Introduction
Due to increases in lifespans and advancements in
diagnostic techniques, the incidence of multiple primary
malignancies is increasing. Depending on the criteria used, the
reported frequency of multiple primary malignancies ranges from 2.4
to 17% (1). Aetiological factors
that may predispose patients to multiple primary malignancies can
be grouped into host related, lifestyle factors and environmental
influences. The International Agency for Research on Cancer defines
synchronous multiple primary malignancies as two or more malignant
tumors diagnosed within the same individual concurrently or within
6 months of each other (2).
Commonly occurring combinations include head and neck with lung
cancer (3), skin with head and neck
cancer (4), skin with lung cancer
(5), breast with ovarian cancer
(6) and breast with endometrial
cancer (7). The malignancies can
share the same histology or be completely different entities.
Long-term survival with multiple primary malignancies is variable
and is influenced by cancer type and stage at diagnosis (1). The present report described a case of
synchronous multiple squamous cell carcinomas (SCCs) of the
stomach, skin and gingiva with metastases to the liver, lung,
spleen, kidney, bone and brain. These results may provide valuable
data on the treatment of multiple primary malignancies, which could
potentially be used to develop future treatments for these
patients.
Case report
Case presentation
A 67-year-old male patient was referred to Zhuji
People's Hospital of Zhejiang Province (Zhuji, China) in December
2023 with progressive chest tightness and fatigue, accompanied by
melena persisting for almost 1 week. His history included heavy
smoking (~60 cigarettes/day), alcohol consumption (75 ml/day) for
35 years and betelnut chewing for several years. He also had a
3-year history of hypertension and type 2 diabetes mellitus, but no
family history of cancer. A comprehensive medical history was
obtained, and physical examination, laboratory blood analyses and
pulmonary and cardiac function tests were performed. Physical
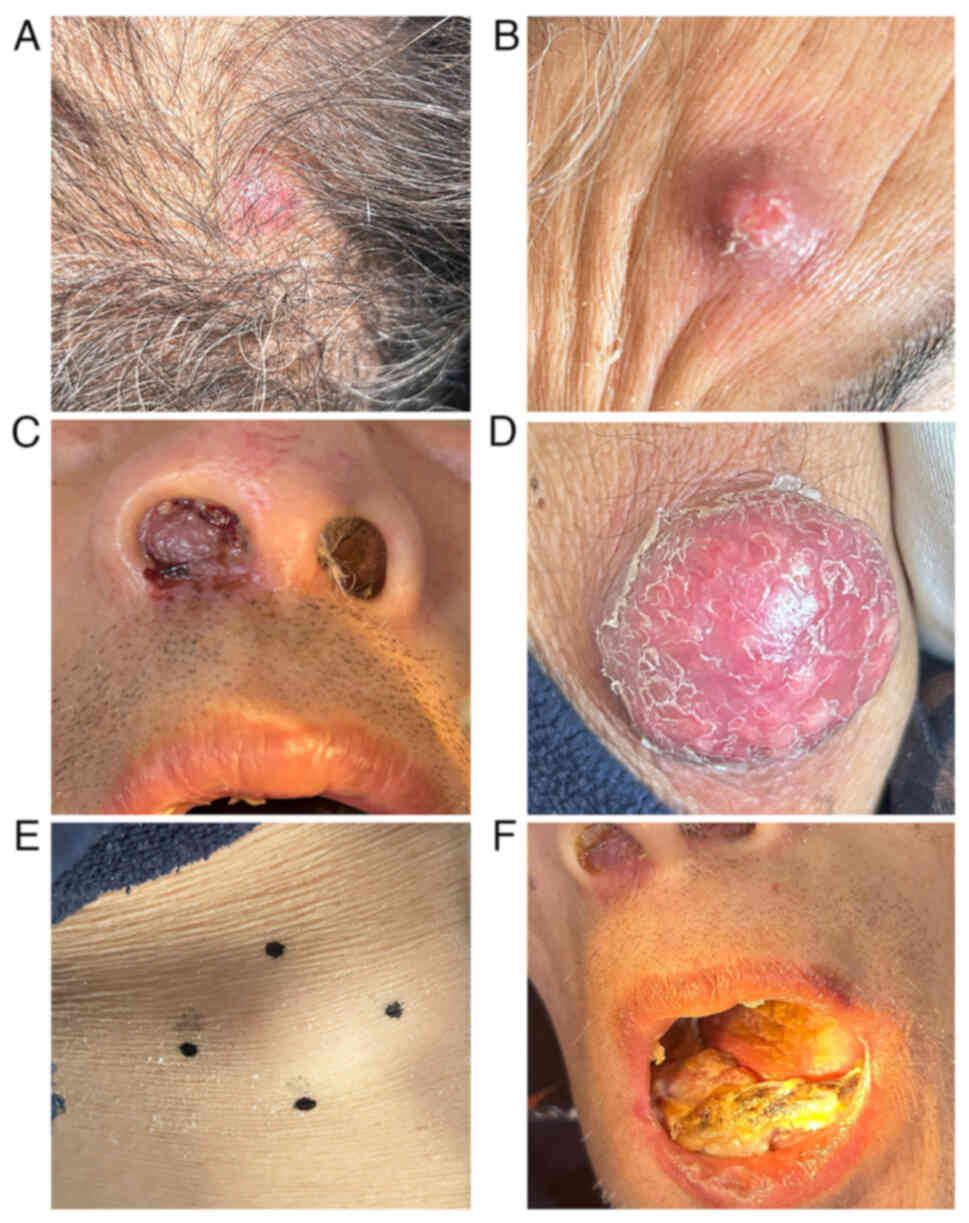
examination revealed an anemic appearance, multiple nodules on the
scalp (Fig. 1A), forehead (Fig. 1B), nasal cavity (Fig. 1C) and right forearm (Fig. 1D), as well as multiple subcutaneous
lumps on the right chest (Fig. 1E)
and left calf. In addition, a large mass was observed on the right
gingiva (Fig. 1F). Laboratory
examinations revealed severe hypochromia with a hemoglobin level of
50 g/l (normal range, 130–175 g/l), a positive fecal occult blood
test, a high serum creatinine level (125 µmol/l; normal range,
57–111 µmol/l), high levels of cytokeratin 19 fragment (cyfra 21-1;
55.1 ng/ml; normal range, 0.0–3.3 ng/ml), neuron specific enolase
(26.2 ng/ml; normal range, 0.0–20.0 ng/ml) and SCC antigen (26.7
µg/l; normal range, 0.0–1.5 µg/l), normal levels of
carcinoembryonic antigen (1.9 µg/l; normal range, 0.0–5.0 µg/l),
α-fetoprotein (1.8 µg/l; normal range, 0.0–7.0 µg/l), carbohydrate
antigen 19-9 (19.3 KIU/l; normal range, 0.0–30.0 KIU/l) and
pro-gastrin releasing peptide (59.4 pg/ml; normal range, 0.0–65.0
pg/ml). The results of pulmonary and cardiac function tests were
normal.
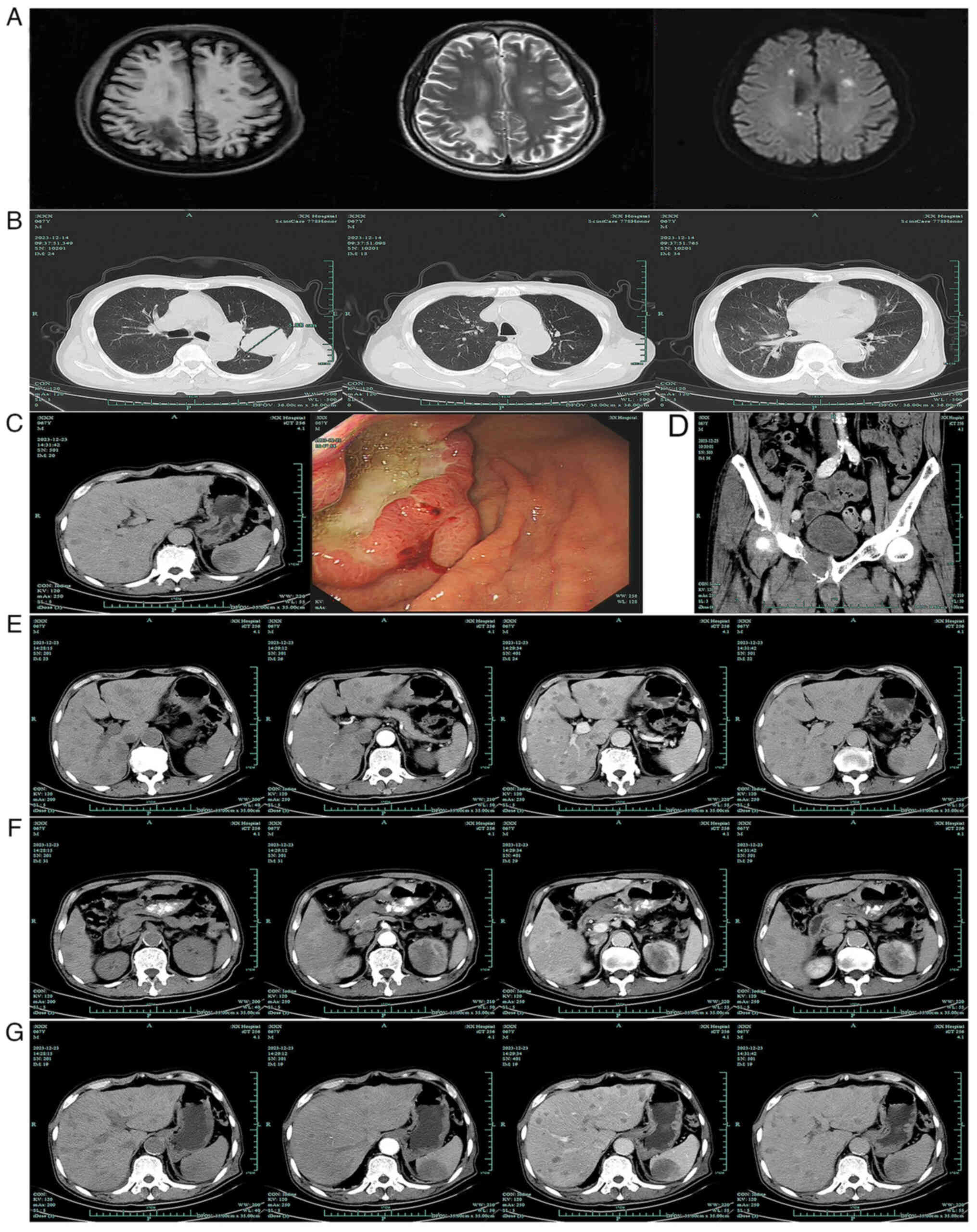
The patient subsequently underwent a chest computed
tomography (CT) plain scan, upper abdominal and pelvic CT scan with
contrast enhancement, brain magnetic resonance imaging with
contrast enhancement and diffusion-weighted imaging. Imaging
revealed the presence of multiple nodules in the frontal, parietal
and temporal lobes of the brain with associated edema (Fig. 2A), a nodule in the left pulmonary
upper lobe invading the pleura (Fig.
2B), local thickening and enhancement of the gastric wall in
the lower part of the gastric corpus and multiple enlarged lymph
nodes around the lesser curvature (Fig.
2C). The endoscopic examination revealed the presence of a
large ulcer with an uneven base in the lesser curvature of the
stomach (Fig. 2C). In addition,
bone of the right superior ramus of the pubis was degraded
(Fig. 2D) and multiple nodules were
observed in the liver (Fig. 2E),
left kidney (Fig. 2F) and spleen
(Fig. 2G). Finally, the patient
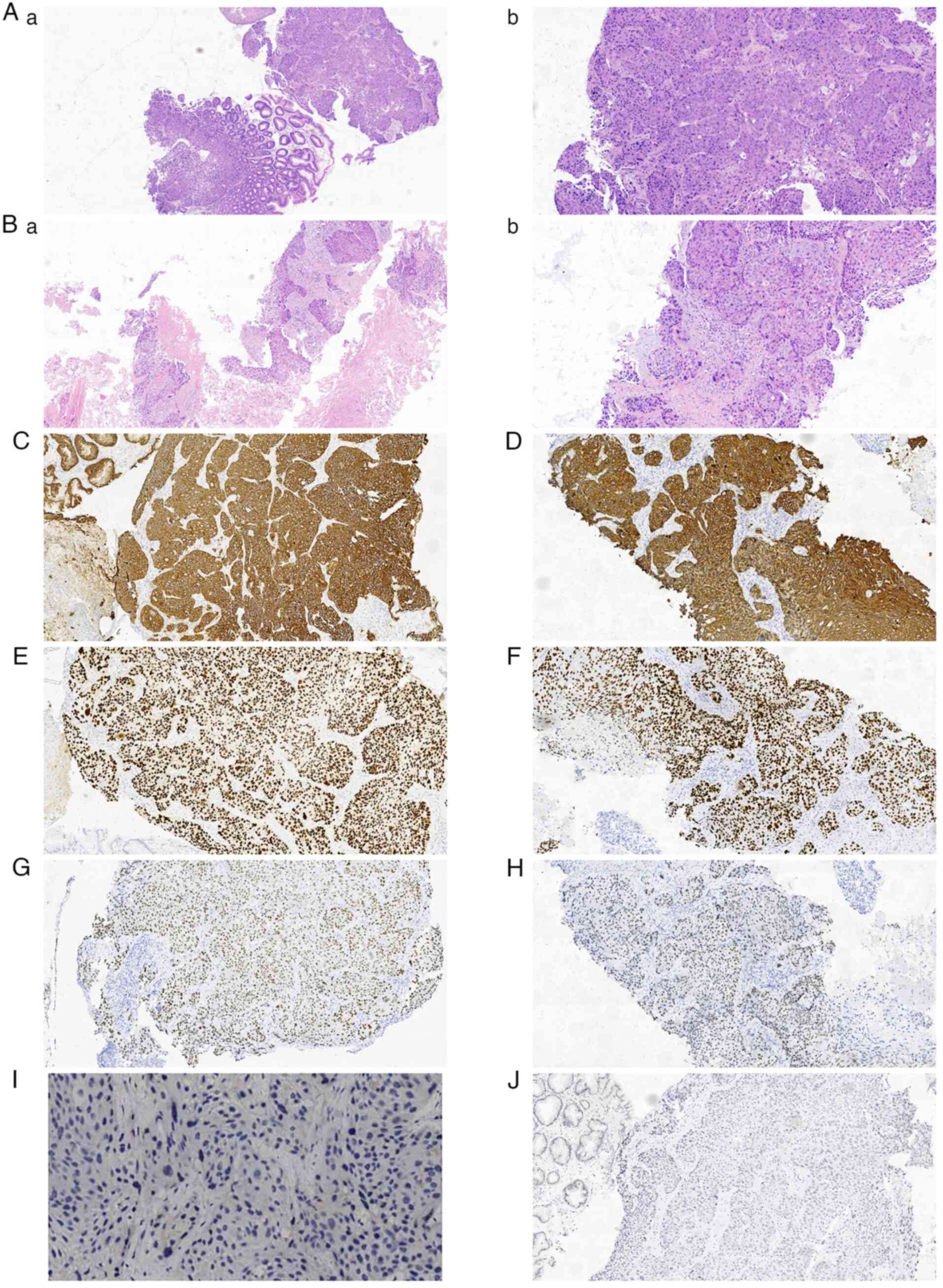
underwent an electronic gastroduodenoscopy with biopsy (Fig. 3A) and a biopsy of the subcutaneous
mass of the right forearm (Fig.
3B). However, he declined biopsies of other sites. Histological
analysis of the two biopsy specimens revealed moderately
differentiated SCC. No Helicobacter pylori was detected in
the gastric biopsy specimens. Immunohistochemical staining of the
biopsy specimens revealed the positive expression of
pan-cytokeratin (panCK; Fig. 3C and
D), P63 (Fig. 3E and F) and P40
(Fig. 3G and H), while the
expression of CK20 and CD56 was negative. In addition, the specimen
from the gastric biopsy was classified as programmed death ligand 1
(PD-L1) negative, with a combined positive score (CPS) of 0
(Fig. 3I), P16 expression was
negative, and P53 overexpression indicated a mutational phenotype.
Furthermore, the immunohistochemistry (IHC) of Epstein-Barr virus
(EBV) was negative (Fig. 3J).
Based on these findings, the patient was diagnosed
with SCC of the stomach, skin and gingiva with brain, lung, liver,
spleen, kidney, superior ramus of the pubis and subcutaneous
metastases, and an Eastern Cooperative Oncology Group (ECOG)
performance status of 2.
After 15 days, treatment was initiated with
intravenous camrelizumab (200 mg over 60 min) on day 1, intravenous
carboplatin (area under the concentration-time curve 5 mg/ml/min
for 30 min) on day 1, and intravenous nab-paclitaxel (100
mg/m2 over 30 min) on days 1 and 8, repeated every 3
weeks. However, the patient suffered from an anaphylactic reaction
to carboplatin, presenting as generalized skin eruption and chest
tightness. Therefore, carboplatin was discontinued and replaced
with cisplatin (25 mg/m2 over 6–8 h) on days 1–3 of the
21-day cycle. After one cycle of camrelizumab plus the
nab-paclitaxel and platinum-based (AP) regimen, physical
examination revealed a marked reduction in the size of the
cutaneous and subcutaneous lesions. However, the performance status
of the patient deteriorated to an ECOG score of 3. Following
discussions with the patient and his family, camrelizumab was
administered alone for the second and third treatment cycle, after
which a palliative approach was pursued. The patient succumbed 3
months after his initial diagnosis.
Histopathological examination
Samples were stained with hematoxylin (room
temperature for 10 min) and eosin (room temperature for 1 min),
before being observed using a light microscope. IHC was performed
as follows: The tissue samples were fixed in 10% neutral buffered
formalin (Zhejiang Jinhua Tonghe Biotechnology Co., Ltd.) at 25°C
for 12 h and then embedded in paraffin. The tissue paraffin block
was cut into 3-µm sections, which were heated at 60°C for 30 min,
and then deparaffinized in three xylene baths successively for 5
min each, and rinsed thrice with anhydrous alcohol, ethanol (95 and
75%) and water for 2 min each. Antigen retrieval was performed
using the DAKO PT Link instrument (Agilent Technologies, Inc.) at
97°C for 20 min. Staining was performed using the DAKO Autostainer
Link 48 system (Agilent Technologies, Inc.). The following primary
antibodies (prediluted by the manufacturer, Guangzhou Anbiping
Medical Laboratory Co., Ltd.) were used: CK (cat. no. IM067; clone:
AE1/AE3), P63 (cat. no. IR383; clone: LBP2-P63), P40 (cat. no.
IR345; clone: LBP2-P40), CK20 (cat. no. IR385; clone: LBP2-CK20),
CD56 (cat. no. IR040; clone: MRQ-42), P16 (cat. no. IM342; clone:
LBP1-P16), P53 (cat. no. IM123; clone: LBP1-P53), EBV (cat. no.
IM077; clone: CS1-4) and PD-L122C3 (cat. no. DAKO SK006). For
detection, an EnVision FLEX+, Mouse, High pH (Link) kit (cat. no.
K8002; DAKO; Agilent Technologies, Inc.), which included blocking
reagent, EnVision FLEX/HRP secondary antibodies and DAB+ chromogen,
was used. Blocking was performed with peroxidase blocking reagent
(cat. no. DAKO SM801) at 25°C for 5 min and all primary antibodies
were incubated with the samples at 25°C for 30 min, followed by
incubation with the prediluted EnVision FLEX/HRP secondary
antibodies (cat. no. DAKO SM802) at 25°C for 20 min and, finally,
staining with DAB+ chromogen (cat. no. DAKO DM827) at 25°C for 5
min. Images were captured using a light microscope.
Discussion
Among invasive forms of non-melanoma skin cancer
(NMSC), cutaneous SCC (CSCC) is the second most prevalent among
White individuals (8,9). It constitutes ~30% of all NMSC cases
(10), demonstrates a sex
disparity, with a higher prevalence in male (9–14%) compared with
female individuals (4–9%) (8,11), and
accounts for 20% of all cutaneous malignancies (9,12).
Established risk factors include chronic inflammation, cicatricial
lesions, lighter skin tone, high cumulative sun exposure and human
papillomavirus (HPV) infection (13–15).
The majority of lesions (80–90%) develop on sun-exposed areas of
the head and neck in older White male individuals (16). Metastasis is rare in CSCC, and the
disease generally follows a favorable clinical course with local
surgical resection (17). However,
CSCC has the potential to spread to regional lymph nodes and
distant organs, with the incidence of systemic metastases ranging
from 1 to 7% (18). Distant
metastases most commonly occur in the lungs, liver, brain, bones
and skin (18,19). Therapeutic outcomes for stage IV
CSCC remain suboptimal, with a reported median progression-free
survival (PFS) of 8 months and overall survival (OS) of 26 months,
resulting in a 5-year survival rate of only 26% (20). Systemic therapy is recommended for
patients with metastatic CSCC who are not candidates for surgery or
radiotherapy (21). The management
modalities include platinum-based chemotherapy, epidermal growth
factor receptor inhibitors and immune checkpoint inhibitors (ICIs)
(21). ICIs, particularly anti-PD-1
antibodies such as cemiplimab and pembrolizumab, are emerging as
first-line options, demonstrating objective response rates (ORR) of
50% in phase II trials (22,23).
Preliminary data have led to the National Comprehensive Cancer
Network Panel suggesting that other anti-PD-1 inhibitors may also
be effective in this setting (21).
Gastric SCC (GSCC) is rare, accounting for
0.04–0.07% of all gastric malignancies (24). The risk factors for GSCC have not
been clearly identified. However, GSCC typically presents at an
advanced stage, metastasizing to the liver, lymph nodes and other
organs, generally leading to poor outcomes (25–28).
No standard chemotherapy or chemotherapeutic regimen has been
defined for GSCC. In addition, the stomach is a rare metastatic
site for SCCs of all primary sites. The occurrence of gastric
metastasis has only been reported in case studies involving
individuals with lung and uterine cervical SCCs (29,30),
as well as an immunodeficient patient diagnosed with CSCC and
gastric metastasis (31).
SCC of the gingiva is uncommon, accounting for
<6% of oral cavity SCCs (OCSCCs) (32). It most commonly occurs in
middle-aged and older male individuals (33). Smoking, alcohol and betel quid
chewing are well-established risk factors (33). SCCs of the mandibular gingiva are
more common than those of the maxillary gingiva (34). ICIs combined with platinum-based
chemotherapy are considered the preferred first-line systemic
therapy for patients with unresectable and metastatic SCC of the
head and neck (SCCHN) (35).
However, metastatic lesions in the oral cavity are extremely rare,
and account for ~3% of all malignancies in adults (36). The most common primary sources of
oral cavity metastases are lung and breast carcinomas (37).
IHC is a powerful technique that uses specific
antibody-antigen binding to detect the localization of specific
antigens in cells and tissue (38).
It is an essential ancillary technique in clinical diagnostics
within anatomic pathology (39).
IHC is an indispensable complement to an epidemiology- and
morphology-driven approach to tumor diagnosis, helping to determine
the site of origin of metastatic tumors and detect tiny tumor foci
that may be inconspicuous on routine H&E staining (38,40).
Guidelines for the standardization and analytic validation of
immunohistochemical tests have been established by the College of
American Pathologists (39,41). panCK is a useful biomarker for
epithelial and epithelial-derived cells (42). SCCs are typically detected using
CK5/6, P63 and P40, whereas CK7 and CK20 are commonly used as
markers of adenocarcinoma, including gastric cancer (43,44).
In the present case, the gastric and subcutaneous tumors were
positive for panCK, P63 and P40, but negative for CK20. These
immunostaining results supported a diagnosis of SCC. PD-L1
expression is an important biomarker for predicting the therapeutic
effect of anti-PD-1 monoclonal antibodies. The KEYNOTE-055 study
revealed that a higher CPS is associated with an increased ORR and
improved survival outcomes (45).
P16 expression as detected by IHC is a widely used surrogate
biomarker that shows strong agreement with HPV status, as
determined by the assessment of HPV E6/E7 mRNA expression (46). HPV infection is a predominant cause
of SCC of the oropharynx, but is less common in OCSCCs (47). Patients with locally advanced
HPV-positive SCCHN exhibit an improved treatment response, OS and
PFS when compared with those with HPV-negative tumors (48). The wild-type P53 staining pattern is
characterized by nuclear staining in 1–80% of tumor cells with
varying intensities, while abnormal P53 expression includes
overexpression, complete absence and cytoplasmic staining patterns.
The overexpression pattern is associated with nonsynonymous
missense mutations in the P53 gene, leading to excessive nuclear
accumulation of P53 protein. This results in the detection of
diffuse, strong nuclear positivity in >80% of tumor cells by IHC
(49,50). Tumors with a complete absence of P53
staining are often associated with frameshift or nonsense mutations
that result in a truncated P53 protein (51). The P53 cytoplasmic staining pattern
is characterized by tumor cells showing distinct moderate or strong
cytoplasmic staining, with variable or absent nuclear staining
(50). In the present study, P53
IHC revealed an abnormal overexpression pattern, indicative of a
mutational phenotype.
Camrelizumab is a high-affinity PD-1 antibody that
exerts antitumor activity with a favorable safety profile in
various malignancies (52,53). Camrelizumab, in combination with
docetaxel and cisplatin, may be also an option for
recurrent/metastatic oral SCC (R/M OSCC), as suggested by the
results of an open-label, single-arm, phase Ib trial (54). The trial indicated that the
combination of camrelizumab and TP regimen chemotherapy was well
tolerated and potentially improved the median OS, PFS and ORR in
the first-line treatment of patients with R/M OSCC.
In the current case, the patient had a history of
smoking, alcohol and betel quid chewing. Intraoral examination
revealed a cauliflower-like mass on the right mandibular gingiva.
Although, no pathological biopsy was performed, given the clinical
presentation and epidemiological features, a primary gingival
malignancy was suspected. In addition, histopathological
examination confirmed that the skin and stomach lesions were SCCs,
as evidenced by the positive immunohistochemical expression of P40,
P63 and panCK. The other lesions were considered secondary
malignancies. Based on these findings, camrelizumab plus AP regimen
chemotherapy was selected in accordance with systemic therapy
guidelines for CSCC and SCCHN (21,35).
Notably, discriminating histologically between
primary tumors and metastases in the present case was challenging,
if not impossible. However, it may be hypothesized that triple
primary skin, stomach and gingival tumors metastasized to the
brain, liver, lung, spleen, kidney, bone and subcutaneous tissue.
Anti-PD-1 antibodies combined with a nab-paclitaxel and carboplatin
regimen may be an option for metastatic SCC in patients with a
performance status of 0–1. However, in the present case, the
patient had an unsatisfactory outcome due to multiple organ
metastases and poor performance status.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and Technology
Planning Project of Medicine and Health in Zhuji City, Zhejiang
Province, China (grant no. 2020YW021).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MT, XC and LL carried out data analyses and drafted
the manuscript. BC, WH and XZ performed the follow-up. YC designed,
coordinated and supervised the study and critically reviewed and
discussed the manuscript. MT and YC confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki and was approved by the Institutional
Ethics Committee of Zhuji People's Hospital of Zhejiang Province
(approval no. 20241109; Zhuji, China).
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this paper.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vogt A, Schmid S, Heinimann K, Frick H,
Herrmann C, Cerny T and Omlin A: Multiple primary tumours:
Challenges and approaches, a review. ESMO Open. 2:e0001722017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moertel CG, Dockerty MB and Baggenstoss
AH: Multiple primary malignant neoplasms. I. Introduction and
presentation of data. Cancer. 14:221–230. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Müller M, Li J, Giger R and Elicin O: Head
and neck cancer with synchronous nodules of the lung as a
diagnostic and therapeutic challenge-a systematic review. Oral
Oncol. 145:1065292023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dür C, Salmina C, Borner U, Giger R and
Nisa L: Relevance of intraparotid metastases in head and neck skin
squamous cell carcinoma. Laryngoscope. 131:788–793. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Altundag K, Yalcin S, Ozkaya O and Guler
N: Synchronous squamous cell carcinoma of the stomach, the lung and
the skin. Onkologie. 27:291–293. 2004.PubMed/NCBI
|
|
6
|
Filippakis GM, Lagoudianakis EE,
Genetzakis M, Antonakis P, Papadima A, Boussiotou A, Katergiannakis
V and Manouras A: Squamous cell carcinoma arising in a mature
cystic teratoma of the ovary with synchronous invasive lobular
breast cancer: Case report. Eur J Gynaecol Oncol. 27:537–540.
2006.PubMed/NCBI
|
|
7
|
Studziński Z and Branicka D: The analysis
of coexistence of endometrial cancer with other malignant and
benign neoplasms with endometriosis. Ginekol Pol. 69:273–278.
1998.(In Polish). PubMed/NCBI
|
|
8
|
Corchado-Cobos R, García-Sancha N,
González-Sarmiento R, Pérez-Losada J and Cañueto J: Cutaneous
squamous cell carcinoma: From biology to therapy. Int J Mol Sci.
21:29562020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Que SKT, Zwald FO and Schmults CD:
Cutaneous squamous cell carcinoma: Incidence, risk factors,
diagnosis, and staging. J Am Acad Dermatol. 78:237–247. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang MS, Azin M and Demehri S: Cutaneous
squamous cell carcinoma: The frontier of cancer immunoprevention.
Annu Rev Pathol. 17:101–119. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alam M and Ratner D: Cutaneous
squamous-cell carcinoma. N Engl J Med. 344:975–983. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmitz L and Kanitakis J: Histological
classification of cutaneous squamous cell carcinomas with different
severity. J Eur Acad Dermatol Venereol. 33 (Suppl 8):S11–S15. 2019.
View Article : Google Scholar
|
|
13
|
Heath CR and Usatine RP: Squamous cell
carcinoma. Cutis. 112:97–98. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kallini JR, Hamed N and Khachemoune A:
Squamous cell carcinoma of the skin: Epidemiology, classification,
management, and novel trends. Int J Dermatol. 54:130–140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karagas MR, Nelson HH, Sehr P, Waterboer
T, Stukel TA, Andrew A, Green AC, Bavinck JN, Perry A, Spencer S,
et al: Human papillomavirus infection and incidence of squamous
cell and basal cell carcinomas of the skin. J Natl Cancer Inst.
98:389–395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Veness MJ: High-risk cutaneous squamous
cell carcinoma of the head and neck. J Biomed Biotechnol.
2007:805722007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmults CD, Karia PS, Carter JB, Han J
and Qureshi AA: Factors predictive of recurrence and death from
cutaneous squamous cell carcinoma: A 10-year, single-institution
cohort study. JAMA Dermatol. 149:541–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jambusaria-Pahlajani A, Miller CJ, Quon H,
Smith N, Klein RQ and Schmults CD: Surgical monotherapy versus
surgery plus adjuvant radiotherapy in high-risk cutaneous squamous
cell carcinoma: A systematic review of outcomes. Dermatol Surg.
35:574–585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weinberg AS, Ogle CA and Shim EK:
Metastatic cutaneous squamous cell carcinoma: An update. Dermatol
Surg. 33:885–899. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu GA and Lynn Su Chang A: Overall and
progression-free survival of stage 4 cutaneous squamous cell
carcinoma at a single large referral center. J Am Acad Dermatol.
73:165–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology (NCCN
Guidelines©). Squamous Cell Skin Cancer, version 2.2025.
https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdfApril
3–2025
|
|
22
|
Rischin D, Khushalani NI, Schmults CD,
Guminski AD, Chang ALS, Lewis KD, Lim AML, Hernandez-Aya LF, Hughes
BGM, Schadendorf D, et al: Phase II study of cemiplimab in patients
(pts) with advanced cutaneous squamous cell carcinoma (CSCC):
Longer follow-up. J Clin Oncol. 38 (Suppl 15):S100182020.
View Article : Google Scholar
|
|
23
|
Grob JJ, Gonzalez R, Basset-Seguin N,
Vornicova O, Schachter J, Joshi A, Meyer N, Grange F, Piulats JM,
Bauman JR, et al: Pembrolizumab monotherapy for recurrent or
metastatic cutaneous squamous cell carcinoma: A single-arm phase II
trial (KEYNOTE-629). J Clin Oncol. 38:2916–2925. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muto M, Hasebe T, Muro K, Boku N, Ohtsu A,
Fujii T, Ono M, Taijiri H, Mukai K and Yoshida S: Primary squamous
cell carcinoma of the stomach: A case report with a review of
Japanese and Western literature. Hepatogastroenterology.
46:3015–3018. 1999.PubMed/NCBI
|
|
26
|
Dursun M, Yaldiz M, Işikdoğan A, Yilmaz G,
Canoruç F, Ormeci N and Yilmaz S: Primary squamous cell carcinoma
of the stomach: A case report and review of the literature. Eur J
Gastroenterol Hepatol. 15:329–330. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mori M, Iwashita A and Enjoji M: Squamous
cell carcinoma of the stomach: Report of three cases. Am J
Gastroenterol. 81:339–342. 1986.PubMed/NCBI
|
|
28
|
Volpe CM, Hameer HR, Masetti P, Pell M,
Shaposhnikov YD and Doerr RJ: Squamous cell carcinoma of the
stomach. Am Surg. 61:1076–1078. 1995.PubMed/NCBI
|
|
29
|
He Y, Cui Y, Duan X, Liu C and Cai X:
Primary lung squamous cell carcinoma with gastric metastasis: A
case report. Thorac Cancer. 10:373–377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oriuchi M, Uno K, Fujishima F, Takeuchi A,
Lee S, Hatta W, Asano N, Koike T, Imatani A and Masamune A: A rare
case of gastric squamous-cell carcinoma metastasized from the
cervix. Clin J Gastroenterol. 13:1062–1065. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reise-Filteau M, Carter M, DeCoste R and
Kohansal A: Metastasis of cutaneous squamous cell carcinoma to the
stomach: A rare entity. BMJ Case Rep. 13:e2387312020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krolls SO and Hoffman S: Squamous cell
carcinoma of the oral soft tissues: A statistical analysis of
14,253 cases by age, sex, and race of patients. J Am Dent Assoc.
92:571–574. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chi AC, Day TA and Neville BW: Oral cavity
and oropharyngeal squamous cell carcinoma-an update. CA Cancer J
Clin. 65:401–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bornstein MM, Andreoni C, Meier T and
Leung YY: Squamous cell carcinoma of the gingiva mimicking
periodontal disease: A diagnostic challenge and therapeutic
dilemma. Int J Periodontics Restorative Dent. 38:253–259. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology (NCCN
Guidelines©). Head and Neck Cancers, version 2.2025.
https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdfApril
3–2025
|
|
36
|
Will TA, Agarwal N and Petruzzelli GJ:
Oral cavity metastasis of renal cell carcinoma: A case report. J
Med Case Rep. 2:3132008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aguirre A, Rinaggio J and Diaz-Ordaz E:
Lingual metastasis of renal cell carcinoma. J Oral Maxillofac Surg.
54:344–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Magaki S, Hojat SA, Wei B, So A and Yong
WH: An Introduction to the performance of immunohistochemistry.
Methods Mol Biol. 1897:289–298. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin F and Chen Z: Standardization of
diagnostic immunohistochemistry: Literature review and geisinger
experience. Arch Pathol Lab Med. 138:1564–1577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bellizzi AM: An algorithmic
immunohistochemical approach to define tumor type and assign site
of origin. Adv Anat Pathol. 27:114–163. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fitzgibbons PL, Bradley LA, Fatheree LA,
Alsabeh R, Fulton RS, Goldsmith JD, Haas TS, Karabakhtsian RG,
Loykasek PA, Marolt MJ, et al: Principles of analytic validation of
immunohistochemical assays: Guideline from the College of American
pathologists pathology and laboratory quality center. Arch Pathol
Lab Med. 138:1432–1443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Linder S, Olofsson MH, Herrmann R and
Ulukaya E: Utilization of cytokeratin-based biomarkers for
pharmacodynamic studies. Expert Rev Mol Diagn. 10:353–359. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moll R: Molecular diversity of
cytokeratins: Significance for cell and tumor differentiation. Acta
Histochem Suppl. 41:117–127. 1991.PubMed/NCBI
|
|
44
|
Nobre AR, Albergaria A and Schmitt F: p40:
A p63 isoform useful for lung cancer diagnosis-a review of the
physiological and pathological role of p63. Acta Cytol. 57:1–8.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bauml J, Seiwert TY, Pfister DG, Worden F,
Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A, et al:
Pembrolizumab for platinum- and cetuximab-refractory head and neck
cancer: Results from a single-arm, phase II study. J Clin Oncol.
35:1542–1549. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Agalliu I, Gapstur S, Chen Z, Wang T,
Anderson RL, Teras L, Kreimer AR, Hayes RB, Freedman ND and Burk
RD: Associations of oral α-, β-, and γ-human papillomavirus types
with risk of incident head and neck cancer. JAMA Oncol. 2:599–606.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen X, Gao L, Sturgis EM, Liang Z, Zhu Y,
Xia X, Zhu X, Chen X, Li G and Gao Z: HPV16 DNA and integration in
normal and malignant epithelium: Implications for the etiology of
laryngeal squamous cell carcinoma. Ann Oncol. 28:1105–1110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fullerton ZH, Butler SS, Mahal BA,
Muralidhar V, Schoenfeld JD, Tishler RB and Margalit DN: Short-term
mortality risks among patients with oropharynx cancer by human
papillomavirus status. Cancer. 126:1424–1433. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Köbel M and Kang EY: The many uses of p53
immunohistochemistry in gynecological pathology: Proceedings of the
ISGyP companion society session at the 2020 USCAP Annual9 meeting.
Int J Gynecol Pathol. 40:32–40. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jia H, Wu S, Ma G, Yang P, Li X, Zeng M,
Ji X and Xing X: p53 Immunohistochemistry staining patterns and
prognosis significance in 212 cases of non-endometrioid endometrial
cancer. Pathol Res Pract. 263:1555952024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vermij L, Léon-Castillo A, Singh N, Powell
ME, Edmondson RJ, Genestie C, Khaw P, Pyman J, McLachlin CM,
Ghatage P, et al: p53 immunohistochemistry in endometrial cancer:
Clinical and molecular correlates in the PORTEC-3 trial. Mod
Pathol. 35:1475–1483. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou C, Chen G, Huang Y, Zhou J, Lin L,
Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al: Camrelizumab plus
carboplatin and pemetrexed versus chemotherapy alone in
chemotherapy-naive patients with advanced non-squamous
non-small-cell lung cancer (CameL): A randomised, open-label,
multicentre, phase 3 trial. Lancet Respir Med. 9:305–314. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang J, Xu J, Chen Y, Zhuang W, Zhang Y,
Chen Z, Chen J, Zhang H, Niu Z, Fan Q, et al: Camrelizumab versus
investigator's choice of chemotherapy as second-line therapy for
advanced or metastatic oesophageal squamous cell carcinoma
(ESCORT): A multicentre, randomised, open-label, phase 3 study.
Lancet Oncol. 21:832–842. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ju H, Wei D, Wu Y, Liu Y, Ding Q, Rui M,
Fan Z, Yao Y, Hu J and Ren G: A pilot study of camrelizumab with
docetaxel and cisplatin for the first line treatment in
recurrent/metastatic oral squamous cell carcinoma. MedComm (2020).
4:e3122023. View Article : Google Scholar : PubMed/NCBI
|