Introduction
In 2020, 732,210 people had colorectal cancer in 185
countries, accounting for 339,022 deaths (1). Among colorectal cancer, locally
advanced rectal cancer (LARC) poses significant treatment
challenges due to its high risk of local recurrence and distant
metastasis. In Western countries, neoadjuvant therapies, including
chemoradiotherapy (CRT) and total neoadjuvant therapy (TNT), are
the standard of care for LARC, aiming to facilitate tumor
downstaging, improve local control, and reduce the risk of distant
metastasis (2,3). Furthermore, CRT has also been widely
utilized in the treatment of various locally advanced solid tumors,
including gastrointestinal malignancies, head and neck,
gynecological, lung, and genitourinary cancers, as well as for
glioblastoma and sarcoma (4).
While CRT has significantly improved outcomes for
LARC, treatment responses remain highly variable among patients.
One of the factors that may influence the efficacy of CRT is
cigarette smoking. Recent studies have shown that cigarette smoking
during radiotherapy for glottic carcinomas and anal cancers is
associated with poor therapeutic responses (5,6).
Smoking during radiotherapy has been linked to increased hypoxia,
reduced radiosensitivity, and impaired treatment outcomes. However,
the effect of smoking on CRT for rectal cancer remains unclear. In
our institution, patients with LARC are strictly instructed to quit
smoking before radiotherapy, and all patients are monitored for
smoking cessation by measuring carbon monoxide (CO) levels in their
exhaled breath using a Smokerlyzer prior to initiation of
neoadjuvant CRT.
This study aimed to describe the outcomes of
neoadjuvant CRT under strict non-smoking conditions, as confirmed
by measuring CO levels, followed by total mesorectal excision (TME)
in patients with LARC. By assessing treatment response rates and
survival outcomes, this study seeks to provide valuable insights
into the potential benefits of smoking cessation on neoadjuvant CRT
efficacy for LARC. These findings could inform improvements in the
treatment outcomes of patients with LARC undergoing neoadjuvant
CRT.
Materials and methods
Ethics
This retrospective single-center observational study
was approved by the institutional review board of Osaka General
Medical Center (approval no. 2020-069; Osaka, Japan). Informed
consent was obtained through an opt-out method on the website, by
which any patient could refuse inclusion in the study.
Patients
We retrospectively included the data of patients
with LARC (clinical stage II or III) who underwent neoadjuvant CRT
and radical surgery between January 2014 and December 2019. The
inclusion criteria were as follows: i) Histologically confirmed
adenocarcinoma of the rectum, ii) tumors located below the
peritoneal reflection, and iii) cT3/T4 and/or cN+ rectal cancer
without distant metastasis. The clinical stage was determined based
on imaging studies, including endoscopy, multi-slice computed
tomography (CT), and magnetic resonance imaging (MRI). Lymph nodes
with a short-axis diameter ≥10 mm on CT or MRI and/or a
high-intensity spot on positron emission tomography CT images were
considered suspicious for metastasis. Pre- and post-operative
staging was performed according to the 9th edition of the Japanese
Society for Cancer of the Colon and Rectum classification (JSCCR)
(7). For lymph node assessment,
lateral lymph nodes (LLNs) (i.e., obturator and sacral lymph nodes)
were defined as regional lymph nodes. Metastasis in the inguinal
lymph node was defined as distant unless the tumor extended to the
anal canal.
Monitoring smoking cessation
Prior to the initiation of neoadjuvant CRT, smoking
cessation guidance was provided to all patients who smoked at
diagnosis. Complete smoking cessation was monitored using a
Smokerlyzer (Bedfont Scientific Ltd., Kent, UK) (8), which quantified expiratory CO levels
to detect whether patients had smoked within the past few days.
Smoking cessation was defined as a maintained expiratory CO level
of 0–3 ppm without an increase for 4 consecutive weeks. For
patients with CO levels >3 ppm, CRT was postponed for 4 weeks.
Four consecutive weeks after smoking cessation, CO levels were
measured to ensure that smoking cessation was maintained.
Treatment strategy
CRT comprised a total radiation dose of 50.0 Gy (2.0
Gy/day, 5 days per week, for 5 weeks) and
tegafur/gimeracil/oteracil (TS-1). The bilateral pelvic area, apart
from the primary tumor and regional lymph nodes, was included in
the radiation target area. Concomitant chemotherapy with TS-1
(80–120 mg/day) was orally administered on the day of radiotherapy
for 5 weeks. Imaging examinations were performed 4–6 weeks after
CRT, and the clinical stage was preoperatively determined.
Surgical resection was performed 8–12 weeks after
completion of CRT. All patients underwent laparoscopic TME. Lateral
lymph node dissection (LLND) was performed only in patients with
suspected LLN metastases based on pretreatment images, regardless
of the response of the lymph nodes to CRT. LLND was performed only
on the side of the suspected LLNs; furthermore, the internal iliac,
external iliac, and obturator regions were dissected following the
standard LLND procedure. In the case of suspected bilateral
metastases to LLNs, bilateral LLND was performed. Temporary
ileostomy was performed at the discretion of each surgeon, as
recommended by the multidisciplinary team.
Adjuvant chemotherapy was considered for patients
with high-risk stages II and III. The decision was made by the
attending physician.
Evaluation of clinical and
pathological response to CRT
The clinical response to CRT was evaluated based on
the Response Evaluation Criteria in Solid Tumors (RECIST 1.1).
The pathological primary tumor response to CRT was
evaluated using a grading scale according to the JSCCR 9th edition.
Grade 0 represents no response to treatment, grade 1a a tumor size
reduction of 1/3, grade 1b a tumor size reduction of 1/3-2/3, grade
2 a tumor size reduction >2/3, and grade 3 complete tumor
ablation. Grade 3 corresponds to pathological complete response
(pCR) (7).
Follow-up
The patients were followed up at 3-month intervals
during the first 3 years and at 6-month intervals thereafter for up
to 5 years. Tumor markers carcinoembryonic antigen (CEA) and
carbohydrate antigen 19-9 were assessed at each follow-up visit. CT
scans were performed at 6-month intervals. Total colonoscopies were
performed annually.
Statistical analysis
Continuous parameters are presented as mean, median,
standard deviation, and interquartile range. Univariate and
multivariate logistic regression analyses were used to evaluate
factors associated with pCR. The Kaplan-Meier method was used to
evaluate survival outcomes, and the log-rank test was used to
assess the estimated survival rate. The hazard ratio (HR) was
calculated using a Cox proportional hazards model, followed by the
calculation of the 95% confidence interval (CI). Variables were
included in the models based on the existing knowledge regarding
risk factors for 5-year overall survival (OS). All statistical
analyses were performed using R version 1.60 (SAS Institute Inc.,
Cary, NC, USA) and GraphPad Prism version 6.01 for Windows
(GraphPad Software, San Diego, CA, USA). Statistical significance
was assessed using 95% CI. Two-tailed P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
Overall, 28 patients were included, with their
characteristics summarized in Table
I. The median age was 66 years, and 20 patients were male.
Median tumor size and median tumor location from the anal verge
were 32.5 and 50 mm, respectively. Sixteen patients were diagnosed
with clinical stage III. Seven patients smoked at diagnosis. The
average expiratory CO level was 8 (range, 8–30) ppm. After smoking
cessation guidance, smoking cessation was confirmed based on an
expiratory CO level <3 ppm within a month before CRT
initiation.
 | Table I.Patients' characteristics (n=28). |
Table I.
Patients' characteristics (n=28).
| Variable | Value |
|---|
| Median age, years
(range) | 66 (45–89) |
| Sex, n (%) |
|
| Male | 20 (71.4) |
|
Female | 8 (28.6) |
| Median BMI,
kg/m2 (range) | 22.4 (16.9–35.9) |
| History of smoking, n
(%) | 18 (64.3) |
| Median expiratory CO,
ppm (range) |
|
| Smoker at
diagnosis (n=7) | 8 (8–30) |
| After
smoking cessation (n=7) | 2 (1–3) |
| Median pretreatment
CEA, ng/ml (range) | 3.7 (1–47.1) |
| Median pretreatment
Hb, g/dl (range) | 13.0
(7.3–15.5) |
| Median tumor size,
mm (range) | 32.5 (0–70) |
| Median tumor
location from AV, mm (range) | 50 (0–100) |
| Circumferential
involvement (<1/3), n (%) | 3 (10.7) |
| Clinical N stage, n
(%) |
|
|
Negative | 12 (42.9) |
|
Positive | 16 (57.1) |
| Adverse events
during CRT, n (%) |
|
|
Diarrhea (Grade 2) | 1 (3.6) |
| Ileus
(Grade ≥3) | 1 (3.6) |
|
Neutropenia (Grade ≥3) | 1 (3.6) |
| Clinical response
to CRT, n (%) |
|
|
cPR | 24 (85.7) |
|
cSD | 3 (10.7) |
| cPD
(occurrence of liver metastasis) | 1 (3.6) |
| Adjuvant
chemotherapy |
|
|
CAPOX | 2 (7.1) |
|
TS-1 | 12 (42.9) |
|
UFT/LV | 1 (3.6) |
CRT
All patients successfully underwent the scheduled
CRT. During CRT, one patient experienced diarrhea (grade 2), with
the TS-1 dose being reduced. A second and third patient experienced
ileus (grade ≥3) and neutropenia (grade ≥3), respectively, with CRT
treatment being temporarily discontinued in both patients. Clinical
partial response was achieved in 24 patients, whereas one patient
developed liver metastasis during CRT.
Surgical outcomes
Table II summarizes
the surgical outcomes. Fourteen patients underwent abdominoperineal
resection. LLND was performed in four patients with suspected LLN
metastasis before CRT. There was no conversion to open surgery.
Curative resection of the primary tumor was performed in all 28
patients, with a surgically sufficient circumferential resection
margin. Among them, one patient who developed liver metastasis
during neoadjuvant CRT underwent curative hepatectomy. There were
no major complications defined as grade ≥3 according to the
Clavien-Dindo classification.
 | Table II.Surgical outcomes (n=28). |
Table II.
Surgical outcomes (n=28).
| Variable | Value |
|---|
| Surgical procedure,
n (%) |
|
|
APR | 14 (50.0) |
|
LAR | 13 (46.4) |
|
ISR | 1 (3.6) |
| With
LLND | 4 (14.3) |
| Median operation
time, min (range) | 367.5
(203–552) |
| Median estimated
blood loss, ml (range) | 120 (0–1420) |
| Conversion to open
surgery, n | 0 |
| Curative resection
for primary tumor, n (%) | 28 (100) |
| Postoperative
complication, n (%) |
|
|
SSI | 9 (32.1) |
|
Anastomotic leakage | 1 (3.6) |
|
Paralytic ileus | 3 (10.7) |
| Urinary
dysfunction | 2 (7.1) |
|
Lymphatic leakage | 2 (7.1) |
| Median
postoperative hospital stay, days (range) | 17 (9–77) |
| Postoperative
mortality, n | 0 |
| Sites of
recurrence, n (%) |
|
|
Local | 1 (3.6) |
|
Lung | 3 (10.7) |
|
Liver | 3 (10.7) |
| Lateral
lymph node | 0 |
Pathological findings
The pathological findings are summarized in Table III. Overall, pCR was achieved in
8/28 patients (28.6%; 95% CI, 15.1–47.2). LLN metastasis was
detected in one out of four patients who underwent LLND. None of
the patients showed a positive circumferential resection
margin.
 | Table III.Pathological findings (n=28). |
Table III.
Pathological findings (n=28).
| Variable | Value |
|---|
| Histological type,
n (%) |
|
|
Well-/moderately
differentiated | 26 (92.9) |
|
Mucinous/poorly
differentiated/signet | 2 (7.1) |
| ypT, n (%) |
|
|
ypT0 | 8 (28.6) |
|
ypT1 | 0 |
|
ypT2 | 6 (21.4) |
|
ypT3 | 14 (50.0) |
|
ypT4 | 0 |
| ypN, n (%) |
|
|
ypN0 | 20 (71.4) |
|
ypN1 | 7 (25.0) |
|
ypN2 | 0 |
|
ypN3 | 1 (3.6) |
| Pathological
complete response, n (%) | 8 (28.6) |
| Median number of
lymph nodes resected (range) | 12 (2–34) |
| Location of lymph
node metastasis, n (%) |
|
|
Mesorectum | 7 (25.0) |
|
LLN | 1 (3.6) |
| Positive
lymphovascular invasion, n (%) | 9 (32.1) |
| Positive
circumferential resection margin, n | 0 |
Survival outcomes and
clinicopathological factors associated with pCR
The median follow-up period was 60.1 months. The
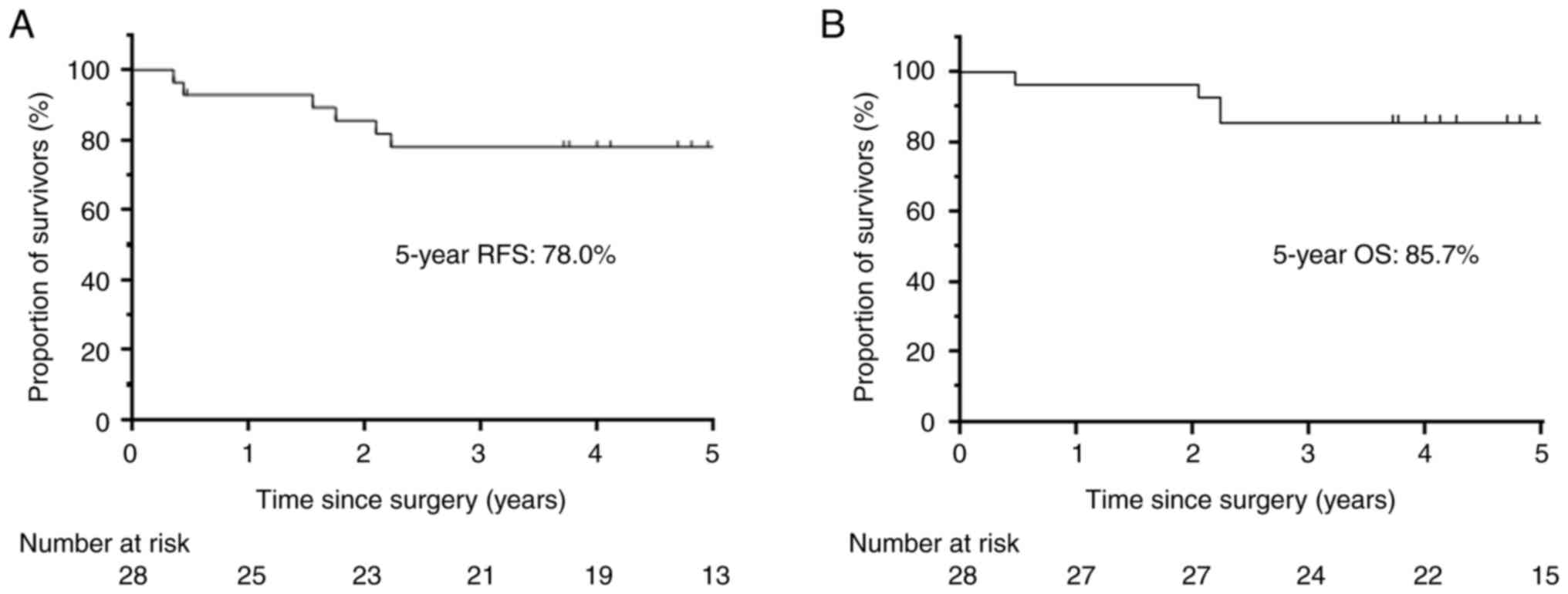
5-year recurrence-free survival and OS rates were 78.0% (95% CI,
57.4–89.5) and 85.7% (95% CI, 66.3–94.4), respectively (Fig. 1). There was no significant
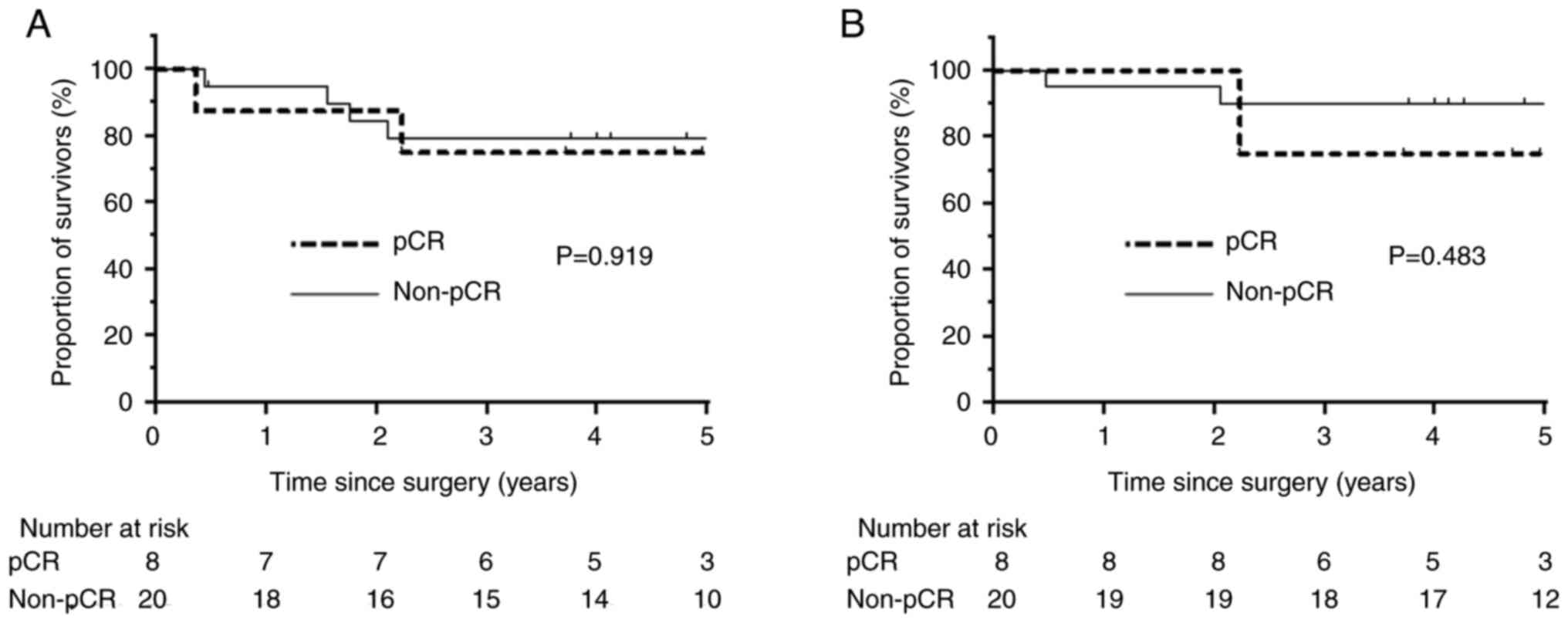
difference in survival between patients with and without pCR
(Fig. 2). One patient had local
recurrence; three, including one with pCR, had lung recurrence; and
three, including one with pCR, developed liver recurrence. No
patient developed local recurrence at the LLN. Moreover, there was
no significant difference in survival between smokers and
non-smokers at diagnosis (Fig.
S1).
Table IV shows the
clinicopathological factors associated with pCR. Univariate
analysis showed that pretreatment hemoglobin (Hb) (>13.0 g/dl)
was associated with pCR (HR: 7.00; 95% CI: 1.09–45.2; P=0.0408).
Multivariate logistic regression analysis showed that clinically
negative N stage before treatment was significantly associated with
pCR (HR: 18.9; 95% CI, 1.63–218.0; P=0.0187). However, being a
non-smoker was not associated with pCR (HR: 0.417; 95% CI,
0.0687–2.53; P=0.341).
 | Table IV.Univariate and multivariate logistic
regression analyses of factors associated with pCR. |
Table IV.
Univariate and multivariate logistic
regression analyses of factors associated with pCR.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age |
|
|
|
|
| ≤75
years | Reference | 0.643 |
|
|
| >75
years | 0.571
(0.054–6.08) |
|
|
|
| Sex |
|
|
|
|
|
Female | Reference | 0.792 |
|
|
|
Male | 1.290
(0.199–8.29) |
|
|
|
| BMI |
|
|
|
|
| ≤25
kg/m2 | Reference | 0.3410 |
|
|
| >25
kg/m2 | 2.400
(0.396–14.600) |
|
|
|
| Smoking at
diagnosis |
|
|
|
|
|
Smoker | Reference | 0.341 |
|
|
|
Non-smoker | 0.417
(0.0687–2.53) |
|
|
|
| Pretreatment CEA
levels |
|
|
|
|
| ≤5
ng/ml | Reference | 0.9010 |
|
|
| >5
ng/ml | 1.110
(0.203–6.11) |
|
|
|
| Pretreatment
Hb |
|
|
|
|
| ≤13.0
g/dl | Reference | 0.0408 | Reference | 0.110 |
|
>13.0 g/dl | 7.00
(1.090–45.20) |
| 5.99
(0.665–54.0) |
|
| Tumor size |
|
|
|
|
| ≤50
mm | Reference | 0.475 |
|
|
| >50
mm | 0.429
(0.0418–4.39) |
|
|
|
| Tumor location from
AV |
|
|
|
|
| >40
mm | Reference | 0.63 |
|
|
| ≤40
mm | 1.50
(0.288–7.81) |
|
|
|
| Circumferential
involvement |
|
|
|
|
|
≤1/3 | Reference | 0.847 |
|
|
|
>1/3 | 0.778
(0.0605–10.00) |
|
|
|
| Clinical N
stage |
|
|
|
|
|
Positive | Reference | 0.0103 | Reference | 0.0187 |
|
Negative | 21.0
(2.05–215) |
| 18.9
(1.63–218.0) |
|
| Histological
type |
|
|
|
|
|
Well/moderate | Reference | 0.500 |
|
|
|
Mucinous/poor/signet | 2.71
(0.149–49.50) |
|
|
|
| CRT |
|
|
|
|
|
Completion of CRT | Reference | 0.847 |
|
|
|
Incompletion of CRT | 1.29
(0.100–16.5) |
|
|
|
Discussion
To our knowledge, this is the first study to report
the oncological outcomes in patients with LARC who underwent
neoadjuvant CRT under strict non-smoking conditions. Neoadjuvant
CRT under non-smoking conditions achieved favorable pCR rates and
comparable survival outcomes. Additionally, a clinically negative N
stage before treatment was significantly associated with pCR to
neoadjuvant CRT.
Smoking creates hypoxic environment given an
increase in the degree of carboxyhemoglobin saturation in the blood
and tissue. Chronic hypoxia compromises the effects of
radiotherapy, resulting in limited treatment efficacy (9). Browman et al (10) reported that patients with head and
neck cancer who continued smoking during radiotherapy (n=53) had a
lower complete response rate (45% vs. 74%, P=0.008) and poorer
2-year survival (39 vs. 66%, P=0.005) than those who did not smoke
or quit before treatment (n=62). Their study suggested that smoking
during therapy reduced the response to radiotherapy in patients
with head and neck cancer. To improve the clinical outcomes of
radiotherapy, complete smoking cessation, confirmed by monitoring
expiratory CO levels using a Smokerlyzer, has contributed towards a
lower relapse rate and better prognosis in various cancers
(5,6). Thus, we used Smokerlyzer to monitor
smoking cessation. Smokers at diagnosis who quit smoking before
radiotherapy showed expiratory CO levels within the reference range
and had outcomes similar to those of non-smokers, highlighting the
potential benefit of smoking cessation before radiotherapy.
Neoadjuvant CRT reduces the local recurrence rate in
patients undergoing LARC, with previous studies reporting pCR rates
of 5.3% (11), 14.6% (12), 15.6% (13), and 19.2% (14). In this study, clinical partial
response was achieved in 24/28 patients (85.7%); pCR was achieved
in 8/28 patients (28.6%), which was higher than that achieved in
these previous studies. A good pathological response to neoadjuvant
CRT is associated with prolonged OS (14,15).
Furthermore, achieving pCR after CRT is a valid surrogate of a
favorable outcome regarding local control, distant recurrence,
disease-free survival, and OS (14). Therefore, we investigated the
predictive factors associated with pCR to neoadjuvant CRT.
Univariate analysis showed that high pretreatment Hb (>13.0
g/dl) was associated with an increased pCR rate. Additionally,
multivariate analysis revealed that clinically negative N stage
before treatment was significantly associated with an increased pCR
rate. These results are consistent with previous reports showing
that pCR is associated with smaller tumor size, clinically negative
N stage, higher radiation dose, and lower pretreatment CEA level
(13,16–18).
However, predictive factors of the pathological response to
neoadjuvant CRT in patients with LARC are lacking.
Another possible reason for the high pCR rate in
this study is the maintenance of the Hb level. A previous
retrospective study suggested that Hb levels were significantly
associated with disease-free survival in patients with rectal
cancer who received neoadjuvant CRT (19). Moreover, clinical downstaging was
achieved in 55 and 35% of patients with Hb levels >12 g/dl and
<12 g/dl, respectively. Accordingly, we attempted to maintain
the pretreatment Hb level by transfusion or prescribing oral iron
tablets. We speculate that maintaining both high Hb levels and low
CO levels under non-smoking conditions significantly contributed to
high pCR rates and favorable outcomes in this study.
In our study, only one patient had local recurrence,
and no recurrence was observed in the LLN region. However, distant
metastases developed in 6/28 (21.4%) patients, including one
patient who developed liver metastasis during neoadjuvant CRT.
These results indicated that despite achieving good local control,
including in the LLN region, distant metastases were not adequately
controlled. An alternative treatment strategy known as TNT shows
promise for improving both pCR rates and disease-free survival in
patients with LARC (20). Recent
evidence suggests that TNT may also increase the likelihood of
achieving a complete clinical response, allowing for a
non-operative management strategy in selected cases (21,22).
Although TNT is promising for improving oncological outcomes,
further studies are required to determine its long-term effects on
local control and survival.
In our cohort, 14 patients (50%) underwent
abdominoperineal resection (APR), while only 1 (3.6%) underwent
intersphincteric resection (ISR). The high frequency of APR was
primarily due to tumor invasion into the sphincter complex, making
sphincter preservation unfeasible in many cases. Additionally, some
patients who were eligible for ISR or low anterior resection (LAR)
opted for APR to avoid potential postoperative functional
impairment. LAR and ISR are associated with LAR syndrome, which
includes symptoms such as frequent defecation, urgency, and fecal
incontinence. Furthermore, neoadjuvant CRT can exacerbate these
functional impairments by inducing radiation-associated fibrosis
and neuropathy, which negatively impact sphincter control and
anorectal compliance. Given these concerns, APR was chosen as a
definitive approach to ensure a better long-term quality of life in
selected patients.
This study has some limitations. First, this was a
single-arm single-center retrospective observational study. Second,
this study had a small sample size. Therefore, a large multicenter
prospective study is required to evaluate the outcomes in patients
with LARC who are treated with neoadjuvant CRT under non-smoking
conditions. Third, we did not compare between current smokers and
non-smokers during radiotherapy. This is because all the included
patients successfully quit smoking prior to the initiation of
neoadjuvant CRT. Based on the established benefit of smoking
cessation for radiotherapy in other tumors, our institutional
policy is to guide all patients quitting smoking before
radiotherapy and monitor smoking cessation before starting
neoadjuvant CRT. Another limitation is that all patients underwent
laparoscopic TME, whereas robotic-assisted surgery was not
performed in any case. In Japan, robotic-assisted surgery for
rectal cancer has been covered by the national insurance system
since 2018, whereas robotic-assisted surgery for colon cancer was
included in 2022. However, during our study period (2014–2019),
robotic-assisted surgery was not widely available owing to high
costs and limited institutional experience. Recent studies suggest
that robotic-assisted surgery offers superior outcomes,
particularly in male patients with mid- to low-rectal cancer, owing
to its enhanced precision and improved ergonomics in narrow pelvic
spaces (23–25).
In conclusion, neoadjuvant CRT under strict
non-smoking conditions, as confirmed by measuring expiratory CO
levels, yielded favorable pCR rates and comparable survival
outcomes in patients with LARC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AI, YK, YN, TK, MH, YO, YMo, SS, YMi, AT, MM, and KF
contributed to the conception and design. Material preparation,
data collection and analysis were performed by AI, YK and YN. AI,
YK and YN confirm the authenticity of all the raw data. The first
draft of the manuscript was written by AI and YK, and all authors
commented on previous versions of the manuscript. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
This retrospective single-center observational study
was approved by the Institutional Review Board of Osaka General
Medical Center (approval no. 2020-069). Informed consent was
obtained through an opt-out method on the website, by which any
patient could refuse inclusion in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APR
|
abdominoperineal resection
|
|
CO
|
carbon monoxide
|
|
CEA
|
carcinoembryonic antigen
|
|
CRT
|
chemoradiotherapy
|
|
CT
|
computed tomography
|
|
CI
|
confidence interval
|
|
HR
|
hazard ratio
|
|
Hb
|
hemoglobin
|
|
ISR
|
intersphincteric resection
|
|
JSCCR
|
Japanese Society for Cancer of the
Colon and Rectum classification
|
|
LLND
|
lateral lymph node dissection
|
|
LLNs
|
lateral lymph nodes
|
|
LARC
|
locally advanced rectal cancer
|
|
LAR
|
low anterior resection
|
|
MRI
|
magnetic resonance imaging
|
|
OS
|
overall survival
|
|
pCR
|
pathological complete response
|
|
TS-1
|
tegafur/gimeracil/oteracil
|
|
TME
|
total mesorectal excision
|
|
TNT
|
total neoadjuvant therapy
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sauer R, Becker H, Hohenberger W, Rödel C,
Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF,
et al: Preoperative versus postoperative chemoradiotherapy for
rectal cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Garrido-Laguna I, et al: NCCN guidelines insights: Rectal cancer,
version 6.2020. J Natl Compr Canc Netw. 18:806–815. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rallis KS, Lai Yau TH and Sideris M:
Chemoradiotherapy in cancer treatment: Rationale and clinical
applications. Anticancer Res. 41:1–7. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tatekawa S, Shimamoto S, Miyata Y, Yoshino
Y, Hirata T, Tamari K, Seo Y, Isohashi F, Yamamoto Y, Uno A, et al:
Monitoring expiratory carbon monoxide to study the effect of
complete smoking cessation on definitive radiation therapy for
early stage glottic carcinoma. Acta Oncol. 60:582–588. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lerman J, Hennequin C, Etienney I,
Abramowitz L, Goujon G, Gornet JM, Guillerm S, Aparicio T, Valverde
A, Cattan P and Quéro L: Impact of tobacco smoking on the patient's
outcome after (chemo)radiotherapy for anal cancer. Eur J Cancer.
141:143–151. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Japanese Society for Cancer of the Colon
and Rectum, . Japanese classification of colorectal, appendiceal,
and anal carcinoma: The 3d English edition [secondary publication].
J Anus Rectum Colon. 3:175–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deveci SE, Deveci F, Açik Y and Ozan AT:
The measurement of exhaled carbon monoxide in healthy smokers and
non-smokers. Respir Med. 98:551–556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nordsmark M and Overgaard J: Tumor hypoxia
is independent of hemoglobin and prognostic for loco-regional tumor
control after primary radiotherapy in advanced head and neck
cancer. Acta Oncol. 43:396–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Browman GP, Wong G, Hodson I, Sathya J,
Russell R, McAlpine L, Skingley P and Levine MN: Influence of
cigarette smoking on the efficacy of radiation therapy in head and
neck cancer. N Engl J Med. 328:159–163. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akagi T, Inomata M, Fujishima H, Fukuda M,
Konishi T, Tsukamoto S, Teraishi F, Ozawa H, Tanaka K, Hida K, et
al: Preoperative chemoradiotherapy versus surgery alone for
advanced low rectal cancer: A large multicenter cohort study in
Japan. Surg Today. 50:1507–1514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huh JW, Kim HR and Kim YJ: Clinical
prediction of pathological complete response after preoperative
chemoradiotherapy for rectal cancer. Dis Colon Rectum. 56:698–703.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maas M, Nelemans PJ, Valentini V, Das P,
Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R,
Haustermans K, et al: Long-term outcome in patients with a
pathological complete response after chemoradiation for rectal
cancer: A pooled analysis of individual patient data. Lancet Oncol.
11:835–844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sadahiro S, Suzuki T, Tanaka A, Okada K,
Saito G, Kamijo A, Akiba T and Kawada S: Phase II study of
preoperative concurrent chemoradiotherapy with S-1 plus bevacizumab
for locally advanced resectable rectal adenocarcinoma. Oncology.
88:49–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomono A, Yamashita K, Kanemitsu K, Sumi
Y, Yamamoto M, Kanaji S, Imanishi T, Nakamura T, Suzuki S, Tanaka K
and Kakeji Y: Prognostic significance of pathological response to
preoperative chemoradiotherapy in patients with locally advanced
rectal cancer. Int J Clin Oncol. 21:344–349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garland ML, Vather R, Bunkley N, Pearse M
and Bissett IP: Clinical tumour size and nodal status predict
pathologic complete response following neoadjuvant
chemoradiotherapy for rectal cancer. Int J Colorectal Dis.
29:301–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang CM, Huang CW, Ma CJ, Yeh YS, Su WC,
Chang TK, Tsai HL, Juo SH, Huang MY and Wang JY: Predictive value
of FOLFOX-based regimen, long interval, hemoglobin levels and
clinical negative nodal status, and postchemoradiotherapy CEA
levels for pathological complete response in patients with locally
advanced rectal cancer after neoadjuvant chemoradiotherapy. J
Oncol. 2020:94376842020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Sukhni E, Attwood K, Mattson DM,
Gabriel E and Nurkin SJ: Predictors of pathologic complete response
following neoadjuvant chemoradiotherapy for rectal cancer. Ann Surg
Oncol. 23:1177–1186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berardi R, Braconi C, Mantello G,
Scartozzi M, Del Prete S, Luppi G, Martinelli R, Fumagalli M,
Valeri G, Bearzi I, et al: Anemia may influence the outcome of
patients undergoing neo-adjuvant treatment of rectal cancer. Ann
Oncol. 17:1661–1664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Conroy T, Bosset JF, Etienne PL, Rio E,
François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O,
Gargot D, et al: Neoadjuvant chemotherapy with FOLFIRINOX and
preoperative chemoradiotherapy for patients with locally advanced
rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised,
open-label, phase 3 trial. Lancet Oncol. 22:702–715. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asoglu O, Bulut A, Aliyev V, Piozzi GN,
Guven K, Bakır B and Goksel S: Chemoradiation and consolidation
chemotherapy for rectal cancer provides a high rate of organ
preservation with a very good long-term oncological outcome: A
single-center cohort series. World J Surg Oncol. 20:3582022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Asoglu O, Goksoy B, Aliyev V, Mustafayev
TZ, Atalar B, Bakir B, Guven K, Demir G and Goksel S: Watch and
wait strategy for rectal cancer: How long should we wait for a
clinical complete response? Surg Technol Int. 40:130–139. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aliyev V, Goksel S, Bakir B, Guven K and
Asoglu O: Sphincter-saving robotic total mesorectal excision
provides better mesorectal specimen and good oncological local
control compared with laparoscopic total mesorectal excision in
male patients with mid-low rectal cancer. Surg Technol Int.
38:160–166. 2021.PubMed/NCBI
|
|
24
|
Aliyev V, Piozzi GN, Shadmanov N, Guven K,
Bakır B, Goksel S and Asoglu O: Robotic and laparoscopic
sphincter-saving resections have similar peri-operative,
oncological and functional outcomes in female patients with rectal
cancer. Updates Surg. 75:2201–2209. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aliyev V, Piozzi GN, Huseynov E,
Mustafayev TZ, Kayku V, Goksel S and Asoglu O: Robotic male and
laparoscopic female sphincter-preserving total mesorectal excision
of mid-low rectal cancer share similar specimen quality,
complication rates and long-term oncological outcomes. J Robot
Surg. 17:1637–1644. 2023. View Article : Google Scholar : PubMed/NCBI
|