated in a flow diagram
presented in Fig. 1.
Study characteristics
A total of 4,459 patients with RCC were included in
the present meta-analysis. Table I
presents the main characteristics of the 21 included studies, which
were published between 2021 and 2024. Among the 21 studies, 7
reported on localized/non-metastatic RCC, while 11 focused on
metastatic RCC and 3 on mixed RCC. Additionally, of the 21 studies,
18 reported OS data, 6 reported RFS or PFS data and 6 reported CSS
data. The histological types included clear cell RCC, papillary
RCC, non-clear cell RCC and mixed types. The cut-off values for NLR
ranged from 2.33 to 4.0. The HRs and 95% CIs for the 21 studies
were derived from multivariate Cox regression analyses and
Kaplan-Meier survival curves. The mean age of the patients ranged
from 57 to 73 years and the mean follow-up period ranged from 15.3
to 93.5 months. The NOS scores were 7 or 8, indicating that the
included studies were of a moderate to high quality (Table SI).
 | Table I.Characteristics of the studies
included in the meta-analysis. |
Table I.
Characteristics of the studies
included in the meta-analysis.
| First author,
year | Country | Sample size, n | Histology type | Metastatic
state | Mean age,
years | Treatment | Cut-off value,
determination method | Outcome | Mean follow-up,
months | NOS score | (Refs.) |
|---|
| Allenet et
al, 2022 | France | 786 | non-hereditary
RCC | Non-metastatic | N/A | Surgery | 2.70, based on
previous study | OS, RFS | 48.0 | 8 | (27) |
| Parosanu et
al, 2023 | Romania | 38 | ccRCC | Metastatic | 62.8 | Targeted therapy
and/or surgery | 3.00, ROC
curve | OS | 15.3 | 8 | (39) |
| Korkmaz et
al, 2023 | Turkiye | 110 | RCC | Metastatic | 65.0 | Surgery | 2.33, ROC
curve | OS, PFS | N/A | 7 | (28) |
| Parosanu et
al, 2023 | Romania | 74 | RCC | Metastatic | 62.5 | Surgery +
immunotherapy | 3.00, ROC
curve | OS, CSS | 15.3 | 7 | (40) |
| Nagamoto et
al, 2023 | Japan | 55 | RCC | Mixed | 66.0 | Immunotherapy | 2.90, ROC
curve | OS, CSS | 44.2 | 8 | (41) |
| Asif et al,
2023 | UK | 203 | Small renal cell
cancer | Non-metastatic | 73.0 | Surgery | 2.82, ROC
curve | OS, CSS, RFS, MFS
OS, | 93.5 | 8 | (32) |
| Ni et al,
2022 | China | 425 | RCC | Mixed | 65.0 | Surgery or
conservative treatment | 2.90, ROC
curve | CSS | 32.7 | 8 | (42) |
| Chaker et
al, 2022 | Tunis | 202 | RCC | Non-metastatic | 59.5 | Immunotherapy | 3.20, ROC
curve | RFS, MFS | 39.8 | 8 | (43) |
| Tucker et
al, 2021 | USA | 110 | ccRCC | Metastatic | 61.0 | Surgery | 3.42, ROC
curve | PFS, OS | N/A | 8 | (29) |
| Wang et al,
2023 | China | 198 | RCC | Metastatic | 57.0 | Surgery or surgery
+ drugsa | 3.11, ROC
curve | OS | N/A | 7 | (44) |
| Shang et al,
2021 | China | 203 | non-ccRCC | Non-metastatic | 61.0 | Image-guided
cryoablation or radiofrequency ablation | 4.00, data on
follow-up and blood counts | CSS | 46.0 | 8 | (7) |
| Wang et al,
2023 | China | 210 | RCC | Metastatic | 59.0 | Immunotherapy | 2.85, ROC
curve | OS, PFS | N/A | 8 | (45) |
| Young et al,
2024 | UK | 132 | ccRCC | Metastatic | 63.0 | Targeted drug
therapy | 3.00, univariate
analysis in ORR and DCR | OS | N/A | 8 | (31) |
| Khan et al,
2022 | USA | 158 | RCC | Metastatic | 61.3 | Surgery +
Immunotherapy | 3.50, based on
previous study | OS | N/A | 8 | (46) |
| Cheng et al,
2023 | China | 444 | ccRCC | Non-metastatic | 58.0 | Immunotherapy | 3.40, ROC
curve | RFS, CSS, OS | 70.0 | 8 | (47) |
| Zhang et al,
2023 | China | 328 | RCC | Non-metastatic | 57.0 | Surgery | 2.52, ROC
curve | OS | 64.0 | 7 | (48) |
| Cordeiro et
al, 2022 | Brazil | 187 | ccRCC | Non-metastatic | 63.4 | Immunotherapy | 4.00, ROC
curve | RFS | 48.7 | 8 | (30) |
| Anpalakhan et
al, 2023 | UK | 200 | RCC | Mixed | 69.7 | Surgery | 3.40, ROC
curve | OS | N/A | 7 | (49) |
| Rebuzzi et
al, 2022 | Turkiye | 306 | RCC | Metastatic | 70.0 | Surgery | 3.20, ROC
curve | OS, PFS | N/A | 8 | (50) |
| Aslan et al,
2022 | Italy | 52 | RCC | Metastatic | 65.0 | Immunotherapy | 3.40, median value
of NLR | OS, PFS | N/A | 8 | (51) |
| Ueda et al,
2022 | Japan | 38 | RCC | Metastatic | 68.0 | Surgery or surgery
+ drugsa | 3.00, based on
previous study | OS, PFS | N/A | 8 | (52) |
NLR and OS in RCC
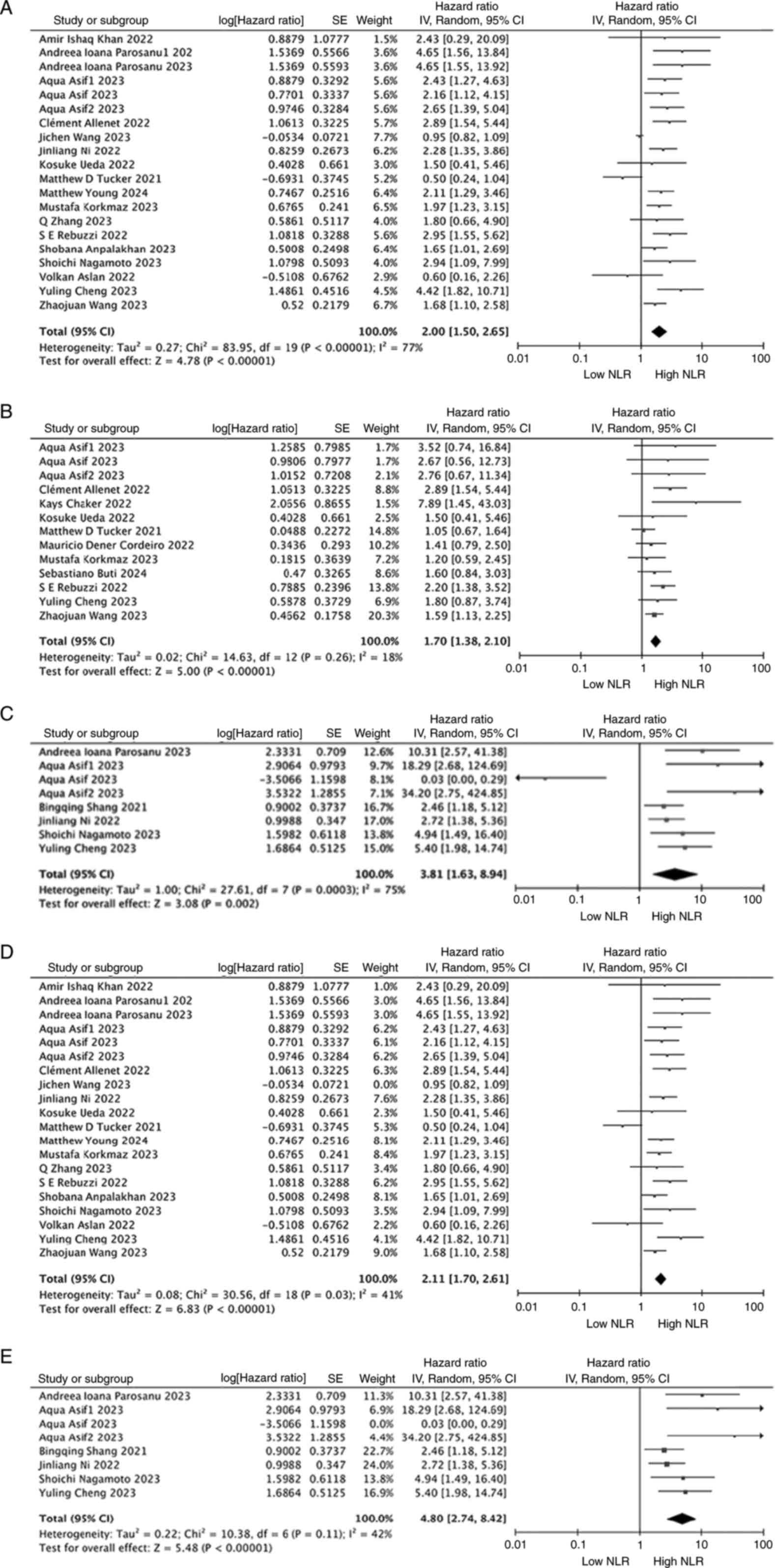
In total, 18 studies involving 3,867 patients with
RCC assessed the association between NLR and OS. The forest plot
utilizing a random-effects model to investigate the association
between NLR and OS demonstrated that in the overall population, a
high NLR was significantly associated with a shorter OS time (HR,
2.00; 95% CI, 1.50-2.65; P<0.00001; Fig. 2A). To explore whether individual
studies influenced the heterogeneity and conclusions, a sensitivity
analysis was conducted by sequentially excluding each study. After
excluding the study by Wang et al (45), the heterogeneity among the RCC
studies decreased (I2=41%, P<0.00001; Fig. 2D). Overall, the sensitivity analysis
results did not alter the above conclusions, confirming the
robustness of the findings.
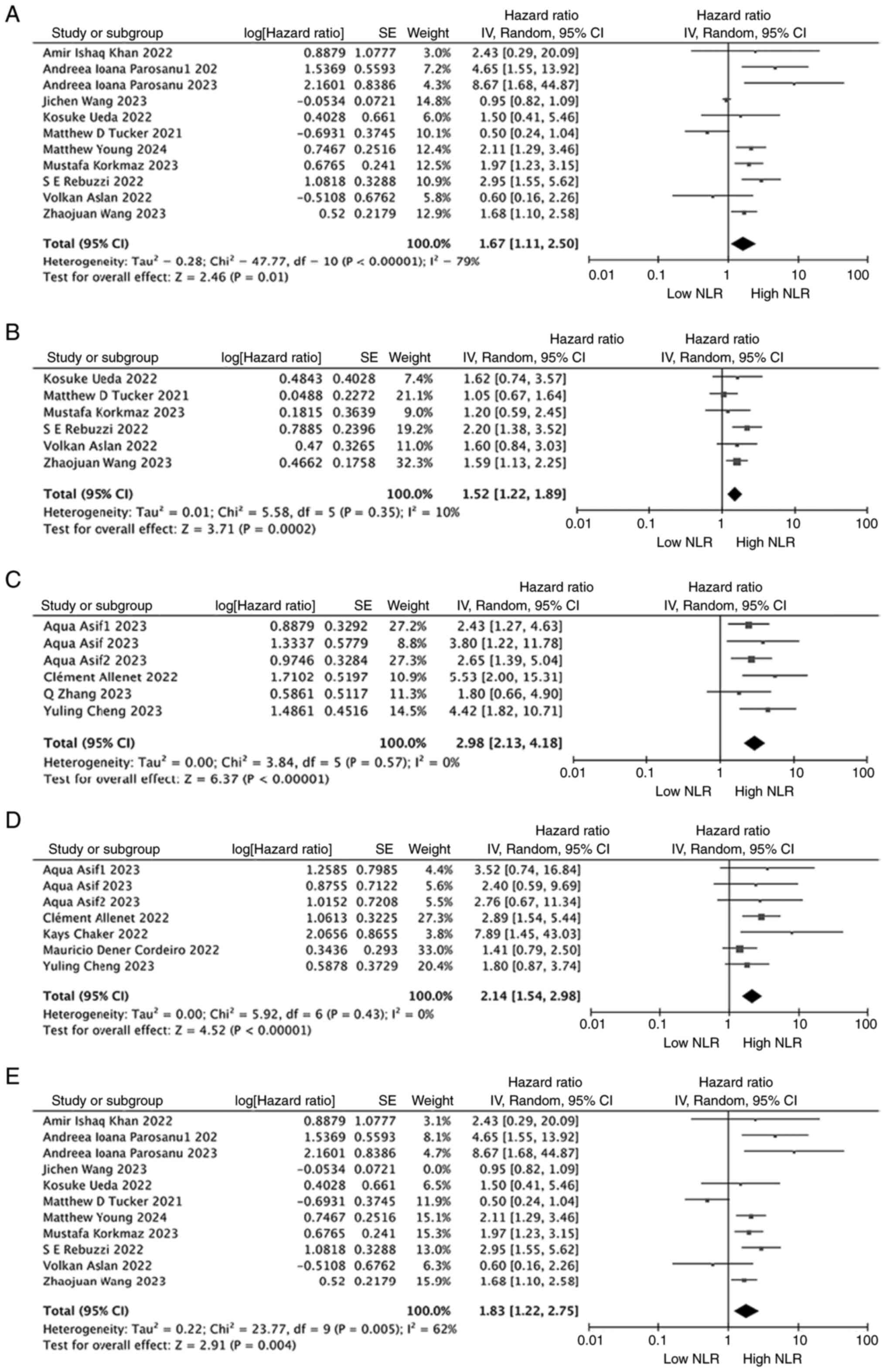
When evaluating the relationship between NLR and OS
in non-metastatic RCC, 4 studies that included 1,761 patients were
examined. In 11 studies involving 1,426 patients with metastatic
RCC, a similar relationship between NLR and OS was observed. The
meta-analysis showed that a high NLR was significantly associated
with poorer OS in both patients with non-metastatic (HR, 2.98; 95%
CI, 2.13–4.18; P<0.00001; I2=0%; Fig. 3C) and metastatic RCC (HR, 1.67; 95%
CI, 1.11–2.50; P=0.001; I2=79%; Fig. 3A). Notably, heterogeneity remained
significant in the metastatic RCC population (I2=79%,
P=0.001; Fig. 3A). The results
indicated that the studies by Wang et al (45), Tucker et al (29) and Aslan et al (51) (Fig.
3A) influenced the heterogeneity. Therefore, a sensitivity
analysis was performed in patients with metastatic RCC. The results
showed that excluding any single study, except for the study by
Wang et al (45), did not
significantly affect the heterogeneity. However, after removing the
study by Wang et al (45),
there was a significant effect on heterogeneity (Fig. 3E).
Due to the involvement of different study
characteristics, subgroup analyses to explore the potential sources
of heterogeneity in the metastatic RCC cohort were further
performed after excluding the study by Wang et al (45) (Table
II). In the subgroup analysis based on sample size,
heterogeneity was higher in patients with clear cell RCC (HR, 1.63;
95% CI, 0.51–5.16; P=0.41; I2=86%) and Caucasian
patients (HR, 2.29; 95% CI, 1.09–4.81; P=0.03; I2=76%).
Therefore, the main sources of heterogeneity may be histological
type (clear cell carcinoma) and ethnicity (Caucasian population),
as these factors had the highest I2 values, indicating
the greatest variability in study results under these
conditions.
 | Table II.Subgroup analysis for overall
survival in patients with metastatic renal cell carcinoma. |
Table II.
Subgroup analysis for overall
survival in patients with metastatic renal cell carcinoma.
|
|
|
|
|
| Heterogeneity |
|---|
|
|
|
|
|
|
|
|---|
|
Subgroupa | No. of studies | No. of
patients | HR (95% CI) | P-value | I2,
% | P-value |
|---|
| Overall | 11 | 1426 | 1.67
(1.11–2.50) | 0.01 | 79 | <0.00001 |
| Studies for
subgroup analysis | 10 | 1228 | 1.83
(1.22–2.75) | 0.004 | 62 | 0.005 |
| Ethnicity |
|
|
|
|
|
|
|
Caucasian | 6 | 818 | 2.29
(1.09–4.81) | 0.03 | 76 | 0.001 |
|
Asian | 4 | 410 | 1.69
(1.25–2.28) | 0.0006 | 0 | 0.43 |
| Sample size |
|
|
|
|
|
|
|
≥200 | 2 | 516 | 2.11
(1.23–3.62) | 0.007 | 51 | 0.15 |
|
<200 | 8 | 712 | 1.74
(0.98–3.06) | 0.006 | 67 | 0.003 |
| Histology type |
|
|
|
|
|
|
| Clear
cell carcinoma | 3 | 280 | 1.63
(0.51–5.16) | 0.41 | 86 | 0.0007 |
|
Others | 7 | 948 | 1.95
(1.49–2.54) | <0.00001 | 29 | 0.21 |
| Mean age,
years |
|
|
|
|
|
|
|
≥65 | 4 | 506 | 2.00
(1.41–2.85) | 0.0001 | 37 | 0.19 |
|
<65 | 6 | 722 | 1.96
(1.03–3.72) | 0.04 | 73 | 0.002 |
| Treatment |
|
|
|
|
|
|
| Surgery
or surgery + drugs | 5 | 686 | 2.59
(1.83–3.66) | <0.00001 | 14 | 0.33 |
|
Drugs | 5 | 542 | 1.20
(3.68–2.13) | 0.53 | 68 | 0.01 |
| NLR cut-off
value |
|
|
|
|
|
|
|
≥2.75 | 8 | 1066 | 2.00
(1.21–3.31) | 0.007 | 67 | 0.004 |
|
<2.75 | 2 | 162 | 1.29
(0.42–3.92) | 0.66 | 63 | 0.1 |
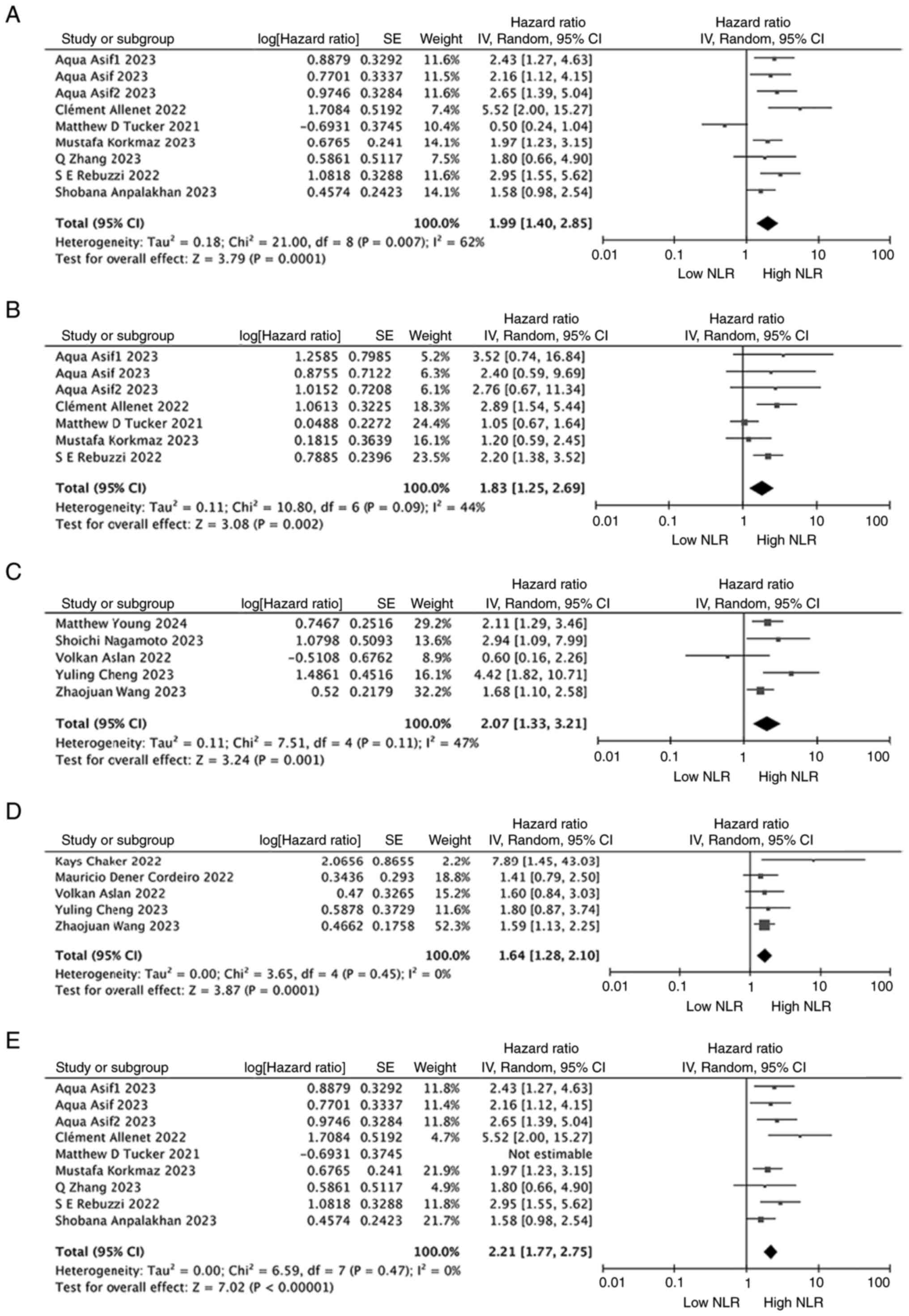
When evaluating the relationship between NLR and OS
in patients with RCC who underwent only surgical treatment, there
were 7 relevant studies but the study by Asif et al
(32) contained 3 datasets with
different patient cohorts (preoperative, intraoperative and
postoperative) and these datasets were therefore included in the
analysis separately. Thus, 9 studies/cohorts involving 2,043
surgically treated patients were examined. In 5 studies involving
893 patients with non-surgically treated RCC, a similar
relationship between NLR and OS was observed. The meta-analysis
showed that a high NLR was significantly associated with poorer OS
in both patients with surgically (HR, 1.99; 95% CI, 1.40–2.85;
P=0.0001; I2=62%; Fig.
4A) and non-surgically (HR, 2.07; 95% CI, 1.33–3.21; P=0.001;
I2=47%; Fig. 4C) treated
RCC. Notably, heterogeneity remained significant in the surgically
treated RCC population (Fig. 4A).
Therefore, a sensitivity analysis was conducted for studies
analyzing patients with surgically treated RCC. The results
indicated that the study by Tucker et al (29) influenced the heterogeneity, and
after excluding this study, the heterogeneity among the studies
decreased (I2=0%, P<0.00001; Fig. 4E). Overall, the sensitivity analysis
results did not alter the aforementioned conclusions, confirming
the robustness of the findings.
NLR and RFS/PFS in RCC
Due to the potential overlap in biological
significance between RFS and PFS in specific clinical contexts
(such as postoperative adjuvant therapy for solid tumors), and
since some studies did not strictly distinguish between these
endpoints, RFS and PFS were combined in this analysis.
Additionally, merging the two endpoints improved statistical power
and reduced bias from small sample sizes when individual analyses
of RFS or PFS were infeasible. When examining the association
between NLR and RFS/PFS, 11 studies involving 2,648 patients were
selected. The forest plot of the meta-analysis showed that a high
NLR was associated with poorer RFS/PFS in the overall population
(HR, 1.70; 95% CI, 1.38–2.10; P<0.00001; I2=18%;
Fig. 2B).
Upon further assessment of the relationship between
NLR and PFS in patients with metastatic RCC, a meta-analysis based
on 6 studies involving 826 patients indicated that a high NLR was
significantly associated with poorer PFS (HR, 1.52; 95% CI,
1.22–1.89; P=0.0002; I2=10%; Fig. 3B). Regarding the relationship
between NLR and RFS in patients with non-metastatic RCC, 5 studies
involving 1,822 patients were examined. The forest plot showed that
a high NLR was significantly associated with poorer RFS (HR, 2.14;
95% CI, 1.54–2.98; P<0.00001; I2=0%; Fig. 3D).
When the relationship between NLR and RFS/PFS in
patients with RCC who underwent only surgical treatment was
examined, 5 studies involving 1,515 patients were included. The
forest plot showed that a high NLR was significantly associated
with poorer RFS/PFS (HR, 1.83; 95% CI, 1.25–2.69; P=0.002;
I2=44%; Fig. 4B). For
the relationship between NLR and RFS/PFS in patients with RCC who
did not undergo surgery, based on 5 studies involving 1,095
patients, the forest plot showed that a high NLR was significantly
associated with poorer RFS/PFS (HR, 1.64; 95% CI, 1.28–2.10;
P<0.0001; I2=0%; Fig.
4D).
NLR and CSS in RCC
In total, 6 studies involving 1,404 patients
reported data on the association between NLR and CSS. The forest
plot of the meta-analysis indicated that a high NLR was
significantly associated with poorer CSS (HR, 3.81; 95% CI,
1.63–8.94; P=0.002; I2=75%; Fig. 2C). Of these 6 included studies, 3
studies involved non-metastatic RCC, 1 study involved metastatic
RCC and 2 studies involved mixed type. Therefore, the association
between NLR and CSS in patients with non-metastatic and metastatic
RCC was not further investigated separately.
Additionally, a sensitivity analysis was conducted
to explore whether any single study influenced heterogeneity and
the overall conclusion. After excluding the preoperative cohort in
the study by Asif et al (32), the heterogeneity among the
non-metastatic RCC studies notably changed (I2=42%,
P<0.0001; Fig. 2E). However, the
recalculated HR did not alter the aforementioned conclusions,
confirming the robustness of the results.
OS and RFS/PFS in patients treated
with ICIs
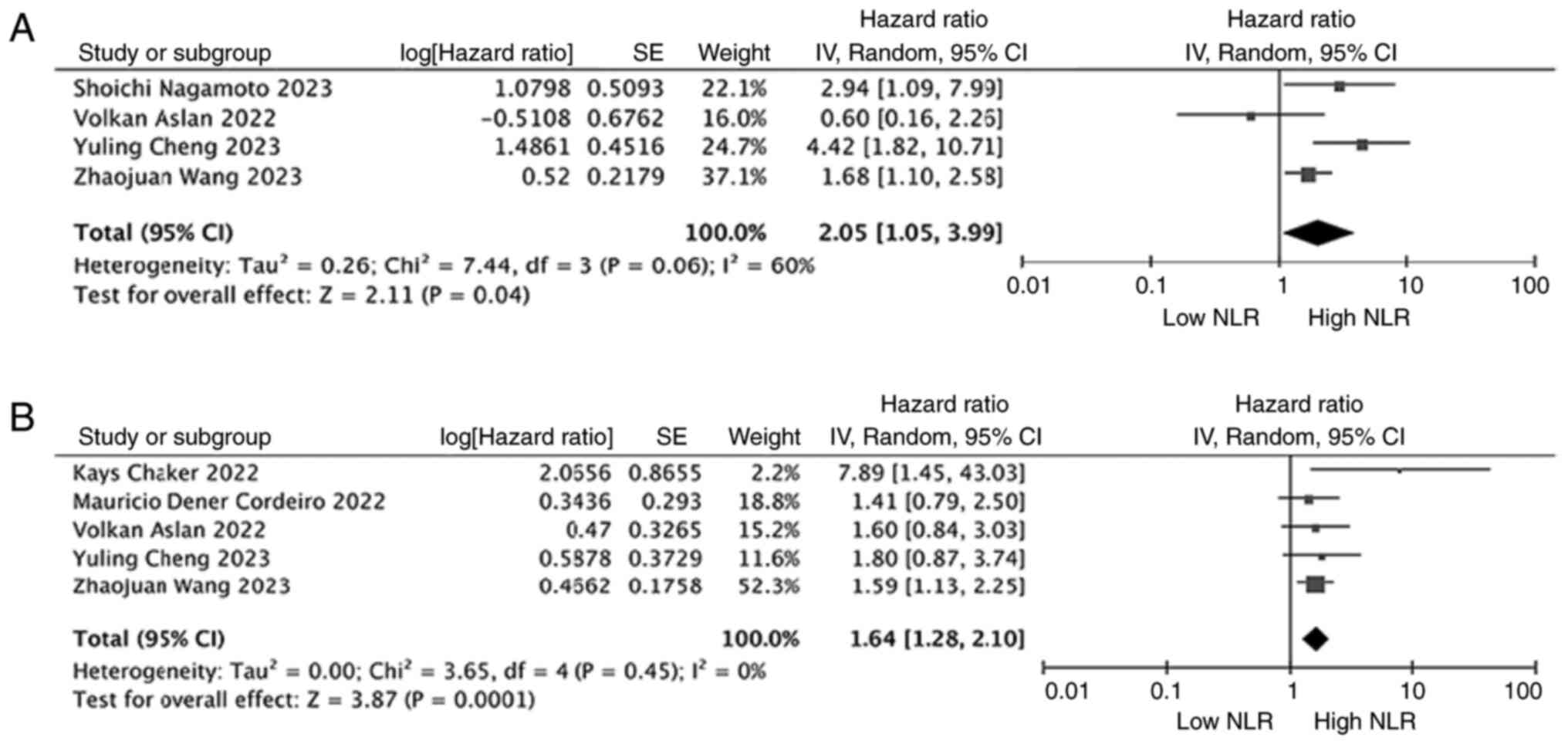
The prognostic value of NLR in patients with RCC who
were treated exclusively with ICIs was also examined. In examining
the relationship between NLR and OS in patients with RCC treated
with ICIs only, 4 studies involving 761 patients were included. The
results of the meta-analysis showed that high NLR was associated
with poorer OS (HR, 2.05; 95% CI, 1.05–3.99; P=0.04;
I2=60%; Fig. 5A). In
examining the relationship between NLR and RFS/PFS in patients with
RCC treated with ICIs only, 5 studies involving 1,095 patients were
included. The results of the meta-analysis showed that high NLR was
associated with poorer RFS/PFS (HR, 1.64; 95% CI, 1.28–2.10;
P=0.001; I2=0%; Fig.
5B).
Publication bias
The publication bias for OS, RFS/PFS and CSS was
assessed without considering the staging of patients with RCC. For
OS and CSS, the funnel plots were asymmetric (Fig. S1A and B). Egger's test also
indicated the presence of publication bias (both P<0.001).
Therefore, the trim-and-fill method was employed to test the
asymmetry of the funnel plot by hypothesizing the existence of
unpublished studies. The recalculated results demonstrated that a
high NLR was significantly associated with OS and CSS, with
statistical significance (P<0.05) after trimming and filling.
Furthermore, the combined results before and after trimming had
P<0.05, suggesting the stability of the results (Fig. S2A and B). For RFS/PFS, the funnel
plot was relatively symmetric (Fig.
S1C). Additionally, Egger's test showed no significant
publication bias (P=0.667).
Discussion
Inflammatory factors in the human body have a
crucial role in the occurrence, development and prognosis of tumors
(53). The inflammatory response is
a defense mechanism of the body against injury and infection, but
chronic inflammation can induce abnormal cell proliferation, DNA
damage and immune escape, thereby promoting the occurrence and
development of tumors (53,54). Inflammatory factors in the tumor
microenvironment, such as tumor necrosis factor-α, interleukins
(such as IL-6 and IL-1β) and C-reactive protein (55–57),
not only participate in the proliferation, invasion and metastasis
of tumor cells but also affect the tumor's response to treatment.
Therefore, the levels of inflammatory factors are often closely
related to the prognosis of patients with cancer, with higher
levels often indicating a poorer clinical prognosis (53,58).
Investigating the mechanisms by which inflammatory factors affect
tumors can help reveal the patterns of tumor occurrence and
development, and provide new insights and targets for early
diagnosis, personalized treatment and the prognosis assessment of
tumors.
NLR is a simple and reliable marker that can be used
to predict immune responses to infectious and non-infectious
stimuli and serves as a reliable indicator of cancer-associated
inflammation, as well as a predictor of tumor survival and
treatment outcomes (59,60). NLR has an important role in the
prognosis of RCC. This may be due to the fact that NLR reflects the
inflammatory response of the body, which serves a notable role in
tumor progression and metastasis (8). The present study systematically
evaluated the impact of NLR on the prognosis of patients with RCC
through a systematic review and meta-analysis of 4,459 patients.
The results showed that a high NLR was significantly associated
with a poor OS, RFS/PFS and CSS in patients with RCC. Additionally,
the results of the present study indicated that a high NLR was
significantly associated with a poor OS and RFS/PFS in patients
with RCC, regardless of the metastasis status or treatment type. In
the meta-analysis of metastatic RCC, the association between NLR
and OS demonstrated significant heterogeneity (I2=79%).
Despite sensitivity analyses, the heterogeneity remained high. To
further investigate the source of heterogeneity, subgroup analyses
based on the characteristics of the included studies were
performed, which demonstrated the stability and reliability of the
results. Overall, the results from the pooled data of the present
systematic review and meta-analysis suggest that NLR may be used as
a prognostic indicator for patients with RCC, aiding in clinical
decision-making and the selection of individualized treatment
strategies.
Neutrophils are a key component of the acute phase
of inflammation and are associated with cancer development.
Neutrophils can directly influence tumor cells, promoting cancer
progression, and indirectly modify the tumor microenvironment to
facilitate cancer metastasis (61).
Moreover, neutrophils can release vascular endothelial growth
factor, affecting tumor development (60,62).
By contrast, lymphocytes have an important role in the antitumor
immune response. Increased lymphocyte infiltration in the tumor
region is associated with improved responsiveness to treatment and
prognosis in patients with solid tumors (60). Additionally, lymphopenia (reduction
in CD4+ T cells) can impair lymphocyte-mediated
antitumor responses (63).
Therefore, NLR not only reflects the patient's inflammatory
response but also represents a decrease in antitumor immunity, with
elevated NLR often indicating lower survival rates and more
aggressive disease in patients with cancer.
The results of the present study align with several
others (26,64–68),
indicating that patients with high NLR typically have poorer
outcomes, which further underscores the potential clinical value of
NLR. This similarity may be attributed to the use of the same
inclusion criteria and measurement tools in all studies, as well as
the comparable sample sizes, which likely led to the consistency of
the findings. Moreover, these similar results provide a solid
evidence base for future research, as NLR, a simple and easily
obtainable inflammatory marker, has been repeatedly validated in
various studies as being closely associated with patient prognosis.
Looking ahead, multicenter prospective studies are needed to
further confirm the applicability and feasibility of NLR in
different populations. For patients with high NLR, future clinical
research could explore interventions such as anti-inflammatory
treatments or immune modulation therapies to reduce NLR levels,
thereby improving prognosis.
Although NLR demonstrates considerable predictive
value for prognosis in various cancer types [such as colorectal
(14), prostate (15), uroepithelial (16), penile (17), lung (18) and breast (19) cancer], the use of this single marker
has its limitations. These limitations include non-specificity
(interference by infection or coexisting disease), variability in
measurement time points and methods, lack of consistent thresholds
and confounding effects of therapeutic interventions on
inflammatory signals. Future studies should focus on the combined
use of NLR with other inflammatory markers, molecular biomarkers
and clinical pathological features (such as TNM staging and tumor
markers) to build more accurate prognostic models. This integrated
approach could provide essential insights for personalized
treatment strategies. In the context of personalized therapy, NLR,
as a marker of immune-inflammatory response, could assist in
predicting the response of patients to immunotherapy or targeted
therapies. Future research may investigate the relationship between
NLR and treatment response, exploring whether treatment plans can
be tailored based on NLR levels, thereby improving therapeutic
efficacy and minimizing unnecessary side effects.
Despite the notable potential of NLR as a prognostic
indicator in clinical practice, its translation into routine
clinical use faces several challenges. Future studies need to
address issues such as standardizing NLR measurement methods and
managing the heterogeneity arising from factors such as ethnicity,
age, sex and histological type. Additionally, staging may influence
NLR levels through systemic inflammatory responses and
immunosuppressive status, which is particularly relevant in
patients with advanced disease. Subgroup analyses based on staging
were not performed in the present study, primarily due to sample
size limitations that could have affected the statistical power.
The present study was designed to initially evaluate the overall
prognostic value of NLR. However, future research will aim to
expand the cohort and conduct more targeted analyses to validate
the staging-specific effects. Furthermore, large-scale multicenter
prospective studies will be essential to provide a stronger
evidence foundation for the widespread application of NLR.
The present analysis has several limitations
warranting consideration. Primarily, the reliance on non-randomized
observational designs with limited participant numbers may restrict
generalizability. Although random-effects models were applied to
address variability, residual heterogeneity persisted in stratified
assessments, potentially reflecting unmeasured covariates or
population diversity. While sensitivity analyses mitigated
detection bias, residual selection bias or unmeasured confounders
may persist despite analytical controls. Regarding methodological
validity, statistical adjustments using the trim-and-fill method
indicated that the core findings remained consistent; however,
undetected publication bias in smaller cohorts could still
influence effect estimates. Additionally, the absence of
standardized NLR thresholds remains a critical gap. Current
practices often adopt optimal thresholds derived from receiver
operating characteristic curves or extrapolate values from prior
cohorts, introducing comparability challenges across datasets.
Prospective validation through multicenter collaborations is
imperative to establish NLR criteria consistent with clinical
endpoints (such as progression-free intervals) while accounting for
treatment-era effects and biomarker-temporal dynamics.
In conclusion, the results of the present
meta-analysis suggest that elevated NLR is a potential biomarker
for the prognostic evaluation of patients with RCC. Clinically, for
the treatment of RCC, NLR could be considered in the routine
assessment of patients to more accurately predict the prognosis of
this disease.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to acknowledge the help
provided by Dr Junjie Hu (Department of Urology, Lanxi People's
Hospital) in analyzing the large number of samples.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
KCL conceived the manuscript and performed data
acquisition, data analysis and statistical analysis. XC assisted
with data acquisition, data analysis and manuscript preparation. XC
reviewed the manuscript and polished the grammar. KCL and XC
confirm the authenticity of all the raw data. Both authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Capitanio U and Montorsi F: Renal cancer.
Lancet. 387:894–906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ, Jonasch E, Agarwal N, Bhayani
S, Bro WP, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Fishman
M, et al: Kidney cancer, version 2.2017, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 15:804–834. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang JC and Childs R: Immunotherapy for
renal cell cancer. J Clin Oncol. 24:5576–5583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simonaggio A, Elaidi R, Fournier L, Fabre
E, Ferrari V, Borchiellini D, Thouvenin J, Barthelemy P, Thibault
C, Tartour E, et al: Variation in neutrophil to lymphocyte ratio
(NLR) as predictor of outcomes in metastatic renal cell carcinoma
(mRCC) and non-small cell lung cancer (mNSCLC) patients treated
with nivolumab. Cancer Immunol Immunother. 69:2513–2522. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang B, Guo L, Shen R, Cao C, Xie R,
Jiang W, Wen L, Bi X, Shi H, Zheng S, et al: Prognostic
significance of NLR about NETosis and lymphocytes perturbations in
localized renal cell carcinoma with tumor thrombus. Front Oncol.
11:7715452021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boissier R, Campagna J, Branger N,
Karsenty G and Lechevallier E: The prognostic value of the
neutrophil-lymphocyte ratio in renal oncology: A review. Urol
Oncol. 35:135–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu H, Yao X, Xie X, Wu X, Zheng C, Xia W
and Ma S: Prognostic value of preoperative NLR, dNLR, PLR and CRP
in surgical renal cell carcinoma patients. World J Urol.
35:261–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao
C, Liu W, Deng H, Li J, Ning P and Wang Z: Pyroptosis in
inflammatory diseases and cancer. Theranostics. 12:4310–4329. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee HM, Lee HJ and Chang JE: Inflammatory
cytokine: An attractive target for cancer treatment. Biomedicines.
10:21162022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma N and Jha S: NLR-regulated pathways
in cancer: Opportunities and obstacles for therapeutic
interventions. Cell Mol Life Sci. 73:1741–1764. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Jia H, Yu W, Xu Y, Li X, Li Q and
Cai S: Nomograms for predicting prognostic value of inflammatory
biomarkers in colorectal cancer patients after radical resection.
Int J Cancer. 139:220–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li MX, Liu XM, Zhang XF, Zhang JF, Wang
WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L and Lv Y: Prognostic role
of neutrophil-to-lymphocyte ratio in colorectal cancer: A
systematic review and meta-analysis. Int J Cancer. 134:2403–2413.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu X, Gao X, Li X, Qi X, Ma M, Qin S, Yu
H, Sun S, Zhou D and Wang W: Prognostic significance of
neutrophil-to-lymphocyte ratio in prostate cancer: Evidence from
16,266 patients. Sci Rep. 6:220892016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marchioni M, Primiceri G, Ingrosso M,
Filograna R, Castellan P, De Francesco P and Schips L: The clinical
use of the neutrophil to lymphocyte ratio (NLR) in urothelial
cancer: A systematic review. Clin Genitourin Cancer. 14:473–484.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saputra HM, Hidayatullah F, Kloping YP,
Renaldo J, Chung E and Hakim L: Prognostic value of
neutrophil-to-lymphocyte ratio (NLR) in penile cancer: A systematic
review and meta-analysis. Ann Med Surg (Lond).
81:1043352022.PubMed/NCBI
|
|
18
|
Stares M, Ding TE, Stratton C, Thomson F,
Baxter M, Cagney H, Cumming K, Swan A, Ross F, Barrie C, et al:
Biomarkers of systemic inflammation predict survival with
first-line immune checkpoint inhibitors in non-small-cell lung
cancer. ESMO Open. 7:1004452022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tokumaru Y, Oshi M, Murthy V, Tian W, Yan
L, Angarita FA, Nagahashi M, Matsuhashi N, Futamura M, Yoshida K,
et al: Low intratumoral genetic neutrophil-to-lymphocyte ratio
(NLR) is associated with favorable tumor immune microenvironment
and with survival in triple negative breast cancer (TNBC). Am J
Cancer Res. 11:5743–5755. 2021.PubMed/NCBI
|
|
20
|
Ouyang H, Xiao B, Huang Y and Wang Z:
Baseline and early changes in the neutrophil-lymphocyte ratio (NLR)
predict survival outcomes in advanced colorectal cancer patients
treated with immunotherapy. Int Immunopharmacol. 123:1107032023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kobayashi T, Ito K, Kojima T, Maruyama S,
Mukai S, Tsutsumi M, Miki J, Okuno T, Yoshio Y, Matsumoto H, et al:
Pre-pembrolizumab neutrophil-to-lymphocyte ratio (NLR) predicts the
efficacy of second-line pembrolizumab treatment in urothelial
cancer regardless of the pre-chemo NLR. Cancer Immunol Immunother.
71:461–471. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mandaliya H, Jones M, Oldmeadow C and
Nordman II: Prognostic biomarkers in stage IV non-small cell lung
cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to
monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and
advanced lung cancer inflammation index (ALI). Transl Lung Cancer
Res. 8:886–894. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grassadonia A, Graziano V, Iezzi L, Vici
P, Barba M, Pizzuti L, Cicero G, Krasniqi E, Mazzotta M, Marinelli
D, et al: Prognostic relevance of neutrophil to lymphocyte ratio
(NLR) in luminal breast cancer: A retrospective analysis in the
neoadjuvant setting. Cells. 10:16852021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu X, Tian T, Zhang X, Sun Q, Chen Y and
Jiang W: Neutrophil-to-lymphocyte and hypopharyngeal cancer
prognosis: System review and meta-analysis. Head Neck. 45:492–502.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen S, Zhang L, Yan G, Cheng S, Fathy AH,
Yan N and Zhao Y: Neutrophil-to-lymphocyte ratio is a potential
prognostic biomarker in patients with ovarian cancer: A
meta-analysis. Biomed Res Int. 2017:79434672017.PubMed/NCBI
|
|
26
|
Shao Y, Wu B, Jia W, Zhang Z, Chen Q and
Wang D: Prognostic value of pretreatment neutrophil-to-lymphocyte
ratio in renal cell carcinoma: A systematic review and
meta-analysis. BMC Urol. 20:902020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Allenet C, Klein C, Rouget B, Margue G,
Capon G, Alezra E, Blanc P, Estrade V, Bladou F, Robert G and
Bernhard JC: Can pre-operative neutrophil-to-lymphocyte ratio (NLR)
help predict non-metastatic renal carcinoma recurrence after
nephrectomy? (UroCCR-61 study). Cancers (Basel). 14:56922022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Korkmaz M and Erylmaz MK: Systemic
inflammatory markers predicting the overall survival of patients
using tyrosine kinase inhibitors in the first-line treatment of
metastatic renal cell carcinoma. J Coll Physicians Surg Pak.
33:653–658. 2023.PubMed/NCBI
|
|
29
|
Tucker MD, Brown LC, Chen YW, Kao C,
Hirshman N, Kinsey EN, Ancell KK, Beckermann KE, Davis NB,
McAlister R, et al: Association of baseline
neutrophil-to-eosinophil ratio with response to nivolumab plus
ipilimumab in patients with metastatic renal cell carcinoma.
Biomark Res. 9:802021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cordeiro MD, Ilario EN, Abe DK, Carvalho
PA, Muniz DQB, Sarkis AS, Coelho RF, Guimarães RM, Haddad MV and
Nahas WC: Neutrophil-to-lymphocyte ratio predicts cancer outcome in
locally advanced clear renal cell carcinoma. Clin Genitourin
Cancer. 20:102–106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Young M, Tapia JC, Szabados B, Jovaisaite
A, Jackson-Spence F, Nally E and Powles T: NLR outperforms low
hemoglobin and high platelet count as predictive and prognostic
biomarker in metastatic renal cell carcinoma treated with immune
checkpoint inhibitors. Clin Genitourin Cancer. 22:1020722024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Asif A, Chan VWS, Osman FH, Koe JSE, Ng A,
Burton OE, Cartledge J, Kimuli M, Vasudev N, Ralph C, et al: The
prognostic value of neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio for small renal cell carcinomas after
image-guided cryoablation or radio-frequency ablation. Cancers
(Basel). 15:21872023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lo CK, Mertz D and Loeb M:
Newcastle-Ottawa Scale: Comparing reviewers' to authors'
assessments. BMC Med Res Methodol. 14:452014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist
G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al:
GRADE guidelines: 1. Introduction-GRADE evidence profiles and
summary of findings tables. J Clin Epidemiol. 64:383–394. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parosanu AI, Baston C, Stanciu IM, Parlog
CF and Nitipir C: Second-line treatment of metastatic renal cell
carcinoma in the era of predictive biomarkers. Diagnostics (Basel).
13:24302023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Parosanu AI, Pirlog CF, Slavu CO, Stanciu
IM, Cotan HT, Vrabie RC, Popa AM, Olaru M, Iaciu C, Bratu LI, et
al: The prognostic value of neutrophil-to-lymphocyte ratio in
patients with metastatic renal cell carcinoma. Curr Oncol.
30:2457–2464. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nagamoto S, Urakami S, Oka S, Ogawa K,
Kono K, Sakaguchi K, Kinowaki K, Yamada D and Kume H: Impact of the
neutrophil-to-lymphocyte ratio as a surgical prognostic factor in
renal cell carcinoma with inferior-vena-cava tumor thrombus. Asian
J Surg. 46:192–200. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ni J, Wang Y, Zhang H, Wang K, Song W, Luo
M, Che J, Geng J, Xu Y, Yao X, et al: Combination of preoperative
plasma fibrinogen and neutrophil-to-lymphocyte ratio to predict the
prognosis for patients undergoing laparoscopic nephrectomy for
renal cell carcinoma. Am J Cancer Res. 12:3713–3728.
2022.PubMed/NCBI
|
|
43
|
Chaker K, Ouanes Y, Dali KM, Bibi M,
Messaoudi Y, Mosbehi B, Abid K, Sellami A, Ben Rhouma S and Nouira
Y: The prognostic value of preoperative neutrophil-to-lymphocyte
ratio in patients with non-metastatic renal cell carcinoma. Prog
Urol. 32:585–592. 2022.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang J, Ye J, Zhao X, Li X and Ma X:
Prognostic value and model construction of preoperative
inflammatory markers in patients with metastatic renal cell
carcinoma. World J Surg Oncol. 21:2112023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Z, Qin Y, Chai X, Lu L, Xue P, Lu R,
Miao C, Ma H, Hu X and Yao J: Systemic inflammatory biomarkers
predict survival of patients treated with tyrosine kinase
inhibitors for metastatic renal cell carcinoma. Cancer Control.
30:107327482311975112023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Khan AI, Psutka SP, Patil DH, Hong G,
Williams MA, Bilen MA, Sekhar A, Kissick HT, Narayan VM, Joshi SS,
et al: Sarcopenia and systemic inflammation are associated with
decreased survival after cytoreductive nephrectomy for metastatic
renal cell carcinoma. Cancer. 128:2073–2084. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cheng Y, Kou W and Zhu Y: Preoperative
inflammation-associated blood cell markers in patients with
non-metastatic clear cell renal cell carcinoma: A retrospective
study. Int J Gen Med. 16:3067–3080. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Q, Song HF, Ma BL, Zhang ZN, Zhou
CH, Li AL, Liu J, Liang L, Zhu SY and Zhang Q: Pre-operative
prognostic nutritional index as a predictive factor for prognosis
in non-metastatic renal cell carcinoma treated with surgery.
Beijing Da Xue Xue Bao Yi Xue Ban. 55:149–155. 2023.(In Chinese).
PubMed/NCBI
|
|
49
|
Anpalakhan S, Signori A, Cortellini A,
Verzoni E, Giusti R, Aprile G, Ermacora P, Catino A, Pipitone S, Di
Napoli M, et al: Using peripheral immune-inflammatory blood markers
in tumors treated with immune checkpoint inhibitors: An INVIDIa-2
study sub-analysis. iScience. 26:1079702023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rebuzzi SE, Signori A, Buti S, Banna GL,
Murianni V, Damassi A, Maruzzo M, Giannarelli D, Tortora G, Galli
L, et al: Validation of the Meet-URO score in patients with
metastatic renal cell carcinoma receiving first-line nivolumab and
ipilimumab in the Italian expanded access program. ESMO Open.
7:1006342022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Aslan V, Kılıç ACK, Sütcüoğlu O, Eraslan
E, Bayrak A, Öksüzoğlu B, Tahtacı G, Özdemir N, Üner A, Günel N, et
al: Cachexia index in predicting outcomes among patients receiving
immune checkpoint inhibitor treatment for metastatic renal cell
carcinoma. Urol Oncol. 40:494.e1–e10. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ueda K, Suekane S, Kurose H, Ogasawara N,
Hiroshige T, Chikui K, Uemura K, Nakiri M, Nishihara K, Matsuo M
and Igawa T: Absolute lymphocyte count is an independent predictor
of survival in patients with metastatic renal cell carcinoma
treated with nivolumab. Jpn J Clin Oncol. 52:179–186. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Singh N, Baby D, Rajguru JP, Patil PB,
Thakkannavar SS and Pujari VB: Inflammation and cancer. Ann Afr
Med. 18:121–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mierke CT: The fundamental role of
mechanical properties in the progression of cancer disease and
inflammation. Rep Prog Phys. 77:0766022014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Balkwill F: TNF-alpha in promotion and
progression of cancer. Cancer Metastasis Rev. 25:409–416. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Orange ST, Leslie J, Ross M, Mann DA and
Wackerhage H: The exercise IL-6 enigma in cancer. Trends Endocrinol
Metab. 34:749–763. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hart PC, Rajab IM, Alebraheem M and
Potempa LA: C-reactive protein and cancer-diagnostic and
therapeutic insights. Front Immunol. 11:5958352020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Carnevale S, Ghasemi S, Rigatelli A and
Jaillon S: The complexity of neutrophils in health and disease:
Focus on cancer. Semin Immunol. 48:1014092020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zahorec R: Neutrophil-to-lymphocyte ratio,
past, present and future perspectives. Bratisl Lek Listy.
122:474–488. 2021.PubMed/NCBI
|
|
60
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shaul ME and Fridlender ZG:
Tumour-associated neutrophils in patients with cancer. Nat Rev Clin
Oncol. 16:601–620. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Masucci MT, Minopoli M and Carriero MV:
Tumor associated neutrophils. Their role in tumorigenesis,
metastasis, prognosis and therapy. Front Oncol. 9:11462019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang B, Gu W, Wan F, Shi G and Ye D:
Prognostic significance of the dynamic changes of systemic
inflammatory response in metastatic renal cell carcinoma. Int Braz
J Urol. 45:89–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hu K, Lou L, Ye J and Zhang S: Prognostic
role of the neutrophil-lymphocyte ratio in renal cell carcinoma: A
meta-analysis. BMJ Open. 5:e0064042015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chandrasekaran D, Sundaram S, Maheshkumar
K, Kathiresan N and Padmavathi R: Preoperative
neutrophil-lymphocyte ratio/platelet-lymphocyte ratio: A potential
and economical marker for renal cell carcinoma. J Cancer Res Ther.
18:1635–1639. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ohno Y, Nakashima J, Ohori M, Gondo T,
Hatano T and Tachibana M: Followup of neutrophil-to-lymphocyte
ratio and recurrence of clear cell renal cell carcinoma. J Urol.
187:411–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ito K, Masunaga A, Tanaka N, Mizuno R,
Shirotake S, Yasumizu Y, Ito Y, Miyazaki Y, Hagiwara M, Kanao K, et
al: Impact of inflammatory marker levels one month after the
first-line targeted therapy initiation on progression-free survival
prediction in patients with metastatic clear cell renal cell
carcinoma. Jpn J Clin Oncol. 49:69–76. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen X, Meng F and Jiang R:
Neutrophil-to-lymphocyte ratio as a prognostic biomarker for
patients with metastatic renal cell carcinoma treated with immune
checkpoint inhibitors: A systematic review and meta-analysis. Front
Oncol. 11:7469762021. View Article : Google Scholar : PubMed/NCBI
|