Introduction
Uterine leiomyosarcoma (uLMS) is a type of
interstitial malignant tumor that originates from the smooth muscle
of the uterus, accounting for 40–50% of all cases of uterine
sarcoma and 1–2% of all malignant tumors of the uterine body
(1). Histopathological subtypes of
uLMS include the spindle cell type, which is the most common, the
epithelioid type and the mucous type (2). Uterine myxoid leiomyosarcoma (MLMS) is
an extremely rare, aggressive variant of uLMS, with an annual
incidence of 0.64 cases per 100,000 women (3). This disease primarily affects
perimenopausal women and is uncommon in women of childbearing age
and has an unfavorable evolution and prognosis (4). Due to its non-specific clinical
presentation and lack of distinctive findings in preoperative
auxiliary examinations, Early uterine sarcomas are often
misdiagnosed as benign uterine disease. Therefore, early diagnosis
and timely intervention of MLMS are challenging, which can lead to
considerable psychological and physical distress for patients. In
the present case report, a rare case of MLMS in a woman of
reproductive age is described, and details of its clinical
presentation, diagnosis and treatment are presented.
Case report
A 33-year-old female presented to the Women and
Children's Hospital Affiliated to Ningbo University (Ningbo, China)
in February 2024 with lower abdominal pain. Ultrasonography
revealed an unevenly echogenic region measuring 94×74×88 mm in the
posterior wall myometrial layer, which was predominantly cystic in
nature. Uterine echogenicity was heterogeneous, with a bilateral
endometrial thickness of 13 mm. A three-dimensional scan of the
uterine cavity displayed an inverse triangle shape. Furthermore, a
dark cystic region measuring 76×58×71 mm was identified in the left
ovary, exhibiting a fine internal mesh structure. No abnormalities
were evident in the right adnexal region, and color Doppler flow
imaging (CDFI) revealed no abnormal blood flow signals. The patient
denied any history of hormone drug use, prior surgery or a family
history of tumors.
In February 2024, the patient went to the Gynecology
Department of the Affiliated Women and Children's Hospital of
Ningbo University (Ningbo, China), where a three-dimensional
follow-up ultrasound examination revealed a region of uneven
echogenicity in the posterior wall of the uterus exhibited
measuring 120×62×109 mm, with unclear boundaries and cystic
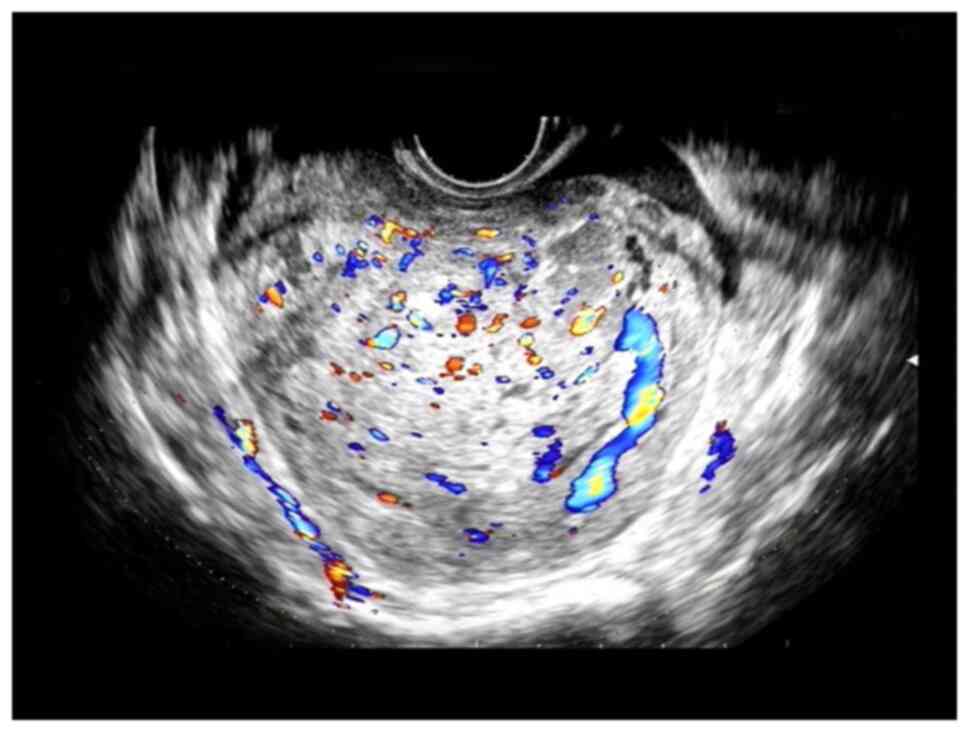
components. CDFI indicated abundant blood flow signals (Fig. 1), which suggested cystic
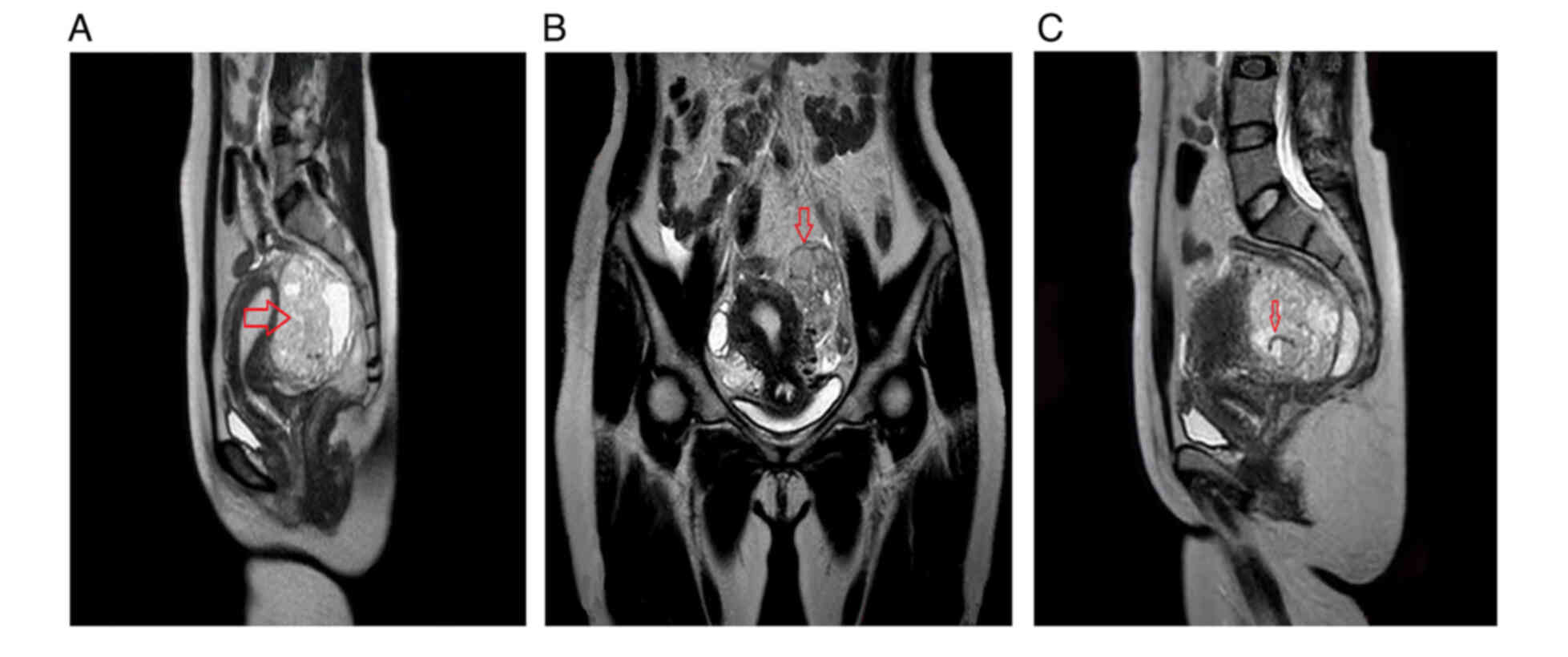
degeneration of uterine myoma. Magnetic resonance imaging (MRI)
indicated the presence of subserous fibroids in the posterior wall
of the uterus, along with degenerative cystic masses in the
bilateral adnexal area and broad ligament fibroids, which warranted
further investigation (Fig. 2).
Preoperative examination of tumor markers, including cancer antigen
125 (CA125), a-fetoprotein (AFP), carbohydrate antigen 19–9,
carcinoembryonic antigen and human epididymis protein 4, yielded
negative results. Subsequently, laparoscopic exploration, pelvic
lymph node biopsy and posterior uterine wall biopsy were performed.
Pathological examination of the frozen section obtained during
surgery revealed that the posterior wall of the uterus was occupied
by tumor cells exhibiting diffuse growth, abundant cytoplasm and
visible mitotic activity. Tumor cells were also identified in the
left external iliac lymph node and the right obturator lymph node,
both of were indicative of metastatic involvement. The surgical
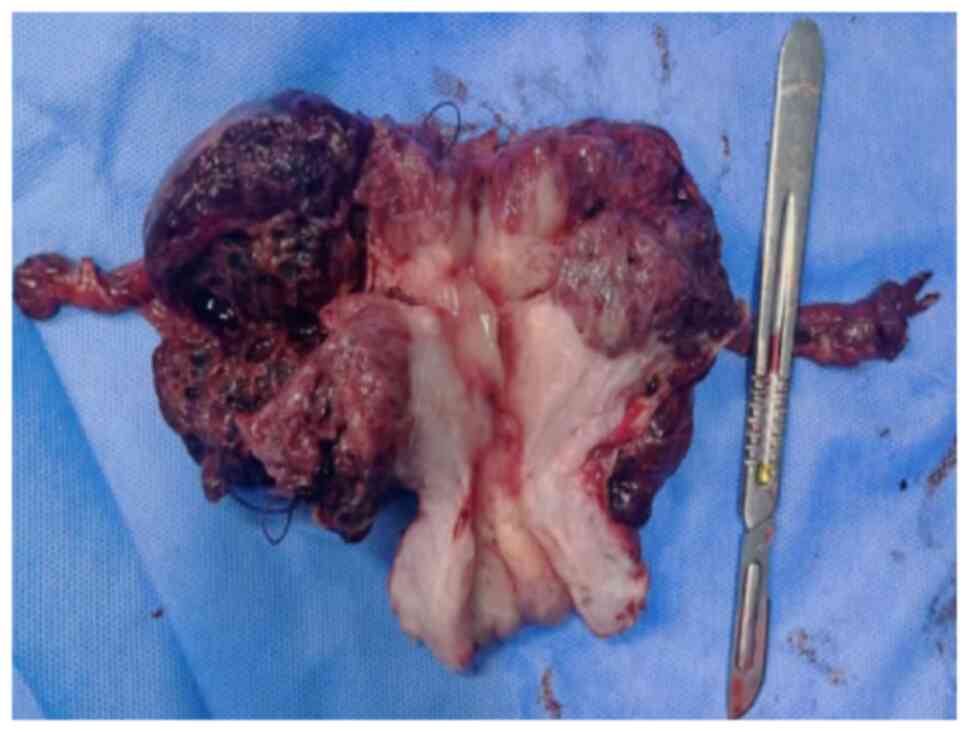
procedure included exploratory laparotomy, total hysterectomy and
bilateral salpingectomy, with preservation of the ovaries (Fig. 3). The patient was discharged from
the hospital 6 days post-surgery.
The final pathological report confirmed that the
posterior wall of the uterus was occupied by a mesenchymal
malignant tumor, identified as MLMS, as corroborated by
immunohistochemical findings (Fig.
4). The tumor measured 70×50×45 mm and had infiltrated the
outer serous membrane. Notably, no tumor involvement was observed
on either side of the uterus. However, tumor involvement was
detected in the extracapsular fibrous adipose tissue of a left
external iliac lymph node. Two right obturator lymph nodes were
assessed: One showed tumor involvement while the other exhibited
involvement of its extrinsic fibrous adipose tissue.
Immunohistochemistry was performed to assist in diagnosis. Tissue
was immersed in 10% neutral formalin at room temperature for >20
h, after which it was embedded in paraffin blocks. The paraffin
blocks were sectioned to a thickness of 4 µm and baked at 60°C for
30–60 min. Paraffin sections were immersed in fresh xylene and
descending ethanol solutions. The tissue was then rinsed with tap
water and PBS and heated to 100°C. Following treatment with 3%
H2O2 for 10 min at room temperature, 10%
normal goat serum (Scientific Phygene) was added at 37°C for 30 min
to block nonspecific binding. Primary antibodies against Ki-67
(dilution 1:800; cat. no. ZM-0165 Beijng Zhongshan Golden Bridge),
ER (Ready to use; cat. no. 790-4324; Roche), PR (Ready to use; cat.
no. 790-4296; Roche) and Desmin (dilution 1:200; cat. no. M0760;
DAKO) were added and incubated at 37°C for 2 h. The samples were
then washed 3 times with PBS (2 min). Subsequently, added
horseradish peroxidase (HRP)-labeled as the secondary antibody
(dilution 1:200; cat. no. K4001; DAKO) and incubated at 37°C for 30
min. The samples were washed again 3 times with PBS with each wash
lasting 2 min. A volume of 10 µl freshly prepared DAB solution was
added, samples were rinsed with tap water and counterstained with
hematoxylin for 30–60 sec at room temperature, followed by rinsing
with tap water to restore the blue color. The samples were
dehydrated by using ethanol gradients. Sections were treated with
xylene 3 times for 2 min each to achieve transparency. Finally, the
sections were sealed with neutral gum and observed under a light
microscope (Olympus Corporation). Immunohistochemical analysis
revealed Ki-67 (+50%), pan-cytokeratin (CK) (−), cell adhesion
molecule 5.2 (−), CK7 (−), estrogen receptor (clonal number SP1)
(+), progesterone receptor (clonal number 1E2) (+), CD10 (−),
cyclin D1 (−), desmin (+), smooth muscle actin (SMA) (−), caldesmon
(−), Melan-A (A103) (−), human melanoma black 45 (−), calretinin
(−), CK5/6 (−), D2-40 (−), Wilms tumor (+), Ets-related gene (−),
CD31 (−), ALK (clone D5F3) (−), myogenic differentiation 1 (−),
myogenin (−), Sal-like 4 (−), Octamer-binding transcription factor
¾ (−), AFP (−), S-100 (−), SRY-box-10 (−), CD56 (+), inhibin A (−),
P53 (clone number DO-7) (normal coloring); BCL6 corepressor (−) and
actin (−) (Fig. 5). Based on the
staging and classification of uterine sarcoma according to the 2009
International Federation of Gynecology and Obstetrics guidelines
(5), a diagnosis of MLMS (stage
IIIC) was established.
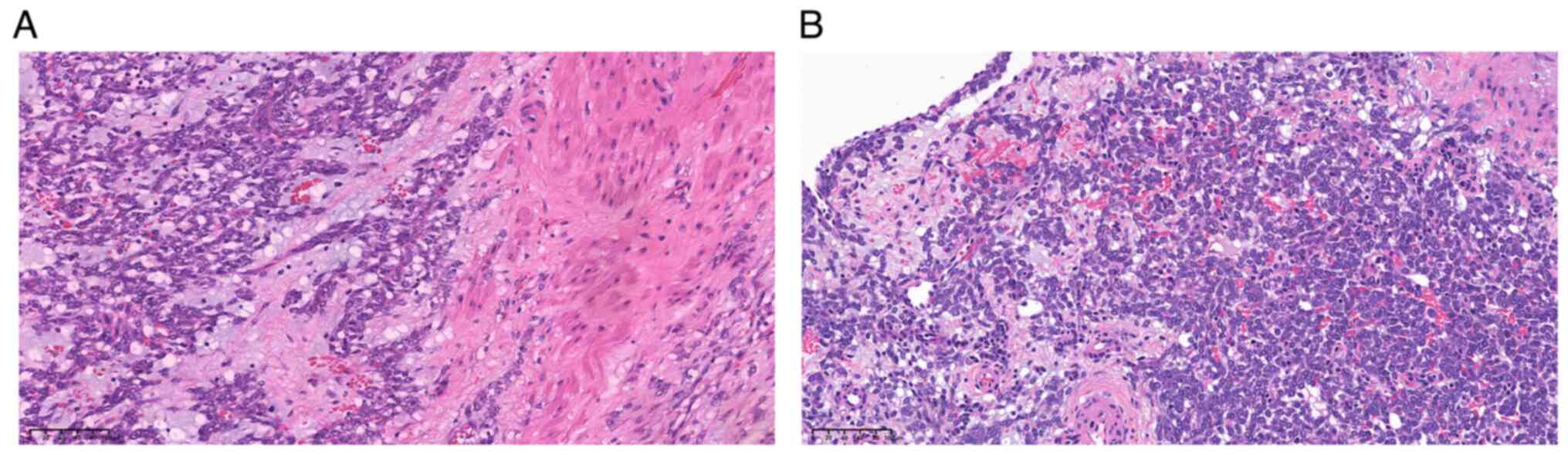
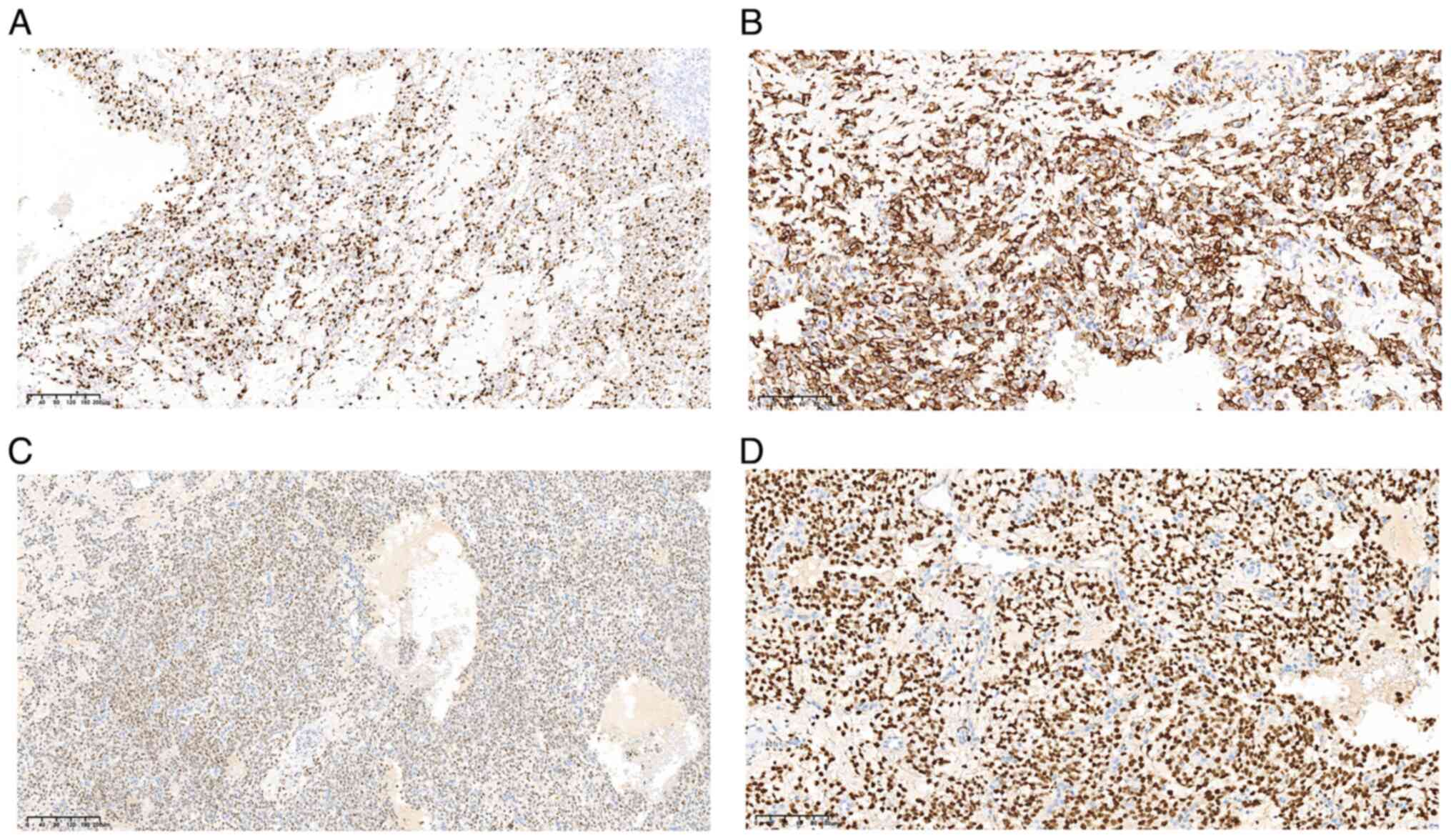 | Figure 5.Immunohistochemical analysis of the
excised tumor. Neoplastic cells exhibit the expression of (A) Ki-67
with ~50% positive nuclei (magnification, ×10; scale bar, 200 µm),
and the positive expression of (B) desmin (magnification, ×20;
scale bar, 100 µm), (C) estrogen receptor (magnification, ×10;
scale bar, 200 µm) and (D) progesterone receptor (magnification,
×20; scale bar, 100 µm). |
At 3 weeks post-surgery, the patient underwent
positron emission tomography/computed tomography for further
evaluation. The results indicated a high likelihood of vaginal
stump inflammation, a right paravascular cystic lesion,
physiological changes in the bilateral accessory area, and a small
amount of pelvic effusion. In April 2024, laparoscopic bilateral
oophorectomy, decompression of pelvic adhesions, appendectomy and
greater omental biopsy were performed. Pathological findings were
chronic appendicitis, degeneration and old hemorrhage in the left
ovarian tissue, and corpus luteum hemorrhage in the right ovarian
tissue. In addition, pathological findings from the greater omentum
biopsy showed fibrous adipose tissue with a cystic follicular
structure. The GD regimen, comprising gemcitabine 1.4 g on day 1
and 1.3 g day 8 and docetaxel 100 mg on day 8 was administered
after surgery. As of March 2025, the patient had received six
cycles of GD. Follow-up is performed every 3 months and the patient
is in good condition with no obvious complications or signs of
recurrence.
Discussion
MLMS is a very rare histological subtype of uLMS,
which is highly invasive, prone to recurrence and has a poor
prognosis (6,7). LMS predominantly affects patients
between 50 and 55 years old, with only 15% of cases occurring in
individuals <40 years old (8).
The present case report describes a 33-year-old female of
reproductive age who was diagnosed with MLMS, a very rare clinical
finding. Analysis of this case may improve the awareness and
recognition of MLMS in women of childbearing age, thereby improving
the prospects for early diagnosis and individualized treatment and
ultimately protecting female fertility.
The clinical manifestations of MLMS include abnormal
uterine bleeding (56%), palpable pelvic mass (54%) and/or pelvic
pain (21%) (9). In the present
case, the patient presented with lower abdominal pain, and both MRI
and three-dimensional ultrasound suggested the possibility of
uterine fibroids; however, these findings lack specificity in the
diagnosis of MLMS. The clinical and imaging manifestations of MLMS
are challenging to distinguish from those of benign uterine
fibroids, and ultrasound lacks diagnostic specificity in
distinguishing between the two (10). The CA125 tumor marker has been
proposed as a potential indicator for MLMS, but its clinical
significance remains unclear (11,12).
This underscores the challenges in the preoperative diagnosis of
MLMS (9). Therefore, the
histopathological examination of surgical specimens is essential
for the accurate diagnosis of MLMS (13). At present, the histopathological
diagnosis of MLMS is mainly based on hematoxylin and eosin
staining, with the microscopic observation of tumor cell necrosis,
nuclear pleomorphism, a mitotic rate of ≥0.4/mm2,
equivalent to ≥1/10 high-power fields, and mitotic changes
(4,14). Furthermore, in a previous case,
immunohistochemical analysis revealed α-SMA, desmin and vimentin
positivity (15). MLMS is
characterized by a soft, gelatinous consistency and rich mucoid
matrix, which distinguishes it from other types of tumors.
Therefore, in young women with a history of uterine fibroids and
pelvic symptoms, the presence of visible soft tissue, microscopic
mitosis, cellular polymorphism, a mucus-rich composition and
specific immunohistochemical markers lay a foundation for the
diagnosis of MLMS.
MLMS in women of childbearing age is often
misdiagnosed as benign fibroids. In a previous case report, a
26-year-old woman who underwent myomectomy for uterine fibroids was
initially diagnosed with a borderline/low-grade mucinous
mesenchymal-derived tumor (16).
However, after 4 years of postoperative follow-up, multiple soft
tissue masses were found in the pelvis, leading to the suspicion of
recurrent MLMS. Therefore, total hysterectomy and bilateral
adnexectomy were performed; misdiagnosis led to the permanent loss
of fertility in this patient. For patients of childbearing age
diagnosed with uterine fibroids prior to surgery, laparoscopic
myomectomy offers several benefits over abdominal myomectomy. These
include reduced blood loss, a shorter hospital stay, and reduced
postoperative analgesia requirements, without a significant
increase in the incidence of complications. In addition, obstetric
outcomes are comparable between the two approaches (17). Considering the medical history of
the present case, after the patient had completed a fertility plan,
the imaging results were carefully evaluated, and comprehensive
discussions were held between the medical team and the patient
regarding surgical approach, safety and fertility considerations. A
decision was made to perform transabdominal surgery, based on the
risk of postoperative uterine sarcoma. However, intraoperative
frozen section analysis revealed a malignant tumor. Since the
patient was young, bilateral ovaries were originally preserved,
while a total hysterectomy and bilateral salpingectomy were
performed. However, the final postoperative pathology led to a
diagnosis of MLMS, leading to a subsequent bilateral ovariectomy.
This highlights the importance of individualized treatment plans
tailored to the specific patient.
At present, the main treatment method for MLMS is
surgical intervention. However, postoperative adjuvant therapy,
including radiotherapy, chemotherapy, targeted therapy and
immunotherapy, also play a key role (18). There is no standardized treatment
plan for MLMS, and the effectiveness of adjuvant therapy remains
unclear, with most drugs still undergoing clinical trials. Although
no specific targeted therapies or immunotherapies for MLMS are
available, molecular and protein-level analyses can be helpful in
the identification of patients at risk of relapse (19). Research on immune checkpoint
inhibitors and targeted therapies is ongoing, with the aim of
determining effective treatment methods and ultimately improving
patient prognosis and survival rates. In the present case, no
recurrence occurred in the first 6 months after surgery.
MLMS is a rare pathological type of uterine sarcoma,
and uterine sarcoma is often misdiagnosed as uterine fibroids,
which are more common in women of reproductive age. The low
incidence of MLMS in this age group can lead to delays in its
diagnosis. Sensitive indices to determine the nature of
hematological tumors are currently lacking. Therefore, it is
crucial to identify specific markers for uterine sarcoma in women
of childbearing age. In the present case, blood indicators and
imaging findings were not sensitive in determining the nature of
the tumor. In such rare cases, a multidisciplinary approach
involving obstetrics and gynecology, imaging, oncology and clinical
laboratory medicine are necessary. Multidisciplinary discussions
are essential in the diagnosis and treatment of rare diseases.
Strengthening the early diagnosis and long-term management of MLMS
in women of childbearing age is particularly important. However, it
must be noted that the present discussion is based on a single
case. Future studies with additional cases are necessary to further
investigate MLMS, provide insights into its early diagnosis, and
ultimately improve patient outcomes and survival rates.
In conclusion, MLMS is a rare malignant tumor of the
uterus. In the present case, a 33-year-old patient with MLMS
presented with symptoms similar to those of benign uterine
fibroids, highlighting the challenges of early diagnosis,
particularly in women of reproductive age. The present case report
aims to serve as a reference for the early diagnosis and
individualized treatment of MLMS, as well as to encourage further
research into this disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TW, XW, JW, XL and LP contributed to study
conception and design. XW, JW, XL and LP analyzed data. TW wrote
the manuscript and all authors commented on the manuscript. TW and
XW confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
CA125
|
cancer antigen 125
|
|
CDFI
|
color Doppler flow imaging
|
|
MLMS
|
uterine myxoid leiomyosarcoma
|
|
MRI
|
magnetic resonance imaging
|
|
uLMS
|
uterine leiomyosarcoma
|
References
|
1
|
Mbatani N, Olawaiye AB and Prat J: Uterine
sarcomas. Int J Gynaecol Obstet. 143 (Suppl):S51–S58. 2018.
View Article : Google Scholar
|
|
2
|
Chinese Anticancer Association
Gynecological Tumor Professional Committee, . Diagnosis of uterine
sarcoma in treatment guidelines (2021 edition). China Oncology.
31:513–519. 2021.(In Chinese).
|
|
3
|
Harlow BL, Weiss NS and Lofton S: The
epidemiology of sarcomas of the uterus. J Natl Cancer Inst.
76:399–402. 1986.PubMed/NCBI
|
|
4
|
Istrate-Ofiţeru AM, Zorilă GL, Ruican D,
Petrescu AM, Berbecaru EIA, Roşu GC, Căpitănescu RG, Nagy RD,
Cercelaru L, Edu A, et al: Uterine myxoid leiomyosarcoma-A rare
malignant tumor: The role of complex morphopathological assay.
Review and case presentation. Rom J Morphol Embryol. 62:883–896.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sen A; FIGO, : FIGO 2009 classification
for uterine sarcoma staging: FIGO 2023 endometrial cancer
classification for carcinosarcoma staging. Int J Gynaecol Obstet.
167:12732024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ng WK, Lui PCW and Ma L: Peritoneal
washing cytology findings of disseminated myxoid leiomyosarcoma of
uterus: Report of a case with emphasis on possible differential
diagnosis. Diagn Cytopathol. 27:47–52. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takano Y, Morimura Y, Yamada H, Yanagida
K, Sato A, Suzuki O and Suzuki T: Myxoid leiomyosarcoma of the
uterus. Fukushima J Med Sci. 46:41–47. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gadducci A, Landoni F, Sartori E, Zola P,
Maggino T, Lissoni A, Bazzurini L, Arisio R, Romagnolo C and
Cristofani R: Uterine leiomyosarcoma: Analysis of treatment
failures and survival. Gynecol Oncol. 62:25–32. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui RR, Wright JD and Hou JY: Uterine
leiomyosarcoma: A review of recent advances in molecular biology,
clinical management and outcome. BJOG. 124:1028–1037. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antonio R, Dieg R and Daniele N:
Diagnostic accuracy of ultrasound in the diagnosis of uterine
leiomyomas and sarcomas. J Minim Invasive Gynecol. 31:28–36. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kagami S, Kashimura M, Toki N and Katuhata
Y: Myxoid leiomyosarcoma of the uterus with subsequent pregnancy
and delivery. Gynecol Oncol. 85:538–542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mittal K, Popiolek D and Demopoulos RI:
Uterine myxoid leiomyosarcoma within a leiomyoma. Hum Pathol.
31:398–400. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wankhade R, Sajjanar A, Dawande P and
Noman O: A rare case of uterine myxoid leiomyosarcoma. Cureus.
15:e443032023.PubMed/NCBI
|
|
14
|
Burch DM and Tavassoli FA: Myxoid
leiomyosarcoma of the uterus. Histopathology. 59:1144–1155. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Teng Y, Na S and Yuan Y:
Pleomorphic leiomyosarcoma of the adrenal gland in a young woman: A
case report and review of the literature. Onco Targets Ther.
13:4705–4713. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li RP, Zhang M, Gao J and Guo Y:
Misdiagnosis and mistreatment of uterine myxoid leiomyosarcoma: A
case report and literature review. Asian J Surg. 46:2714–2715.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giannini A, Cuccu I, D'Auge TG, De Angelis
E, Laganà AS, Chiantera V, Caserta D, Vitale SG, Muzii L, D'Oria O,
et al: The great debate: Surgical outcomes of laparoscopic versus
laparotomic myomectomy. A meta-analysis to critically evaluate
current evidence and look over the horizon. Eur J Obstet Gynecol
Reprod Biol. 297:50–58. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berchuck A, Rubin SC, Hoskins WJ, Saigo
PE, Pierce VK and Lewis JL: Treatment of uterine leiomyosarcoma.
Obstet Gynecol. 71:845–850. 1988.PubMed/NCBI
|
|
19
|
Giannini A, Golia D'Augè T, Bogani G,
Laganà AS, Chiantera V, Vizza E, Muzii L and Di Donato V: Uterine
sarcomas: A critical review of the literature. Eur J Obstet Gynecol
Reprod Biol. 287:166–170. 2023. View Article : Google Scholar : PubMed/NCBI
|