Introduction
Owing to advancements in therapeutic approaches, the
overall survival (OS) rates of patients diagnosed with colorectal
cancer (CRC) have increased (1).
However, as survival rates have improved, a corresponding increase
in the incidence of brain metastasis (BM) has been observed in
patients with CRC (2,3), with the reported occurrence of BM
arising from CRC ranging from 0.1–11% (2). Regarding the timing of BM development,
synchronous BM indicates that metastasis occurs around the same
time or shortly after a primary cancer. By contrast, metachronous
BM refers to cases where a significant interval exists between the
diagnosis of a primary tumor and the development of BM (4). The exact time interval for defining
synchronous or metachronous BM remains inconclusive in the
literature.
The impact of the timing of BM development has been
discussed in several studies; however, most of these studies have
primarily focused on patients with BM originating from lung cancer
or renal cell carcinoma (4–6). Despite the emergence of studies
focusing on treatment strategies and prognostic factors for
survival outcomes in patients with BM from CRC, uncertainties
remain regarding treatment strategies and prognostic factors
(7–9), and studies on the impact of
synchronous BM and metachronous BM specifically in patients with
CRC are limited.
According to the literature, synchronous BM is more
likely to be associated with poorly differentiated histology,
right-sided CRC and aggressive histopathological features,
including higher tumor and lymph node stages (10). Distinguishing between synchronous
and metachronous BM may impact the choice of therapeutic
interventions, such as considering more aggressive approaches for
synchronous BM or prioritizing systemic therapy for metachronous
cases (6). Moreover, understanding
the timing of BM can facilitate discussions with patients regarding
their prognosis, enabling clinicians to tailor communication and
support strategies based on the expected disease course. Therefore,
the aim of the present study was to assess whether the timing of BM
development affects the survival outcomes of patients with BM from
CRC by performing a comprehensive review of the published
literature and a meta-analysis to quantitatively assess this
clinical question.
Materials and methods
Study protocol
A systematic review and meta-analysis was performed
according to the guidelines outlined in the Cochrane Handbook for
Systematic Reviews and Interventions (11). Reporting of the results of the
present study adhered to the Preferred Reporting Items for
Systematic Reviews and Meta-Analyses guidelines. The present study
was registered on the PROSPERO online platform to ensure
transparency and accessibility of the research protocol
(registration no. CRD42023430369; http://www.crd.york.ac.uk/prospero/display_record.php?RecordID=430369).
Study selection
A comprehensive search in the Cochrane Library
(https://www.cochranelibrary.com), Embase
(https://www.embase.com) and MEDLINE (https://www.wolterskluwer.com/zh–tw/solutions/ovid/ovid–medline–901)
electronic databases was performed, covering the period from
January 1900 to December 2023. The key words were ‘colorectal
cancer’ and ‘brain metastasis’. No language restrictions were
applied during the search process to ensure inclusivity. A total of
two investigators (TCT and KYC) independently performed the
searches and identified relevant studies for potential inclusion.
Any discrepancies were resolved through consensus between the
investigators or by consulting a senior reviewer (YC).
Eligibility criteria
The following studies were included in the analysis:
i) Prospective, retrospective cohort and case-control studies; ii)
studies specifically focusing on patients diagnosed with BM from
CRC; and iii) studies reporting survival outcomes for patients with
synchronous and metachronous BM. The following studies were
excluded from the analysis: Case reports, editorials, letters to
the editor, review articles and conference abstracts. The
application of these criteria ensured the relevance and
appropriateness of the studies included in the analysis.
Data extraction
The data extraction process was performed
independently by two investigators (TCT and KYC). The following
information was extracted from eligible studies: First author;
publication year; inclusion criteria; definition of synchronous
brain BM; number of patients; and relevant survival outcomes. This
approach ensured the accurate and comprehensive collection of data
from the selected studies.
Quality assessment
To assess the risk of bias in the included
literature, the Risk of Bias In Non-randomized Studies of
Interventions (ROBINS-I) tool was utilized (12). A total of two investigators (TCT and
KYC) independently performed the critical appraisal of the included
studies using this tool. When disagreements between the assessors
occurred, a third investigator (YC) was consulted to reach a
consensus on the item in question.
Statistical analysis
Statistical analyses were performed utilizing the
meta package within R software (version R-4.4.2; http://www.r–project.org/). To evaluate the
comparative survival outcomes of patients with synchronous and
metachronous BM, the hazard ratios (HRs) for OS were acquired based
on the survival analysis results from the included studies. These
HRs were then pooled with the inverse variance method. The
meta-analysis was performed using a random-effects model, and the
effect sizes were presented alongside their corresponding 95%
confidence intervals (CIs). Heterogeneity among the included
studies was assessed using the I2 statistics proposed by
Higgins et al (13).
I2 <25% indicated low heterogeneity,
I2=25–50% denoted moderate heterogeneity and
I2 >50% demonstrated high heterogeneity (14).
Results
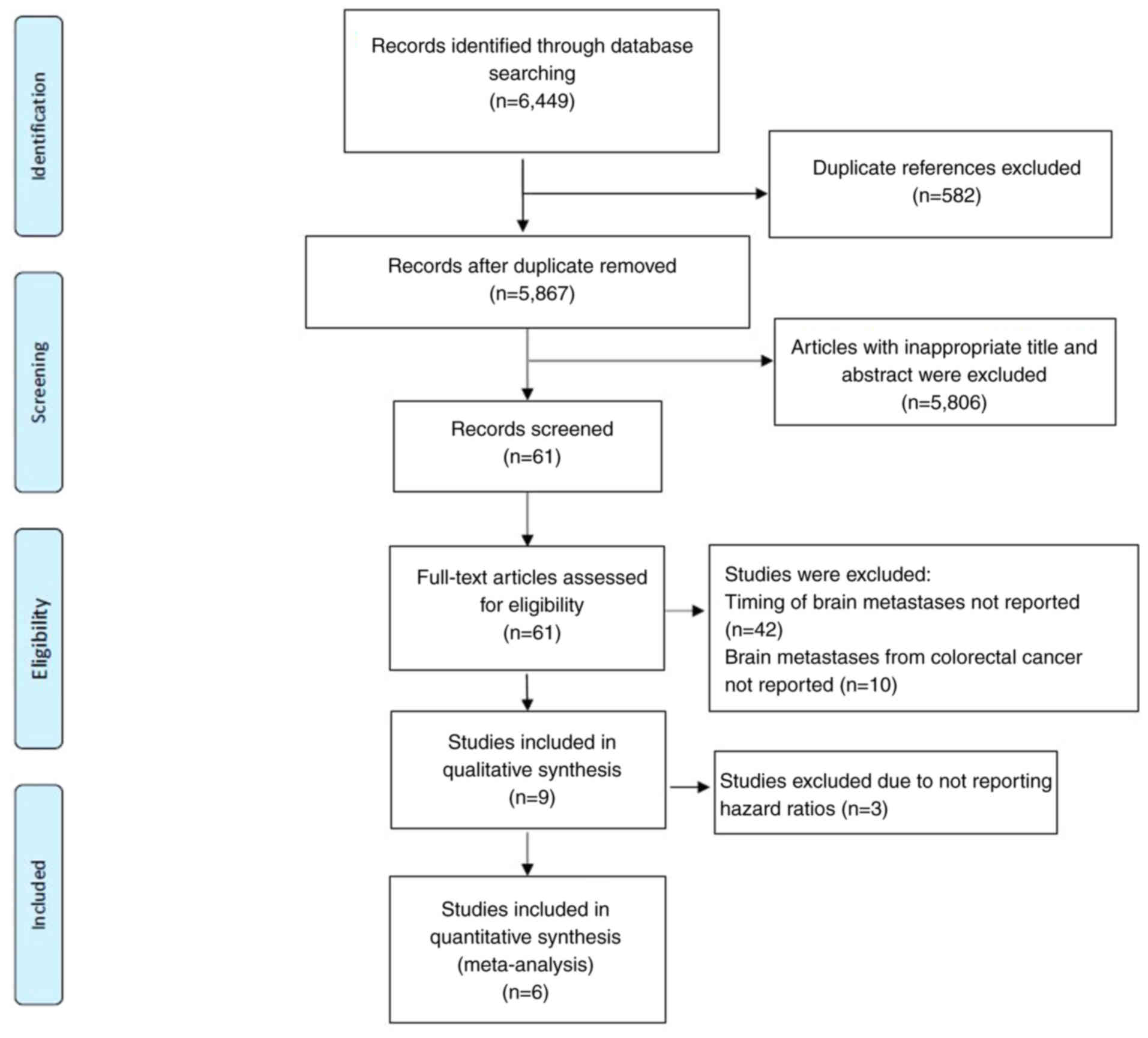
Study selection
The comprehensive search strategy yielded a total of
6,449 references from the Cochrane library, Embase and MEDLINE
electronic databases. After the initial screening of titles and
abstracts, 582 duplicate references and 5,806 references deemed
irrelevant to the study aims were identified and excluded.
Subsequently, the remaining 61 studies underwent a full-text
review. A total of nine studies met the inclusion criteria and were
included in the final analysis. Fig.
1 presents a visual representation of the study selection
process.
Study characteristics
A total of nine retrospective cohort studies
(15–23), published between 2002 and 2023, were
included in the analysis. These studies collectively involved 910
patients diagnosed with BM from CRC. The definition of synchronous
BM varied among the nine studies: In five studies, synchronous BM
was defined as occurring within the first (16), second (22), third (20), sixth (19) or twelfth (17) month after the diagnosis of primary
CRC; however, the remaining four studies (15,18,21,23)
did not provide a specific or explicit definition of synchronous
BM. The basic characteristics of the included studies are presented
in Table I.
 | Table I.Basic characteristics of the included
studies. |
Table I.
Basic characteristics of the included
studies.
|
|
|
|
|
|
| Extracranial
metastases |
|
|
|
|
|
| HR for OS (SBM vs.
MBM) |
|
|---|
|
|
|
|
|
|
|
|
|
| Sample size, n | MST, months |
|
|---|
| First author/s,
year | Inclusion
criteria | SBM definition | Age at initial
diagnosis | Sex | Number of BM |
Negative/positive | Location | Primary tumor
location | Histopathology or
grade |
|
| (Refs.) |
|---|
| SBM | MBM | SBM | MBM |
|---|
| Baek et
al, | Patients with | NA | Median: | Male: | 1: 58 | 6/112 | Lung: | Ascending | Well or | 57 | 61 | 3.4 | 4.6 | NA | (15) |
| 2011 | symptomatic |
| 54 years; | 63 (53%) | (50%); |
| 89 (75%); | colon: | moderately |
|
| (2.5- | (3.3- |
|
|
|
| CRC BM |
| range: | and | 2-3: 31 |
| intra-19 (16%); | differentiated: |
|
|
| 4.30) | 5.9) |
|
|
|
| receiving |
| 19–77 years | female: | (26%); |
| abdominal | Transverse | 89 (75%); |
|
|
|
|
|
|
|
| WBRT, RS |
|
| 55 (47%) | and >3: |
| LN: 42 | colon: 6 (5%); | poorly |
|
|
|
|
|
|
|
| or surgery |
|
|
| 28 (24%); |
| (36%); intra- | descending |
differentiated, |
|
|
|
|
|
|
|
|
|
|
|
| missing: |
| thoracic/ | colon: 5 (4%); | mucinous or |
|
|
|
|
|
|
|
|
|
|
|
| 1 (0.9%) |
| neck LN: | sigmoid | signet ring
cell |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 31 (26%); | colon: | carcinoma: |
|
|
|
|
|
|
|
|
|
|
|
|
|
| bone: 43 | 17 (14%); | 15 (13%); and |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (36%); and | and rectum: | unknown: |
|
|
|
|
|
|
|
|
|
|
|
|
|
| peritoneum: | 72 (61%) | 14 (12%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 21 (18%) |
|
|
|
|
|
|
|
|
| Chen et
al, | Patients with | An interval | <65 years: | Male: | <4: 43 | 16 | -Right colon: | Adeno- |
| 15 | 50 | NA | NA | 0.831 | (16) |
| 2023 | CRC BM | of <1 month | 33 (51%) | 32 (49.2%) | (78.5%) | (24.7%)/ | 10 (15.4%) | carcinoma: |
|
|
|
|
| (0.438- |
|
|
| under | between the | and | and | and ≥4: | 49 | and left | 62 (95.4%); |
|
|
|
|
| 1.575) |
|
|
| treatment | diagnosis of | ≥65 years: | female: | 22 (21.5%) | (75.3%) |
| colon: | mucinous |
|
|
|
|
|
|
|
| (local, | CRC and the | 32 (49%) | 33 (50.8%) |
|
|
| 55 (84.6%) |
adenocarcinoma: |
|
|
|
|
|
|
|
| systemic or | development |
|
|
|
|
|
| 1 (1.5%); and |
|
|
|
|
|
|
|
| combination | of BM |
|
|
|
|
|
| carcinoma: |
|
|
|
|
|
|
|
| therapy) |
|
|
|
|
|
|
| 2 (3.1%) |
|
|
|
|
|
|
| Lu et al,
2019 | Patients with | BM within | ≤60 years: | Male: | 1: 44 (55%) | 13 | Lung | Right colon: | NA | 6 | 74 | 22 | 6 | 2.16 | (17) |
|
| CRC BM | 12 months | 50 (62.5%) | 52 (65%) | and ≥2: | (16.25%)/- | metastasis: | 9 (11.25%); |
|
|
| (0.5- | (4.5- | (0.71- |
|
|
| receiving | of diagnosis | and | and | 36 (45%) |
| 56 (70%) | left colon: |
|
|
| 43.5) | 7.5) | 6.63) |
|
|
| single | of the | >60 years: | female: |
|
| and other | 21 (26.25%); |
|
|
|
|
|
|
|
|
| treatment | primary | 30 (37.5%) | 28 (35%) |
|
| organ | and rectum: |
|
|
|
|
|
|
|
|
| including | CRC |
|
|
|
| metastases: | 50 (62.5%) |
|
|
|
|
|
|
|
|
| neurosurgery, |
|
|
|
|
| 11 (13.75%) |
|
|
|
|
|
|
|
|
|
| WBRT or RS, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| chemotherapy, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| or multi- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| disciplinary |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| treatment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| including |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| neurosurgery |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| plus chemo- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| therapy or |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| radiotherapy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| plus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| chemotherapy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Magni et
al, | Patients with | NA | Median: | Male: | 1: 22 | 2 (5%)/ | -Colon: | G1-G2: 20 |
| 7 | 34 | 21.4 | 4.2 | 0.52 | (18) |
| 2014 | CRC BM |
| 58; range: | 25 (61%) | (53.7%); | 39 (95%) |
| 17 (41.5%) | (48.8%); G3: 7 |
|
| (2.3-u) | (3.2- | (0.144- |
|
|
| undergoing |
| 23-75; | and | ≥2: 17 |
|
| and rectum: | (17.1%); and |
|
|
| 5.1) | 1.89) |
|
|
| surgical |
| ≤65 years: | female: | (41.5%); |
|
| 24 (58.5%) | unknown: |
|
|
|
|
|
|
|
| resection, |
| 29 (70.7%) | 12 (39%) | and |
|
|
| 14 (34.1%); |
|
|
|
|
|
|
|
| WBRT, SRT |
| and |
| unknown: |
|
|
| KRAS mutated: |
|
|
|
|
|
|
|
| and systemic |
| >65 years: |
| 2 (4.9%) |
|
|
| 17 (41.5%); |
|
|
|
|
|
|
|
| chemotherapy |
| 12 (29.3%) |
|
|
|
|
| KRAS wild |
|
|
|
|
|
|
|
| with or |
|
|
|
|
|
|
| type: 11 |
|
|
|
|
|
|
|
| without |
|
|
|
|
|
|
| (26.8%);and |
|
|
|
|
|
|
|
| biological |
|
|
|
|
|
|
| KRAS not |
|
|
|
|
|
|
|
| agents |
|
|
|
|
|
|
| reported |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 13 (31.7%) |
|
|
|
|
|
|
| Mege et
al, | Patients with | BM | <65 years: | Male: | 1: 23 (82%) | NA | - | Colon: | NA | 12 | 16 | Median |
| 0.5 | (19) |
| 2013 | CRC BM | diagnosed | 15 (54%) | 13 (46%) | and 2: |
|
| 15 (54%) |
|
|
| OS:12 |
| (0.1- |
|
|
| undergoing | within | and | and | 5 (18%) |
|
| and rectum: |
|
|
|
|
| 2.6) |
|
|
| surgical | 6 months | >65 years: | female: |
|
|
| 13 (46%) |
|
|
|
|
|
|
|
|
| resection with | after the | 13 (46%) | 15 (54%) |
|
|
|
|
|
|
|
|
|
|
|
|
| or without | diagnosis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| postoperative | of CRC |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| WBRT or RS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Roussile et
al, | Patients with | BM | <65 years: | Male: | 1: 198 | 49 | - | Ascending | Well or | 58 | 300 | 9.7 | 4.8 | NA | (20) |
| 2021 | CRC BM | diagnosed | 160 (44.7%) | 205 | (56.9%); | (13.9%)/ |
| colon: 71 | moderately |
|
|
|
|
|
|
|
| undergoing | within | and | (57.3%) | ≥2: 151 | 303 |
| (20.3%); |
differentiated: |
|
|
|
|
|
|
|
| surgical | 3 months | ≥65 years: | and | (43.1%); | (86.1%) |
| descending | 224 (89.2%); |
|
|
|
|
|
|
|
| resection or | after the | 198 (55.3%) | female: | and |
|
| colon: | poorly |
|
|
|
|
|
|
|
| RS with or | diagnosis |
| 153 | missing: |
|
| 130 (37.3%); |
differentiated: |
|
|
|
|
|
|
|
| without | of CRC |
| (42.7%) | 96 (27.6%) |
|
| and rectum: | 27 (10.8%); |
|
|
|
|
|
|
|
| postoperative |
|
|
|
|
|
| 148 (42.4%) | and missing: |
|
|
|
|
|
|
|
| WBRT |
|
|
|
|
|
|
| 107 (42.8%) |
|
|
|
|
|
|
| Schoeggl et
al, | Patients with | NA | Median: | Male: | 1: 24 | 22 (63%)/ | - | NA | NA | NA | NA | 4.5 | 5.9 | NA | (21) |
| 2002 | CRC BM |
| Radiosurgery, | 23 (65.7%) | (68.6%) | 13 (37%) |
|
|
|
|
|
|
|
|
|
|
| receiving |
| 69 years and | and | and ≥2: |
|
|
|
|
|
|
|
|
|
|
|
| RS with or |
| radiosurgery | female: | 11 (31.4%) |
|
|
|
|
|
|
|
|
|
|
|
| without |
| with WBRT, | 12 (34.3%) |
|
|
|
|
|
|
|
|
|
|
|
|
| WBRT |
| 63 years |
|
|
|
|
|
|
|
|
|
|
|
|
| Tanriverdi et
al, | Patients with | BM | ≤65 years: | Male: | 1: 15 | 15 (11%)/ | - | Rectum: 74 | Grade 2: 21 | 19 | 114 | 26.4 | 25.0 | 1.14 | (22) |
| 2014 | CRC BM | diagnosed | 54 (41%) | 70 (53%) | (11%); | 118 (89%) |
| (56%); recto- | (16%); grade
3: |
|
| (20.0- | (21.5- | (0.31- |
|
|
| undergoing | within | and | and | 2-3: 41 |
|
| sigmoid and | 93 (70%); and |
|
| 32.9) | 28.4) | 4.13) |
|
|
| surgical | 2 months | >65 years: | female: | (31%); |
|
| sigmoid colon: | unknown: |
|
|
|
|
|
|
|
| resection, | after the | 79 (59%) | 63 (47%) | and >3: |
|
| 23 (17%); | 19 (14%) |
|
|
|
|
|
|
|
| WBRT, RS | diagnosis |
|
| 77 (58%) |
|
| transverse |
|
|
|
|
|
|
|
|
| or supportive | of CRC |
|
|
|
|
| colon: |
|
|
|
|
|
|
|
|
| care |
|
|
|
|
|
| 12 (9%); and |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ascending |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| colon and |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| cecum: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 24 (18%) |
|
|
|
|
|
|
|
| Wang et
al, | Patients with | NA | Median, | Male: | 1: 37 | 20 | - | Colon | CEA negative: | 4 | 61 | NA | NA | 0.802 | (23) |
| 2021 | CRC BM |
| 63 years; | 41 (63.1%) | (56.9%) | (30.8%)/ |
| cancer: | 25 (38.5%); |
|
|
|
| (0.231- |
|
|
| with or |
| range: | and | and ≥2: | 45 |
| 24 (36.9%) | CEA positive: |
|
|
|
| 2.783) |
|
|
| without |
| 37–72 years | female: | 28 (43.1%) | (59.2%) |
| and rectal | 35 (53.8%); |
|
|
|
|
|
|
|
| surgical |
|
| 24 (36.9%) |
|
|
| cancer: | and unknown: |
|
|
|
|
|
|
|
| resection |
|
|
|
|
|
| 41 (63.1%) | 5 (7.7%) |
|
|
|
|
|
|
|
| for BM |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
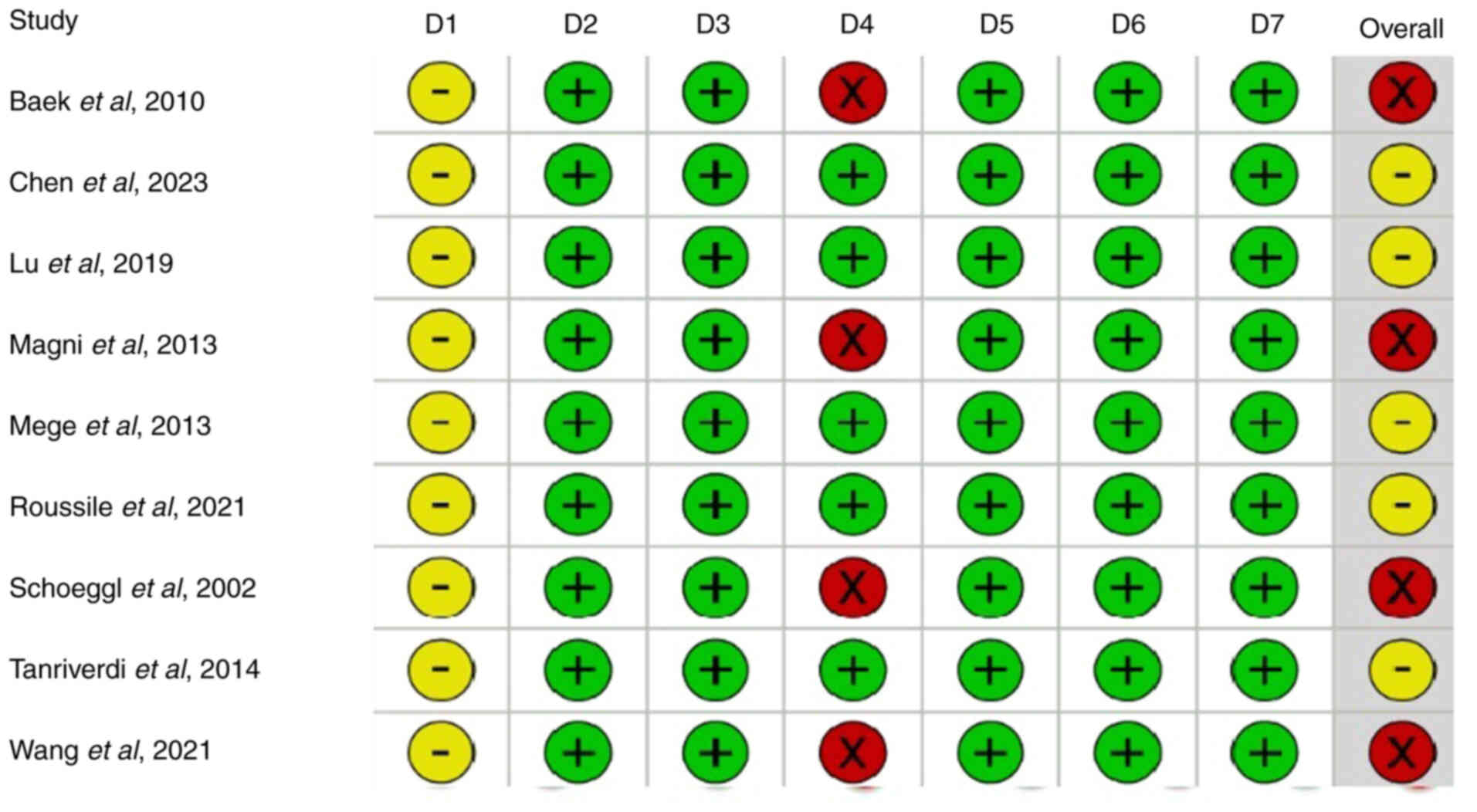
Quality assessment of the included
studies
A quality assessment of the included studies using
the ROBINS-I tool demonstrated that 4 studies were classified as
having a serious risk of bias, whilst the remaining 5 studies were
deemed to have a moderate risk of bias. Fig. 2 presents a graphical representation
of the risk-of-bias assessment.
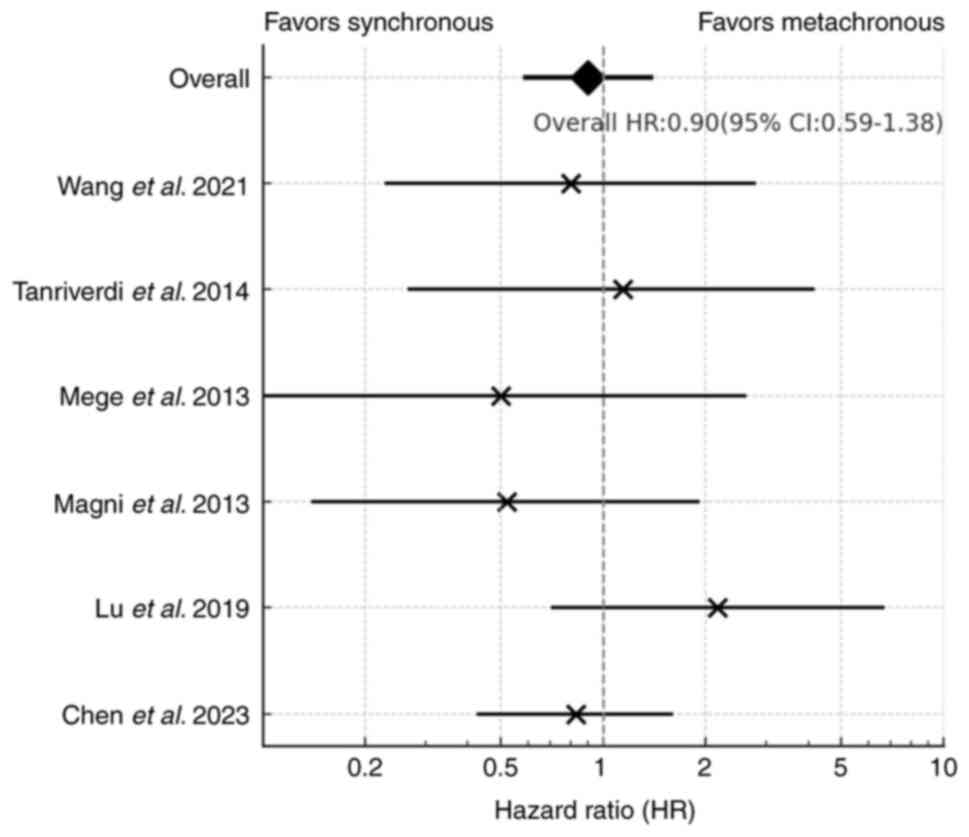
Comparative survival outcome
A total of three studies (15,20,21)
were excluded from the meta-analysis due to the absence of HR data.
The meta-analysis of six studies that compared the survival
outcomes of patients with synchronous BM and metachronous BM
revealed no statistically significant difference in OS between the
two groups (HR, 0.90; 95% CI, 0.59–1.38; Fig. 3). Notably, no statistical
heterogeneity was observed among these studies
(I2=0%).
Discussion
The present study performed a comprehensive review
of the existing literature and a meta-analysis to assess the
relationship between BM timing and survival outcomes in patients
with CRC. The results demonstrated no significant difference in OS
between patients with synchronous or metachronous BM. This finding
suggests that the timing of BM diagnosis may not be a substantial
factor in OS outcome for patients with CRC, highlighting the
importance of individualized treatment strategies that consider
other patient-specific factors.
Previous studies evaluating this relationship in
different cancer types yielded mixed results. Flannery et al
(24) reported that patients with
non-small-cell lung cancer and solitary metachronous BM had greater
rates of survival compared with those with synchronous BM.
Similarly, Ruste et al (4)
reported lower survival rates in patients with clear cell renal
cell carcinoma with synchronous BM compared with those with
metachronous BM. However, a more recent study by Potthoff et
al (5), reported no significant
impact of BM timing on survival in a broader patient population
with BM from several primary cancers. Notably, the inclusion of
different primary cancer types in their study led to substantial
variations in the characteristics of the included patients. The
present study focused on BM from CRC, and the results demonstrated
that the synchronous or metachronous nature of BM did not
significantly affect the survival outcome.
Currently, the primary local treatment options for
BM from CRC consist of surgical resection, radiotherapy or a
combination of both (8). Surgical
resection is considered feasible and appropriate for specific cases
of BM from CRC. This approach is often employed for solitary or
limited BMs and can help in reducing tumor burden and alleviating
symptoms (25). Radiosurgical
modalities, such as Gamma Knife or CyberKnife, are specialized
techniques that deliver highly focused radiation to the BM while
minimizing the impact on surrounding healthy tissues. These
modalities are commonly used for small and well-defined metastatic
lesions that may not be amenable to surgical resection (26). Whole brain radiotherapy is employed
when multiple metastases are present throughout the brain, and can
be used as adjuvant therapy following surgical resection to target
remaining or potential microscopic metastases (27). We hypothesize that the status of BM
as synchronous or metachronous can potentially impact subsequent
treatment approaches, which in turn may affect OS. Thus, the need
for further research in this area is crucial.
Although the results of the present study
demonstrated that the synchronous or metachronous nature of BM did
not significantly impact the survival outcome, these findings
should be interpreted with caution. Despite no significant
statistical heterogeneity demonstrated in the meta-analysis
results, differences were observed among the included studies. For
instance, in the study by Mege et al (19), the mean OS after BM was 12 months,
which is similar to that reported in the study by Potthoff et
al (5), which was not included
in this research as it included several types of BM rather than
just BM from CRC. In the study by Potthoff et al, the mean
survival was also around 12–13 months. However, Roussile et
al (20), reported a worse
survival rate, with the OS for the synchronous and metachronous
groups demonstrated to be 9.7 and 4.8 months, respectively. These
differences may be attributed to the complex nature of the patients
with cancer themselves and variations in treatment modalities and
patient selection. Furthermore, the present study was unable to
ascertain whether the treatments received by the synchronous and
metachronous groups differed within each study.
The present meta-analysis has several limitations
that should be acknowledged. Firstly, all the studies included in
the analysis were retrospective in nature. As patients with cancer
belong to a complex population, multiple potential factors can
introduce bias and limitations in data collection, which may have
impacted the accuracy and reliability of the findings. For example,
our previous study (8) reported
that surgery for CRC BM had improved survival outcomes compared
with radiotherapy. However, the analysis in the present study
focused on BM timing. The lack of detailed treatment information in
the present analysis may impact the accuracy of the results.
Secondly, despite the fact that a comprehensive search was
performed, the number of studies that ultimately met the inclusion
criteria and reported synchronous and metachronous BM was
relatively small. Furthermore, certain studies included in the
analysis did not provide comparative results between the two
groups, but rather reported noncomparative measures, such as median
survival times. Therefore, direct comparative data for the analysis
were limited. Thirdly, owing to insufficient information in the
included studies, the present study was unable to determine whether
the distributions of treatments differed between the synchronous
and metachronous BM groups within each study. Fourthly, the
definition of synchronous and metachronous BM varied across the
included studies and certain studies did not explicitly define or
specify the timing criteria for synchronous BM. Lastly, the impact
of primary tumor location on outcomes is also worth exploring, such
as comparisons between the rectum and colon or between the
right-sided and left-sided colon. However, the studies included in
the present analysis did not assess both the timing of BM and the
primary tumor location simultaneously. Therefore, a subgroup
analysis based on the included studies could not be performed and
is an aspect that requires further research in the future.
Nevertheless, despite these limitations, the present study is the
first meta-analysis to assess this clinical question that comprises
large patient samples from multicenter data with low heterogeneity,
to our best knowledge. Further research, including large,
randomized prospective cohort studies with standardized definitions
of synchronous BM and treatment protocols, is warranted.
In conclusion, the findings of the present
meta-analysis indicate that there is no difference in OS between
patients with synchronous and metachronous BM arising from CRC.
Given the limitations of the present study, further research with
prospective designs and larger sample sizes is needed to obtain
more robust and conclusive evidence.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TCT contributed to the conceptualization, formal
analysis and writing original draft. JS and KYC contributed to
statistical analysis consultation, validation and review and
editing the manuscript. HML contributed to analysis and
interpretation of data, supervision and review and editing of the
manuscript. YC contributed to conceptualization, formal analysis,
writing original draft, and review and editing the manuscript. TCT
and YC confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zeineddine FA, Zeineddine MA, Yousef A, Gu
Y, Chowdhury S, Dasari A, Huey RW, Johnson B, Kee B, Lee MS, et al:
Survival improvement for patients with metastatic colorectal cancer
over twenty years. NPJ Precis Oncol. 7:162023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Müller S, Köhler F, Hendricks A, Kastner
C, Börner K, Diers J, Lock JF, Petritsch B, Germer CT and Wiegering
A: Brain metastases from colorectal cancer: A systematic review of
the literature and meta-analysis to establish a guideline for daily
treatment. Cancers (Basel). 13:9002021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nieder C, Spanne O, Mehta MP, Grosu AL and
Geinitz H: Presentation, patterns of care, and survival in patients
with brain metastases: What has changed in the last 20 years?
Cancer. 117:2505–2512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruste V, Sunyach MP, Tanguy R, Jouanneau
E, Schiffler C, Carbonnaux M, Moriceau G, Neidhardt EM, Boyle H,
Robin S, et al: Synchronous brain metastases as a poor prognosis
factor in clear cell renal carcinoma: A strong argument for
systematic brain screening. J Neurooncol. 153:133–141. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Potthoff AL, Heimann M, Lehmann F, Ilic I,
Paech D, Borger V, Radbruch A, Schäfer N, Schuss P, Vatter H, et
al: Survival after resection of brain metastasis: Impact of
synchronous versus metachronous metastatic disease. J Neurooncol.
161:539–545. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reddy SP, Dowell JE and Pan E: Predictors
of prognosis of synchronous brain metastases in small-cell lung
cancer patients. Clin Exp Metastasis. 37:531–539. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bergen ES, Scherleitner P, Ferreira P,
Kiesel B, Müller C, Widhalm G, Dieckmann K, Prager G, Preusser M
and Berghoff AS: Primary tumor side is associated with prognosis of
colorectal cancer patients with brain metastases. ESMO Open.
6:1001682021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang Y, Wong CE, Lee PH, Huang CC and Lee
JS: Survival outcome of surgical resection vs. radiotherapy in
brain metastasis from colorectal cancer: A meta-analysis. Front Med
(Lausanne). 9:7688962022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imaizumi J, Shida D, Narita Y, Miyakita Y,
Tanabe T, Takashima A, Boku N, Igaki H, Itami J and Kanemitsu Y:
Prognostic factors of brain metastases from colorectal cancer. BMC
Cancer. 19:7552019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lan YT, Chang SC, Lin PC, Lin CC, Lin HH,
Huang SC, Lin CH, Liang WY, Chen WS, Jiang JK, et al:
Clinicopathological and molecular features between synchronous and
metachronous metastases in colorectal cancer. Am J Cancer Res.
11:1646–1658. 2021.PubMed/NCBI
|
|
11
|
Higgins JPT, Thomas J, Chandler J,
Cumpston M, Li T, Page MJ and Welch VA: Cochrane handbook for
systematic reviews of interventions version 6.5 (updated August
2024). Cochrane. 2024.Available from:. www.training.cochrane.org/handbook
|
|
12
|
Sterne JA, Hernán MA, Reeves BC, Savović
J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT,
Boutron I, et al: ROBINS-I: A tool for assessing risk of bias in
non-randomised studies of interventions. BMJ. 355:i49192016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higgins JPT and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baek JY, Kang MH, Hong YS, Kim TW, Kim DY,
Oh JH, Lee SH, Park JH, Kim JH and Kim SY: Characteristics and
prognosis of patients with colorectal cancer-associated brain
metastases in the era of modern systemic chemotherapy. J
Neurooncol. 104:745–753. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen CW, Ou TS, Chen WS, Jiang JK, Yang
SH, Wang HS, Chang SC, Lan YT, Lin CC, Lin HH, et al: Anti-VEGF
therapy possibly extends survival in patients with colorectal brain
metastasis by protecting patients from neurologic disability. Clin
Colorectal Cancer. 22:267–279. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu X, Cai Y, Xia L, Ju H and Zhao X:
Treatment modalities and relative survival in patients with brain
metastasis from colorectal cancer. Biosci Trends. 13:182–188. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magni E, Santoro L, Ravenda PS, Leonardi
MC, Bonomo G, Monfardini L, Nolè F and Zampino MG: Brain metastases
from colorectal cancer: Main clinical factors conditioning outcome.
Int J Colorectal Dis. 29:201–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mege D, Ouaissi M, Fuks D, Metellus P,
Peltier J, Dufour H, Regimbeau JM, Dahan L, Sielezneff I and Sastre
B: Patients with brain metastases from colorectal cancer are not
condemned. Anticancer Res. 33:5645–5648. 2013.PubMed/NCBI
|
|
20
|
Roussille P, Auvray M, Vansteene D,
Lecomte T, Rigault E, Maillet M, Locher C, Dior M, Hautefeuille V,
Artru P, et al: Prognostic factors of colorectal cancer patients
with brain metastases. Radiother Oncol. 158:67–73. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schoeggl A, Kitz K, Reddy M and Zauner C:
Stereotactic radiosurgery for brain metastases from colorectal
cancer. Int J Colorectal Dis. 17:150–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanriverdi O, Kaytan-Saglam E, Ulger S,
Bayoglu IV, Turker I, Ozturk-Topcu T, Cokmert S, Turhal S, Oktay E,
Karabulut B, et al: The clinical and pathological features of 133
colorectal cancer patients with brain metastasis: a multicenter
retrospective analysis of the Gastrointestinal Tumors Working
Committee of the Turkish Oncology Group (TOG). Med Oncol.
31:1522014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang D, Chen C, Ge X, Yang Q, Huang Y,
Ling T, Jin T, Yu S, Wang J and Sun L: Factors prognostic for brain
metastases from colorectal cancer: A single-center experience in
China. Cancer Manag Res. 13:6767–6774. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Flannery TW, Suntharalingam M, Kwok Y,
Koffman BH, Amin PP, Chin LS, Nicol B, Fowler Z, Young AB and
Regine WF: Gamma knife stereotactic radiosurgery for synchronous
versus metachronous solitary brain metastases from non-small cell
lung cancer. Lung Cancer. 42:327–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kye BH, Kim HJ, Kang WK, Cho HM, Hong YK
and Oh ST: Brain metastases from colorectal cancer: The role of
surgical resection in selected patients. Colorectal Dis.
14:e378–e385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Navarria P, Minniti G, Clerici E, Comito
T, Cozzi S, Pinzi V, Fariselli L, Ciammella P, Scoccianti S,
Borzillo V, et al: Brain metastases from primary colorectal cancer:
Is radiosurgery an effective treatment approach? Results of a
multicenter study of the radiation and clinical oncology Italian
association (AIRO). Br J Radiol. 93:202009512020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koo T, Kim K, Park HJ, Han SW, Kim TY,
Jeong SY, Park KJ and Chie EK: Prognostic factors for survival in
colorectal cancer patients with brain metastases undergoing whole
brain radiotherapy: Multicenter retrospective study. Sci Rep.
10:43402020. View Article : Google Scholar : PubMed/NCBI
|