Introduction
Preoperative chemoradiotherapy (CRT) has been used
extensively for locally advanced rectal cancer (LARC). As compared
with conventional postoperative CRT, many studies have reported
improved local tumor control, increased respectability, decreased
toxicities and a higher sphincter-preservation rate (1–4).
Preoperative CRT is also able to reduce tumor volume significantly.
Kim et al have reported the mean percent volume reduction
rate of primary tumors as 66.5% using magnetic resonance volumetry
performed before and after CRT (5).
Although the down-staging rate of primary tumors is approximately
50–60% for the use of preoperative CRT (1,2,4), a
significant proportion of patients did not achieve a sufficient
response to preoperative CRT, even with a long interval from CRT to
surgery. To identify patients that may achieve a favourable
response to treatment and who are at risk for treatment failure, as
well as to predict survival prognosis, a number of molecular
markers were recently investigated in patients with LARC. These
molecular markers would allow for the delivery of individualized
treatment regimens. Studies were performed on LARC patients treated
with preoperative CRT. These studies have reported that expression
patterns of biomarkers including Bax, epidermal growth factor
receptor, p21, Ki-67 and caveolin-1 were correlated with response
to CRT, local tumor control or survival (6–10).
We investigated the expression of molecular markers
including p53, pRb, hMLH1 and MDM2. p53 activates genes that induce
apoptosis in DNA-damaged cells (11). In view of survival prognosis, no
studies have reported on a significant correlation of p53
expression, especially for the use of preoperative CRT, while
studies with the use of preoperative radiotherapy alone have
reported that patients with a higher p53 expression had a shorter
survival (12,13). pRb strongly binds E2F and regulates
the cell cycle by inducing G1 arrest (14). Results are conflicting for the
expression of pRb and survival prognosis in patients with
colorectal cancer treated with surgery without the use of
preoperative CRT (15,16). Furthermore, no study has reported on
the clinical significance of pRb expression in patients treated
with preoperative CRT. hMLH1 is a component of the DNA mismatch
repair (MMR) system responsible for the detection and repair of DNA
lesions, e.g., mismatches, small insertions and deletions (17,18).
For LARC treated with preoperative CRT, conflicting findings in a
few studies have been reported for the expression of hMLH1 and
treatment response (19,20), but no correlation with survival has
been reported (20). MDM2 is a
murine double-minute 2 oncogene product that forms a stable complex
with wild-type and mutant p53 proteins (21). The MDM2 oncogene is amplified or
overexpressed in many human types of cancer, and the overexpression
of MDM2 is correlated with a poor prognosis (22,23).
Concerning MDM2 expression in LARC patients treated with
preoperative CRT, no studies reported a significant relationship
with tumor response or survival (24,25).
Few reports exist regarding the significance of the
biomarkers in patients treated with preoperative CRT for rectal
cancer. In this study, we evaluated the expression of these
biomarkers prior to preoperative CRT and attempted to correlate
their expression with treatment outcome.
Materials and methods
Patients and pretreatment evaluation
Between March 2000 and May 2007, >100 patients
with locally advanced rectal cancer received preoperative
chemoradiotherapy at our institution. More than half of them
received pretreatment pathological diagnosis at an outside referral
hospital. Forty-five patients with available biopsy tissue
specimens at our institution or nearby referral hospital were
enrolled in the study. Pretreatment work-up included a complete
history, physical examination, complete blood count, serum
chemistry, determination of the carcinoembryonic antigen (CEA)
level, chest radiography, an abdominal/pelvic CT scan and
colonoscopy with biopsy. Informed consent was obtained from
patients included in the study before treatment.
Treatment and follow-up
Radiation was delivered with 10-MV photons by the
use of a three-field technique (posterior and both lateral fields).
Radiotherapy was delivered 5 days/week, once/day, at a dose of 1.8
Gy/day. Pelvic radiotherapy consisted of a median dose of 43.2 Gy
in 24 fractions over 5 weeks, which was followed by a median boost
dose of 7.2 Gy, administered in 4 fractions to the primary tumor by
the use of three fields. Chemotherapy consisted of 2 cycles of
intravenous bolus 5-FU (500 mg/m2/day) and leucovorin
(20 mg/m2/day) for 5 days each, with or without
cisplatin on the sixth day. At approximately seven weeks after the
completion of CRT, the patients underwent definitive surgery.
Surgical management included the use of a sphincter preservation
approach whenever possible, by use of the total mesorectal excision
technique. Each patient was provided with follow-up including a
complete blood count, serum chemistry and determination of the CEA
level every 3 months. Chest radiography and an abdominal/pelvic CT
scan were performed every 6 months in the first 2 years. Follow-up
was then performed every 6 months thereafter.
Immunohistochemical staining
procedure
Biopsy specimens were obtained via a pretreatment
endoscopy with 1 sample in each case. Specimens were routinely
fixed in 10% buffered formalin and were embedded in paraffin. After
reviewing hematoxylin and eosin-stained slides, serial sections
from each block were used for immunohistochemistry. We carried out
immunohistochemical staining with the use of the Microprobe
Immuno/DNA stainer (Fisher Scientific, Pittsburgh, PA, USA).
Immunohistochemical staining with primary antibodies for p53
(BP53-12, diluted 1:50; Novocastra Laboratories, Newcastle, UK),
human pRb (clone Rb1, diluted 1:40; Dako, Glostrup, Denmark), hMLH1
(G168-15, diluted 1:100; Zymed Laboratories, South San Francisco,
CA, USA) and human MDM2 (clone IF2, diluted 1:100; Oncogene
Research Products, Cambridge, MA, USA) was performed by use of the
avidin-biotin-peroxidase complex method. Sections were
deparaffinized and placed in a microwave oven with a 2.1% citric
acid buffer solution (pH 6.0) for 10 min to retrieve the antigens.
Following microwave processing, the sections were incubated
overnight at 4°C with primary antibodies. The use of a
streptavidin-horseradish peroxidase detection system (Research
Genetics, Huntsville, AL, USA) was then applied to capillary
channels, followed by a 10-min incubation at 50°C. Reaction
products were visualized with diaminobenzidine as a chromogen and
slides were then counterstained with hematoxylin. Rectal cancer
tissue strongly expressing p53 was used as a positive control for
p53 and MDM2, while normal rectal epithelium adjacent to a tumor
was used as a positive control for pRb and hMLH1. For negative
controls, sections were treated similarly with omission of the
primary antibody.
Assessment of p53, pRb, hMLH1 and MDM2
expression
Each slide was examined under the same magnification
(x200, Vanox-S; Olympus, Tokyo, Japan) by one independent
pathologist who moved the microscopic field randomly across
specimens. Immunoreactivity was evaluated according to the
frequency of positive nuclear staining. Semi-quantitative, 4-tier
grading was based on the percentage of tumor cells that stained
positive in the entire tumor boundary: 0, negative; 1, minimal
(<10% staining); 2, moderately stained (10–50% staining) and 3,
markedly stained (≥50% staining). Results were classified into two
groups according to the number of positively stained cancer cells.
Criteria for positive expression were defined as >50% staining
of the tumor tissue for hMLH1 and p53 expression, >10% staining
for pRb expression and >0% for MDM2 expression.
Statistical analysis
The relationship between the clinicopathological
factors and protein expression was analyzed by use of the
Chi-square or Fisher’s exact test, as appropriate. Overall survival
(OS) was defined as the time from the starting date of preoperative
CRT until the date the patient succumbed to any cause or of the
last follow-up; disease-free survival (DFS) until the date of any
recurrence as a first event and loco-regional recurrence-free
survival (LRFS) until the date of the first detection of recurrence
in the pelvis. Survival outcomes were calculated by use of the
Kaplan-Meier method. The prognostic value of the protein expression
was evaluated by use of the log-rank test for univariate analysis
and the Cox proportional hazards model for multivariate analysis.
P<0.05 was considered as statistically significant. Statistical
analyses were conducted by use of the SPSS version 17.0 statistical
software (SPSS, Chicago, IL, USA).
Results
Immunohistochemical analysis of p53, pRb,
hMLH1 and MDM2 expression
A positive expression of p53, pRb, hMLH1 and MDM2
were found in 40, 46.7, 40 and 66.7% of the tissue specimens,
respectively. Fig. 1 shows
representative staining results of these proteins. Correlations
between protein expression and patient clinicopathological factors
are listed in Tables I and II. No significant correlation was
observed for expression and clinicopathological factors including
gender, age, preoperative clinical stage, postoperative
pathological stage or primary tumor response. However, patients
with a negative pRb expression achieved more complete tumor
response and were of a younger age, with a marginal
significance.
 | Table IClinicopathological characteristics of
p53 and pRb protein expression. |
Table I
Clinicopathological characteristics of
p53 and pRb protein expression.
| Characteristics | p53 | P-value | pRb | P-value |
|---|
|
| |
| |
|---|
| Positive | Negative | | Positive | Negative | |
|---|
| Gender | | | 0.72 | | | 1.00 |
| Male | 15 | 21 | | 17 | 19 | |
| Female | 3 | 6 | | 4 | 5 | |
| Age (years) | | | 0.39 | | | 0.08 |
| ≤55 | 9 | 10 | | 6 | 13 | |
| >55 | 9 | 17 | | 15 | 11 | |
| cStagea | | | 0.90 | | | 0.23 |
| I + II | 7 | 10 | | 6 | 11 | |
| IIIb + IIIc | 11 | 17 | | 15 | 13 | |
| pStageb | | | 0.38 | | | 0.12 |
| 0 | 1 | 6 | | 1 | 6 | |
| I + II | 11 | 13 | | 14 | 10 | |
| III + IV | 6 | 8 | | 6 | 8 | |
| Response | | | 0.14 | | | 0.06 |
| CRc | 2 | 8 | | 2 | 8 | |
| Non-CR | 16 | 19 | | 19 | 16 | |
 | Table IIClinicopathological characteristics
of hMLH1 and MDM2 protein expression. |
Table II
Clinicopathological characteristics
of hMLH1 and MDM2 protein expression.
|
Characteristics | hMLH1 | P-value | MDM2 | P-value |
|---|
|
| |
| |
|---|
| Positive | Negative | | Positive | Negative | |
|---|
| Gender | | | 0.72 | | | 0.70 |
| Male | 15 | 21 | | 23 | 13 | |
| Female | 3 | 6 | | 7 | 2 | |
| Age (years) | | | 0.81 | | | 0.14 |
| ≤55 | 8 | 11 | | 15 | 4 | |
| >55 | 10 | 16 | | 15 | 11 | |
| cStagea | | | 0.45 | | | 0.13 |
| I + II | 8 | 9 | | 9 | 8 | |
| IIIb + IIIc | 10 | 18 | | 21 | 7 | |
| pStageb | | | 0.41 | | | 0.26 |
| 0 | 2 | 5 | | 3 | 4 | |
| I + II | 12 | 12 | | 16 | 8 | |
| III + IV | 4 | 10 | | 11 | 3 | |
| Response | | | 0.36 | | | 0.44 |
| CRc | 3 | 7 | | 6 | 4 | |
| Non-CR | 15 | 20 | | 24 | 11 | |
Relationship between protein expression
and survival
After preoperative CRT, pathological complete
response (CR) of primary tumors occurred in 10 patients (22.2%).
Pathological staging was as follows: pT0N0 in 7 patients (15.6%),
pT0N1-2 in 3 patients (6.6%), pT1-2N0 in 7 patients (15.6%),
pT3-4N0 in 18 patients (40%) and pT2-3N1-2 in 10 patients (22.2%),
including 1 patient with pT2N1M1. The follow-up range of the
patients was 11–107 months (median 38.5). The 5-year OS, DFS and
LRFS rates for the patients inclued in the study were 71.3, 66.1
and 60.9%, respectively. The 5-year OS rates for pathological
stages 0 (n=7), I (n=7), II (n=13), III (n=13) and IV (n=1) were
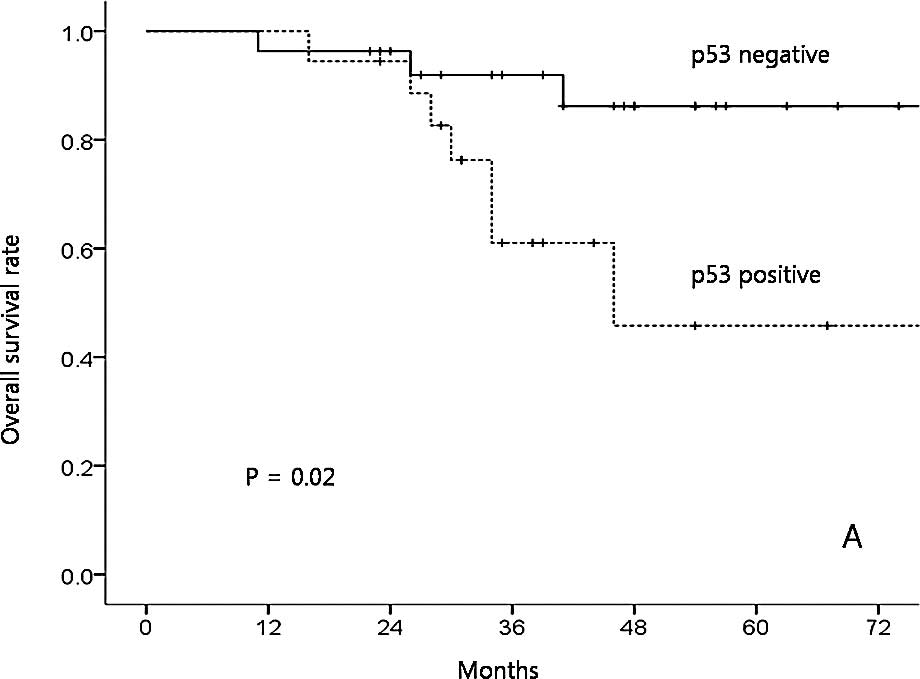
100, 42.9, 73.8, 53.9 and 0%, respectively (p=0.11). Only p53
expression was a significant prognostic factor affecting OS in the
univariate analysis (positive vs. negative expression, 45.8 vs.
86.2%; p=0.02; Fig. 2A). The
expression of hMLH1 (positive vs. negative, 71.2 vs. 71.8%;
p=0.87), MDM2 (positive vs. negative, 72.7 vs. 65%; p=0.31) and pRb
(positive vs. negative, 72.9 vs. 70%; p=0.92) did not affect OS
significantly. The 5-year DFS rates for pathological stages 0, I,
II, III and IV were 100, 57.1, 68.8, 53.8 and 0%, respectively
(p=0.000). p53 expression was a borderline significant prognostic
factor affecting DFS (positive vs. negative, 50 vs. 77%; p=0.06;
Fig. 2B). The expression of hMLH1
(positive vs. negative, 54.3 vs. 74.1%; p=0.24), MDM2 (positive vs.
negative, 69.7 vs. 59.3%; p=0.49) and pRb (positive vs. negative,
59.3 vs. 70.8%; p=0.48) did not affect DFS significantly. The
5-year LRFS rates for pathological stages 0, I, II, III and IV were
85.7, 47.6, 69.7, 41 and 100%, respectively (p=0.31). p53
expression was a borderline significant prognostic factor affecting
LRFS (positive vs. negative, 45.1 vs. 70%; p=0.07; Fig. 2C). The expression of hMLH1 (positive
vs. negative, 69.9 vs. 56.6%; p=0.79), MDM2 (positive vs. negative,
58.2 vs. 58.7%; p=0.50) and pRb (positive vs. negative, 58.8 vs.
62%; p=0.85) did not affect LRFS significantly.
Relationship of the clinicopathological
parameters, protein expression and survival by multivariate
analysis
Multivariate analysis was performed for OS with the
use of 12 covariates. The covariates included age (≤55 vs. >55
years), gender, clinical stage (I + II vs. III), tumor location
from the anal verge (<5 vs. ≥5 cm), interval between
radiotherapy and surgery (≤6 vs. >6 weeks), sphincter-saving
surgery or not, primary tumor response (CR or non-CR), pathological
stage (0, I, II, III and IV) and expression of the four proteins.
Age, MDM2 and p53 expression were significant factors affecting OS
(Table III). No significant
factor was determined for DFS or LRFS by multivariate analysis.
 | Table IIICox proportional hazards model for
overall survival. |
Table III
Cox proportional hazards model for
overall survival.
|
Characteristics | HR | 95% CI | P-value |
|---|
| Age (>55 vs. ≤55
years) | 58.109 | 3.864–873.841 | 0.003 |
| p53 (positive vs.
negative) | 15.347 | 2.209–106.618 | 0.006 |
| MDM2 (positive vs.
negative) | 0.073 | 0.010–0.517 | 0.009 |
| Sphincter-saving
surgery (no vs. yes) | 4.187 | 0.847–20.703 | 0.079 |
Discussion
To identify patients that may achieve favorable
response to treatment and are at risk for treatment failure, as
well as to predict survival prognosis, a number of molecular
markers were recently investigated in LARC patients. These
molecular markers were examined to determine whether they would
allow for the delivery of individualized treatment regimens.
Epidermal growth factor expression has been associated with a poor
response to preoperative chemoradiotherapy and worse DFS (7,8). Rau
et al reported that the expression of p21 appeared to
predict worse DFS, while that of Ki-67 predicted better DFS in
patients treated with neoadjuvant CRT (9). From the four proteins studied, MDM2
and p53 expression was found to be significantly related to OS. In
addition, a negative pRb expression was associated with a more CR.
Although p53 expression was found to have borderline significance
for DFS or LRFS, we consider that the significance of p53
expression for DFS or LRFS may have improved had the analysis been
conducted on a larger number of patients with a longer follow-up
period.
The p53 gene facilitates DNA repair or apoptosis in
response to DNA damage, whereas p53 gene mutations result in
functional abnormalities that lead to radioresistance (26,27).
Shimoji et al reported that p53 immunoreactivity in
esophageal carcinoma specimens, obtained prior to preoperative
radiotherapy, was significantly correlated with radioresistance
(27). In LARC patients treated
with preoperative CRT, some studies have reported a poorer response
in tumors with a higher p53 immunohistochemical staining of
pretreatment biopsy specimens (19,28),
while other studies did not determine any correlation (24,29).
In the present study, pathological complete remission was reported
in 22.2% of the patients. However, our study failed to show any
statistically significant correlation between baseline p53
expression and the pathological response to CRT, although a trend
for a higher pathological CR rate was observed in tumors negative
for p53 expression (Table I).
Concerning survival prognosis, studies of preoperative radiotherapy
alone have reported that patients with a higher p53 expression
showed shorter survival (12,13).
However, other studies have reported no relationship in LARC
patients treated with preoperative CRT (20,30).
Our results showed that p53 was an independent prognostic factor
affecting OS and a marginally significant factor for DFS or
LFRS.
pRb strongly binds E2F and regulates the cell cycle
by inducing G1 arrest (14). pRb is
able to act as a survival factor in colonic epithelial cells by
suppressing apoptosis. The overexpression of pRb in colorectal
tumor cells can cause a loss of sensitivity to apoptotic signaling,
resulting in aberrant cell survival and resistance to therapy
(31). Our study showed that a
trend for a higher pathological CR rate was observed in tumors with
a negative pRb expression (Table
I). Although the difference was not statistically significant,
our results are in accordance with the observation that the
overexpression of pRb may cause a loss of sensitivity to apoptotic
signaling. It remains debatable whether pRb expression is of
clinical significance in various types of cancer (32,33).
At resectable stages, most colorectal cancer types demonstrate an
aberrant expression of pRb and/or p16. One study with surgery alone
has reported that the aberrant expression of pRb and p16, alone and
in combination, is associated with poor prognosis in patients with
colorectal cancer (15), while
other studies have reported no correlation with survival prognosis
(16,34). Our study demonstrated that pRb
expression had no prognostic significance affecting survival, but
had a marginal significance regarding preoperative CRT response,
i.e., the negative pRb expression group showed a more CR of the
primary tumor.
hMLH1 is a component of the DNA MMR system, which
participates in DNA checkpoints and sends apoptotic signals
(35). It is controversial as to
whether radiation sensitivity is affected by the microsatellite
instability or MLH1 status. Davis et al reported that
MLH1-deficient human colon carcinoma cells show lower survival as
compared to MMR-corrected human colon carcinoma cells because of a
deficiency in the G2-M cell cycle checkpoint arrest after radiation
treatment (36). In LARC patients
treated with preoperative CRT, a few studies have shown conflicting
results regarding response to CRT. Charara et al reported
that patients with a lower expression of hMLH1 or higher
microsatellite instability showed better treatment response
(19), while Bertolini et al
reported that patients treated with preoperative CRT and a positive
hMLH1 expression had a higher CR rate (24.3 vs. 9.4%; p=0.055), but
no correlation with survival (20).
We previously reported that a low hMLH1 expression was found to be
associated with poor prognosis in patients treated with definitive
CRT for esophageal cancer (37).
However, in the present study of LARC patients, we did not
determine any significance of hMLH1 expression.
MDM2 is a murine double-minute 2 oncogene product
that forms a stable complex with wild-type and mutant p53 proteins
(21). MDM2 is responsible for
shuttling p53 from the nucleus to the cytoplasm, promoting p53
degradation by proteasomes (38).
MDM2 also binds with pRb, thus inhibiting the growth regulatory
function of pRb in a p53-independent manner (39). The MDM2 oncogene is amplified or
overexpressed in many human types of cancer and has a role in tumor
growth through p53-dependent and -independent mechanisms. Moreover,
MDM2 overexpression is correlated with a poor prognosis in many
cancer types (22,23). Kondo et al reported that
disruption of the p53/MDM2/p14ARF pathway may frequently
participate in colonic carcinogenesis. Moreover, the MDM2
expression status may be a factor in the prediction of potential
invasion and the presence of liver metastases for colorectal
carcinomas (22). Forslund et
al found MDM2 to be amplified in 9% of the 284 colorectal
cancers analyzed and MDM2 gene amplification was significantly
correlated with advanced tumor stage (23). These investigators suggested that
MDM2 is a promising target for cancer therapy in colorectal cancer
for the use of small molecule MDM2 antagonists that can inhibit
p53-MDM2 binding. Concerning the clinical significance of MDM2
expression in LARC, one study reported that MDM2 expression had no
relationship with tumor response to preoperative CRT (24), while another study reported no
correlation with survival in patients treated only with surgery
(25). In the present study, a
positive MDM2 expression was detected in 30 cases (66.7%), showing
a favorable prognosis with statistical significance in the
multivariate analysis for OS. However, we did not consider its
clinical significance, since MDM2 expression was not significant
for any survival in the univariate analysis. Nevertheless, a
further study is needed to elucidate whether the MDM2 oncoprotein
is able to counteract or offset the poor prognostic role of mutant
p53 protein, as well as what possible mechanism exists between
them.
In conclusion, a negative p53 expression was found
to be an independent prognostic marker for improved OS in this
study. Patients with a negative pRb expression had a more CR to
preoperative CRT. We suggest that the expression of p53 is a
potential marker of survival that may be useful for selecting
candidates from LARC patients for more tailored treatment.
Acknowledgements
This study was supported by the CRI-07074-1 grant
from Chonnam National University Hospital Research Institute of
Clinical Medicine. The authors thank Dr Young-Jin Kim, Professor in
the Department of Surgery, Dr Byung-Sik Nah and Dr Woong-Ki Chung,
Professors in the Department of Radiation Oncology, who provided
and cared for the study patients, as well as Dr Jae-Uk Jeong, a
resident in the Department of Radiation Oncology, who collected the
data.
References
|
1
|
Janjan NA, Khoo VS, Abbruzzese J, et al:
Tumor down-staging and sphincter preservation with preoperative
chemoradiation in locally advanced rectal cancer: the M.D. Anderson
Cancer Center experience. Int J Radiat Oncol Biol Phys.
44:1027–1038. 1999. View Article : Google Scholar
|
|
2
|
Kim JS, Cho MJ, Song KS and Yoon WH:
Preoperative chemoradiation using oral capecitabine in locally
advanced rectal cancer. Int J Radiat Oncol Biol Phys. 54:403–408.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sauer R, Becker H, Hohenberger W, et al:
Preoperative versus postoperative chemoradiotherapy for rectal
cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Navarro M, Dotor E, Rivera F, et al: A
Phase II study of preoperative radiotherapy and concomitant weekly
irinotecan in combination with protracted venous infusion
5-fluorouracil, for resectable locally advanced rectal cancer. Int
J Radiat Oncol Biol Phys. 66:201–205. 2006. View Article : Google Scholar
|
|
5
|
Kim YH, Kim DY, Kim TH, et al: Usefulness
of magnetic resonance volumetric evaluation in predicting response
to preoperative concurrent chemoradiotherapy in patients with
resectable rectal cancer. Int J Radiat Oncol Biol Phys. 62:761–768.
2005.PubMed/NCBI
|
|
6
|
Chang HJ, Jung KH, Kim DY, et al: Bax, a
predictive marker for therapeutic response to preoperative
chemoradiotherapy in patients with rectal carcinoma. Hum Pathol.
36:364–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giralt J, De las Heras M, Cerezo L, et al:
The expression of epidermal growth factor receptor results in a
worse prognosis for patients with rectal cancer treated with
preoperative radiotherapy: a multicenter, retrospective analysis.
Radiother Oncol. 74:101–108. 2005. View Article : Google Scholar
|
|
8
|
Kim JS, Kim JM, Li S, et al: Epidermal
growth factor receptor as a predictor of tumor downstaging in
locally advanced rectal cancer patients treated with preoperative
chemoradiotherapy. Int J Radiat Oncol Biol Phys. 66:195–200. 2006.
View Article : Google Scholar
|
|
9
|
Rau B, Sturm I, Lage H, et al: Dynamic
expression profile of p21WAF1/CIP1 and Ki-67 predicts survival in
rectal carcinoma treated with preoperative radiochemotherapy. J
Clin Oncol. 21:3391–3401. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodel F, Capalbo G, Rodel C and Weiss C:
Caveolin-1 as a prognostic marker for local control after
preoperative chemoradiation therapy in rectal cancer. Int J Radiat
Oncol Biol Phys. 73:846–852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adell G, Sun XF, Stal O, Klintenberg C,
Sjodahl R and Nordenskjold B: p53 status: an indicator for the
effect of preoperative radiotherapy of rectal cancer. Radiother
Oncol. 51:169–174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rebischung C, Gerard JP, Gayet J, Thomas
G, Hamelin R and Laurent-Puig P: Prognostic value of p53 mutations
in rectal carcinoma. Int J Cancer. 100:131–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui X, Shirai Y, Wakai T, Yokoyama N,
Hirano S and Hatakeyama K: Aberrant expression of pRb and
p16(INK4), alone or in combination, indicates poor outcome after
resection in patients with colorectal carcinoma. Hum Pathol.
35:1189–1195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ioachim E: Expression patterns of cyclins
D1, E and cyclin-dependent kinase inhibitors p21waf1/cip1, p27kip1
in colorectal carcinoma: correlation with other cell cycle
regulators (pRb, p53 and Ki-67 and PCNA) and clinicopathological
features. Int J Clin Pract. 62:1736–1743. 2008. View Article : Google Scholar
|
|
17
|
Fink D, Aebi S and Howell SB: The role of
DNA mismatch repair in drug resistance. Clin Cancer Res. 4:1–6.
1998.
|
|
18
|
Fishel R: The selection for mismatch
repair defects in hereditary nonpolyposis colorectal cancer:
revising the mutator hypothesis. Cancer Res. 61:7369–7374.
2001.PubMed/NCBI
|
|
19
|
Charara M, Edmonston TB, Burkholder S, et
al: Microsatellite status and cell cycle associated markers in
rectal cancer patients undergoing a combined regimen of 5-FU and
CPT-11 chemotherapy and radiotherapy. Anticancer Res. 24:3161–3167.
2004.
|
|
20
|
Bertolini F, Bengala C, Losi L, et al:
Prognostic and predictive value of baseline and posttreatment
molecular marker expression in locally advanced rectal cancer
treated with neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol
Phys. 68:1455–1461. 2007. View Article : Google Scholar
|
|
21
|
Momand J, Zambetti GP, Olson DC, George D
and Levine AJ: The MDM2 oncogene product forms a complex with the
p53 protein and inhibits p53-mediated transactivation. Cell.
69:1237–1245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kondo I, Iida S, Takagi Y and Sugihara K:
MDM2 mRNA expression in the p53 pathway may predict the potential
of invasion and liver metastasis in colorectal cancer. Dis Colon
Rectum. 51:1395–1402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Forslund A, Zeng Z, Qin LX, et al: MDM2
gene amplification is correlated to tumor progression but not to
the presence of SNP309 or TP53 mutational status in primary
colorectal cancers. Mol Cancer Res. 6:205–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kudrimoti M, Lee EY, Kang Y, Ahmed M and
Mohiuddin M: Genetic markers predictive of response to induction
chemoradiotherapy for locally advanced rectal cancers. J Ky Med
Assoc. 105:18–22. 2007.PubMed/NCBI
|
|
25
|
Tzouvala M, Lazaris AC, Papatheodoridis
GV, et al: Potential role of apoptosis and apoptotic regulatory
proteins in colorectal neoplasia: correlations with
clinico-pathological parameters and survival. Dig Dis Sci.
53:451–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia F, Wang X, Wang YH, Tsang NM, Yandell
DW, Kelsey KT and Liber HL: Altered p53 status correlates with
differences in sensitivity to radiation-induced mutation and
apoptosis in two closely related human lymphoblast lines. Cancer
Res. 55:12–15. 1995.PubMed/NCBI
|
|
27
|
Shimoji H, Miyazato H, Nakachi A, et al:
Expression of p53, bcl-2 and bax as predictors of response to
radiotherapy in esophageal cancer. Dis Esophagus. 13:185–190. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin LC, Lee HH, Hwang WS, et al: p53 and
p27 as predictors of clinical outcome for rectal-cancer patients
receiving neoadjuvant therapy. Surg Oncol. 15:211–216. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Terzi C, Canda AE, Sagol O, et al:
Survivin, p53 and Ki-67 as predictors of histopathologic response
in locally advanced rectal cancer treated with preoperative
chemoradiotherapy. Int J Colorectal Dis. 23:37–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gosens MJ, Dresen RC, Rutten HJ, et al:
Preoperative radiochemotherapy is successful also in patients with
locally advanced rectal cancer who have intrinsically high
apoptotic tumours. Ann Oncol. 19:2026–2032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guy M, Moorghen M, Bond JA, Collard TJ,
Paraskeva C and Williams AC: Transcriptional down-regulation of the
retinoblastoma protein is associated with differentiation and
apoptosis in human colorectal epithelial cells. Br J Cancer.
84:520–528. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ikeguchi M, Ueda T, Fukuda K, Yamaguchi K,
Tsujitani S and Kaibara N: Expression of the murine double-minute
gene 2 oncoprotein in esophageal squamous cell carcinoma as a novel
marker for lack of response to chemoradiotreatment. Am J Clin
Oncol. 25:454–459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garcia del Muro X, Condom E, Vigues F, et
al: p53 and p21 expression levels predict organ preservation and
survival in invasive bladder carcinoma treated with a
combined-modality approach. Cancer. 100:1859–1867. 2004.PubMed/NCBI
|
|
34
|
McKay JA, Douglas JJ, Ross VG, et al:
Analysis of key cell-cycle checkpoint proteins in colorectal
tumours. J Pathol. 196:386–393. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gong JG, Costanzo A, Yang HQ, Melino G,
Kaelin WG Jr, Levrero M and Wang JY: The tyrosine kinase c-Abl
regulates p73 in apoptotic response to cisplatin-induced DNA
damage. Nature. 399:806–809. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Davis TW, Wilson-Van Patten C, Meyers M,
et al: Defective expression of the DNA mismatch repair protein,
MLH1, alters G2-M cell cycle checkpoint arrest following ionizing
radiation. Cancer Res. 58:767–778. 1998.PubMed/NCBI
|
|
37
|
Nam TK, Lee JH, Cho SH, et al: Low hMLH1
expression prior to definitive chemoradiotherapy predicts poor
prognosis in esophageal squamous cell carcinoma. Cancer Lett.
260:109–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Geyer RK, Yu ZK and Maki CG: The MDM2
RING-finger domain is required to promote p53 nuclear export. Nat
Cell Biol. 2:569–573. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiao ZX, Chen J, Levine AJ, Modjtahedi N,
Xing J, Sellers WR and Livingston DM: Interaction between the
retinoblastoma protein and the oncoprotein MDM2. Nature.
375:694–698. 1995. View
Article : Google Scholar : PubMed/NCBI
|