Introduction
The most frequent cutaneous neoplasms in aging
Caucasian populations are basal cell carcinoma (BCC), squamous cell
carcinoma (SCC) and actinic keratosis (AK), which is considered a
carcinoma in situ (CIS) from which an invasive SCC can
develop (1–4). Genesis of all these types of skin
tumours, originating from epidermal keratinocytes or pluripotent
basaloid cells, is initiated and driven by exposure to ultraviolet
light. Accordingly, they are predominantly observed on sun-exposed
skin including forehead, nose, upper lip and lids. Although only
few cases of metastasizing BCCs have been reported, clinically
significant morbidity can be caused by deep and extensive
destructive invasion of the surrounding tissue. In contrast,
approximately 5% of invasive SCCs of the skin can form metastases
by entering lymphatic or haematogenous vessels (5,6).
Therefore, surgical excision of these tumours is a curative
treatment only during their early stages (7,8).
Accurate diagnosis of these tumours and their subclassification
requires, in addition to macroscopic examination, careful
histopathological assessment of the excised specimens. However,
even those currently used methods are not perfectly reliable
(9) and accuracy can be improved by
identification and validation of additional differential molecular
characteristics.
Tetraspanins are potentially useful molecular
markers, since members of this protein family of transmembrane
proteins were often found to be altered during malignant conversion
and tumour progression, in accordance with their roles in a number
of fundamental cellular processes, including adhesion, migration
and intracellular signalling (10,11).
Different expression of a tetraspanin by histologically defined
subtypes has recently been shown for ovarian carcinomas (12).
Correlations between expression of CD9, the
best-studied tetraspanin, and clinical observations or relevant
characteristics of tumour tissues and their cells were reported for
many types of human cancer including melanoma (13). Other types of skin tumours have not
been investigated with the exception of 5 analyzed cases of BCC
(7).
In this initial study, we analysed CD9 expression of
80 epithelial neoplasms to reveal potential differences between
subtypes of carcinomas and between SCCs and their precursor AK
lesions.
Materials and methods
Patients and tumour samples
Tissue samples collected from 80 patients of the
Departments of Ophthalmology and Dermatology of the University
Hospital in Jena after surgical excision of non-melanoma skin
tumours. The tumours were removed from periocular locations or lids
(26; 32.5%), other parts of the head (36; 45%) or other locations
on the body (18; 22.5%). Histopathological assessment of serial
haematoxylin and eosin-stained sections, performed at the
Department of Dermatology by one examiner (M.Z.), provided the
basis for classification of the tumours in superficial, nodular or
sclerosing BCCs, invasive SCCs with the distinct subclass of deeply
invasive tumours and AK-type CIS lesions (Table I). Vertical tumour extension of
>4 mm defined deep invasion in the sub-classification of
invasive SCCs in this study. Mean age of all patients was 72 years.
Patients with BCCs were younger (mean age 69 years) than patients
harbouring SCCs (81 years) or AKs (78 years).
 | Table IExpression of CD9 by different classes
and subtypes of non-melanoma skin tumours. |
Table I
Expression of CD9 by different classes
and subtypes of non-melanoma skin tumours.
| | No.
(CD9-positive) | Staining
intensity |
|---|
| |
|
|
|---|
| Tumour type | No. | Membrane | Intracellular | Periphery | Core |
|---|
| All | 80 | 78 | 57 | | |
| BCCs | 56 | 54 | 41 | 1.91±0.75 | 1.51±0.76 |
| Superficial | 17 | 17 | 13 | 1.88±0.78 | 1.41±0.94 |
| Nodular | 21 | 19 | 11 | 1.71±0.78 | 1.19±0.51 |
| Sclerosing | 18 | 18 | 17 | 2.17±0.62 | 2.00±0.59 |
| SCCs | 14 | 14 | 14 | 3.64±0.50 | 3.43±0.65 |
| Invasive | 8 | 8 | 8 | 3.75±0.46 | 3.63±0.52 |
| Deeply invasive | 6 | 6 | 6 | 3.50±0.50 | 3.16±0.69 |
| AK | 10 | 10 | 2 | 1.63±0.52 | - |
Immunohistochemical staining
Immmunohistochemical staining of sections (4 μm) of
paraffin-embedded tissues was performed as previously described
using a system with signal amplification through a multivalent link
antibody (12). The CD9-specific
primary antibody from NovoCastra (Newcastle upon Tyne, UK) was
diluted 1:40 in Tris-buffered saline and allowed to bind overnight
at 4°C. Of all stained sections, parallel sections were processed
with a similar amount of an isotype-matched (mouse IgG1
from Southern Biotech, Birmingham, USA) control antibody to exclude
non-specific binding.
CD9-specifc staining was assessed after
counterstaining with hematoxylin by light microscopy and a score
was assigned according to a linear scale from 0 (no staining) to 4
for the highest observed intensities. For each slide, scores
indicating observed CD9-specific staining were recorded for several
microscopic fields from the centre of the tumour mass and from the
tumours’ peripheries. In addition, both intracellular and cell
surface staining of CD9 were analysed.
Data analysis
For the histopathologically defined classes and
subtypes of tumours, means of intensity scores and standard
deviations were calculated. To reveal potentially significant
(p<0.05) differences between pairs of groups, the two-sided
Mann-Whitney test was used, and for paired groups of variables from
the same tissue sample (staining at the tumour core vs. periphery)
the Wilcoxon test. Both were included in the SPSS Statistics
(version 16, SPSS, Chicago, IL, USA) software package.
Results
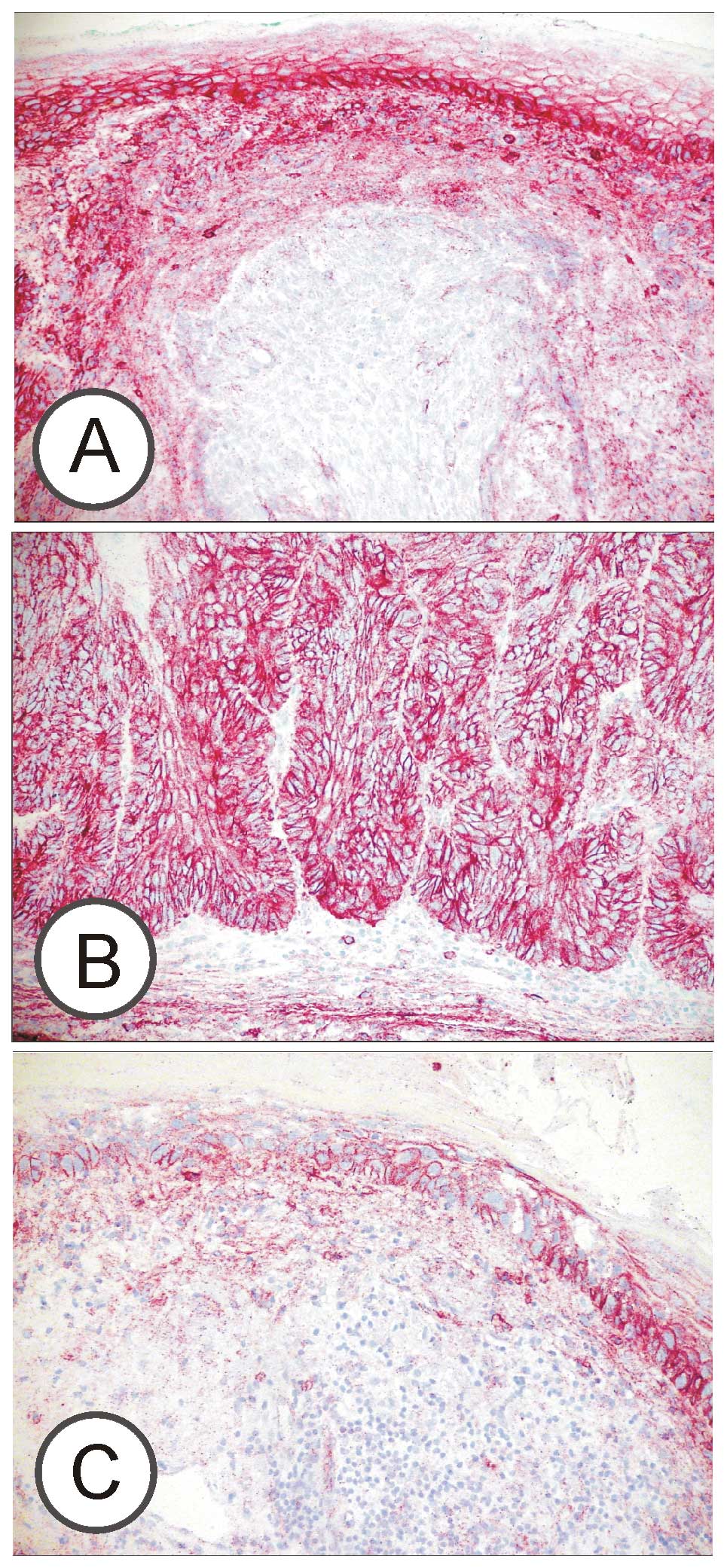
CD9 expression of the main types of non-melanoma
skin tumour cells was determined by immunohistochemical staining of
tissue sections. A moderate to strong CD9-specific staining of the
tumour cells’ plasma membranes was uniquely observed in all BCCs,
SCCs and AK-type carcinomas in situ (Fig. 1). An additional granular
intracellular staining was significantly different between the
investigated types of tumours. All invasive SCCs showed
intracellular CD9, whereas this subcellular location was rarely
(20%) seen in AKs. This difference was calculated with Fisher’s
exact test to be highly (p=0.001) significant. Intracellular
staining was also observed in sclerosing BCCs, but only in a
fraction of superficial and nodal BCCs (Table I).
Semi-quantitative assessment of CD9 present in the
plasma membranes of tumour cells of BCCs (mean staining intensity
1.91) and invasive SCCs (3.64) reflected the different CD9
expression of normal undifferentiated basaloid cells (~2.5) and
keratinocytes (~3.5) from which these tumours most likely
originate. Surprisingly, investigated AKs did not show intense
staining of the plasma membranes typical of normal keratinocytes or
invasive SCCs (p=0.011; Mann-Whitney test) but only moderate (mean
1.63) intensity (Figs. 1 and
2A). Within each group, membrane
stainings of subtypes of BCCs and invasive SCCs were not
significantly different.
Since CD9 is a tetraspanin involved in cell adhesion
and migration, CD9-specific immunoreactivity was assessed both in
the cores of BCCs and invasive SCCs, and at their advancing
borders. Stronger staining was clearly observed at the peripheries
of BCCs (p=0.0005, n=56; Wilcoxon test), for the fewer cases of
invasive SCCs this difference did not yet reach statistical
significance (p=0.11; Fig. 2B).
Discussion
We analyzed expression of the tetraspanin CD9 by
cells forming BCCs, SCCs or AKs which are considered carcinomas
in situ from which invasive SCCs can develop (3,4).
Despite the limited number of cases, significant differences were
observed. In SCC and BCC cells, strong CD9 expression of the normal
cells from which these originate, i.e. keratinocytes and basaloid
precursor cells, appeared to be conserved. However, the
particularly strong CD9 expression of normal keratinocytes was
found to be strongly decreased in AK lesions, which suggests its
down-regulation at the AK stage of carcinogenesis followed by
complete restoration during transition to invasive SCC. One could
speculate that lower amounts of CD9 promote non-invasive expansion
of AK cells through decreased attachment to the extracellular
matrix (ECM) because of the CD9 interaction with integrins in
keratinocytes (14–16) which might also affect proliferation.
At the stage of invasive SCC, up-regulation of CD9 could contribute
to processes that allow invasion through ECM barriers in the tissue
which was observed in advanced stages of cervical carcinomas
(17). However, since a low
expression of CD9 was frequently identified to be an indicator of
poor prognosis of patients and further progression in a number of
tumour types, including melanoma (13) and SCCs of other locations (18–21), a
subsequent study should focus on potential differences of
tetraspanin expression in metastatic cutaneous SCCs. The observed
moderate expression of CD9 in the plasma membranes of BCC cells
reflect amounts of this tetraspanin found in normal basal cells
and, therefore, appeared to be unaltered in the carcinoma cells.
Notably, significantly more CD9 was expressed at advancing borders
of the expanding tumours than in their inner regions. This is in
accordance with CD9’s role in promoting cell migration, which was
demonstrated for various malignant and non-malignant cell types,
e.g. microvascular endothelial cells (22). Since migration is a complex process
involving both local detachment and attachment of cells, increased
expression of a tetraspanin such as CD9 can promote or inhibit
movement of cells, depending strongly on the cell and tissue type
and environmental factors. In BCCs, increased amounts of CD9 at
sites of expansion suggest a stimulation of migration. In AK cells,
stimulation of migration might be achieved by down-regulation of
CD9, before tumour progression gives rise to invasive SCC cells in
which the pro-migratory effect of CD9 dominates again.
In this study we included the most frequent classes
and subtypes of non-melanoma skin tumours with a malignant
potential. Although AKs, invasive SCCs and BCCs differed in their
expression of CD9, there was no indication of subtype-specific
expression among BCC and SCC subtypes which would have been helpful
in their histopathological assessment. For BCCs and SCCs, our
results point to an important role of CD9 at the front of tumour
expansion. The differential expression of CD9 by AK-type carcinomas
in situ and invasive SCCs suggests that it is switched off
and on during the development of a SCC. This observation provides
the basis for further investigation of the roles of tetraspanins in
the pathogenesis of SCCs.
Acknowledgements
We thank D. Lamm and S. Feldrappe (Department of
Dermatology, University of Jena) and J. Windisch (Department of
Gynaecology and Obstetrics, University of Ulm) for their expert
technical assistance.
References
|
1
|
Diepgen TL and Mahler V: The epidemiology
of skin cancer. Br J Dermatol. 146:1–6. 2002. View Article : Google Scholar
|
|
2
|
Leiter U and Garbe C: Epidemiology of
melanoma and nonmelanoma skin cancer - the role of sunlight. Adv
Exp Med Biol. 624:89–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frost CA and Green AC: Epidemiology of
solar keratoses. Br J Dermatol. 131:455–464. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ortonne JP: From actinic keratosis to
squamous cell carcinoma. Br J Dermatol. 146:20–23. 2002. View Article : Google Scholar
|
|
5
|
Kwa RE, Campana K and Moy RL: Biology of
cutaneous squamous cell carcinoma. J Am Acad Dermatol. 26:1–26.
1992. View Article : Google Scholar
|
|
6
|
Chin CW, Foss AJ, Stevens A and Lowe J:
Differences in the vascular patterns of basal and squamous cell
skin carcinomas explain their differences in clinical behaviour. J
Pathol. 200:308–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anthony ML: Surgical treatment of
nonmelanoma skin cancer. AORN J. 71:552–558. 5602000.PubMed/NCBI
|
|
8
|
Neville JA, Welch E and Leffell DJ:
Management of nonmelanoma skin cancer in 2007. Nat Clin Pract
Oncol. 4:462–469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jagdeo J, Weinstock MA, Piepkorn M and
Bingham SF: Reliability of the histopathologic diagnosis of
keratinocyte carcinomas. J Am Acad Dermatol. 57:279–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hemler ME: Tetraspanin functions and
associated microdomains. Nat Rev Mol Cell Biol. 6:801–811. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hemler ME: Targeting of tetraspanin
proteins - potential benefits and strategies. Nat Rev Drug Discov.
7:747–758. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scholz CJ, Kurzeder C, Koretz K, Windisch
J, Kreienberg R, Sauer G and Deissler H: Tspan-1 is a tetraspanin
preferentially expressed by mucinous and endometrioid subtypes of
human ovarian carcinomas. Cancer Lett. 275:198–203. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Si Z and Hersey P: Expression of the
neuroglandular antigen and analogues in melanoma. CD9 expression
appears inversely related to metastatic potential of melanoma. Int
J Cancer. 54:37–43. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baudoux B, Castanares-Zapatero D,
Leclercq-Smekens M, Berna N and Poumay Y: The tetraspanin CD9
associates with the integrin alpha6beta4 in cultured human
epidermal keratinocytes and is involved in cell motility. Eur J
Cell Biol. 79:41–51. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones PH, Bishop LA and Watt FM:
Functional significance of CD9 association with beta 1 integrins in
human epidermal keratinocytes. Cell Adhes Commun. 4:297–305. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okochi H, Kato M, Nashiro K, Yoshie O,
Miyazono K and Furue M: Expression of tetra-spans transmembrane
family (CD9, CD37, CD53, CD63, CD81 and CD82) in normal and
neoplastic human keratinocytes: an association of CD9 with alpha 3
beta 1 integrin. Br J Dermatol. 137:856–863. 1997. View Article : Google Scholar
|
|
17
|
Sauer G, Windisch J, Kurzeder C, Heilmann
V, Kreienberg R and Deissler H: Progression of cervical carcinomas
is associated with down-regulation of CD9 but strong local
re-expression at sites of transendothelial invasion. Clin Cancer
Res. 9:6426–6431. 2003.PubMed/NCBI
|
|
18
|
Erovic BM, Pammer J, Hollemann D,
Woegerbauer M, Geleff S, Fischer MB, Burian M, Frommlet F and
Neuchrist C: Motility-related protein-1/CD9 expression in head and
neck squamous cell carcinoma. Head Neck. 25:848–857. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uchida S, Shimada Y, Watanabe G, Li ZG,
Hong T, Miyake M and Imamura M: Motility-related protein
(MRP-1/CD9) and KAI1/CD82 expression inversely correlate with lymph
node metastasis in oesophageal squamous cell carcinoma. Br J
Cancer. 79:1168–1173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kusukawa J, Ryu F, Kameyama T and Mekada
E: Reduced expression of CD9 in oral squamous cell carcinoma: CD9
expression inversely related to high prevalence of lymph node
metastasis. J Oral Pathol Med. 30:73–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mhawech P, Dulguerov P, Tschanz E, Verdan
C, Ares C and Allal AS: Motility-related protein-1 (MRP-1/CD9)
expression can predict disease-free survival in patients with
squamous cell carcinoma of the head and neck. Br J Cancer.
90:471–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deissler H, Kuhn EM, Lang GE and Deissler
H: Tetraspanin CD9 is involved in the migration of retinal
microvascular endothelial cells. Int J Mol Med. 20:643–652.
2007.PubMed/NCBI
|