Introduction
Treatment for androgen-independent prostate cancer
(AIPC) has been a challenge. During hormone therapy, prostate
cancer initially responds well to androgen ablation; however, this
response is gradually lost and the cancer becomes
hormone-independent. Some patients with AIPC maintain hormone
dependency but are resistant to androgen ablation (1). In this state, low-dose dexamethasone
(DXM) therapy is thought to be effective in addition to second- or
third-line anti-androgens, or anti-androgen withdrawal.
Glucocorticoids are used in the management of AIPC.
In addition to their anti-inflammatory effects, these agents
exhibit antitumor activity. However, their therapeutic role in AIPC
remains unclear (2,3). A few studies have demonstrated
negative effects for prostate cancer suppression with higher doses
of DXM (4,5), or the antagonistic activity of DXM on
prostate cancer cells with specific androgen receptor mutation
(6). However, in most studies, DXM
has exhibited an inhibitory effect on cell growth via intrinsic
glucocorticoid receptors in androgen-independent prostate cancer
cell lines such as PC-3 (3,7) or DU145 (8). In the present study, we
retrospectively evaluated the outcome of oral low-dose DXM therapy
for AIPC in the largest cohort of patients studied thus far.
Materials and methods
We retrospectively studied 99 consecutive patients
with prostate cancer who had been treated with hormone therapy
(surgical castration or luteinizing hormone-releasing hormone
agonist) and had shown biochemical failure [three consecutive
increases in serum prostate-specific antigen (PSA) levels from the
stabilized values]. These patients were treated either at Chiba
University Hospital or Saiseikai Utsunomiya Hospital, and DXM
therapy was started between January 1998 and April 2006. Patient
characteristics are shown in Table
I. Upon biochemical failure, patients were treated with oral
low-dose DXM (initially 1.5 mg/day, then reduced every 2 weeks to
0.5–1.0 mg/day). After 1995, in patients treated with surgical or
medical castration plus anti-androgen (AA) therapy, AA withdrawal
syndrome was assessed for at least 4–8 weeks after the cessation of
AA and prior to DXM being initiated. For these patients,
luteinizing hormone-releasing hormone agonist was not discontinued
(9). Docetaxel was not used in any
of the patients since it was only approved in 2008 in Japan. Serum
PSA levels were determined with the Tandem-R PSA Assay (Hybritech,
Inc., San Diego, CA). The PSA test was performed every 2 weeks in
the first two months, and then every month after the 3rd month of
DXM administration in AIPC patients. Serum levels of interleukin
(IL)-6 were measured in 18 patients before and after DXM
administration using the chemiluminescent enzyme immunoassay
(Cleia, SRL, Inc., Tokyo, Japan). The clinical effect of DXM was
evaluated based on improvement of pain. Pain relief was defined as
the ability of patients to discontinue the use of analgesics
following DXM therapy. Pain scales were not used for assessment.
Patients who showed a ≥50% decline in serum PSA levels were defined
as PSA responders to DXM therapy. The remaining patients were
defined as PSA non-responders. A decrease in PSA was confirmed with
a second PSA level which was also <50%. Treatment failure was
determined when patients showed three consecutive increases in PSA
serum level. No routine bone or CT scans were performed during
treatment.
 | Table ICharacteristics of 99 prostate cancer
patients undergoing low-dose dexamethasone therapy. |
Table I
Characteristics of 99 prostate cancer
patients undergoing low-dose dexamethasone therapy.
| Characteristics | Values |
|---|
| Median age at initial
diagnosis (years) | 70.0 (46–86) |
| Median age at DXM
administration (years) | 74±8.2 (46–89) |
| Median PSA before
hormone therapy (ng/ml) | 243.0
(8.2–29600) |
| Median PSA at DXM
administration (ng/ml) | 140.0
(0.82–5911.0) |
| Median time from
initial PSA failure to administration of DXM (months) | 15.0 (1.0–56.8) |
| Median time to
administration of DXM from the initiation of hormone therapy
(months) | 27.9 (5.3–206.3) |
| Median follow-up from
the initial hormone therapy (months) | 41.9
(11.4–207.3) |
| Clinical stage
(unknown in 1 case) (n) |
| C | 19 |
| D1 | 6 |
| D2 | 73 |
| Gleason score
(unknown in 5 cases) (n) |
| 5 | 7 |
| 6 | 5 |
| 7 | 26 |
| 8 | 19 |
| 9 | 28 |
| 10 | 9 |
| EOD grade (unknown in
1 case) (n) |
| 0 | 27 |
| 1 | 11 |
| 2 | 21 |
| 3 | 33 |
| 4 | 6 |
| Treatment prior to
low-dose DXM (n) |
| Castration only | 1 |
| Maximum androgen
blockade | 98 |
| Number of prior
hormonal agentsa |
| 1 | 17 |
| 2 | 41 |
| 3 | 37 |
| 4 | 2 |
| Prior
chemotherapy |
| UFT | 1 |
| CDDP | 1 |
| Estramustine | 15 |
| Estramustine +
etoposide | 2 |
Statistical analysis
Patient survival was analyzed by the Kaplan-Meier
method. Statistical significance was examined using Student’s
t-test, Chi-square test, one-way analysis of variance (one-way
ANOVA) and the log-rank test. Values are reported as the median or
mean ± SD. Multivariate analysis was performed using a Cox
regression model. P<0.05 was considered to be statistically
significant (Stat-View program).
Results
Clinical response to DXM therapy
PSA response
Of the 99 cases, 40 (40.4%) showed a ≥50% decrease
in serum PSA levels. The PSA decrease was <50% in 25 patients
(25.3%), and the other 34 cases (34.3%) showed an increase in PSA
levels. Eight (8.1%) cases showed a <4 ng/ml decrease in PSA
levels. The PSA response was significantly associated with the time
from initial hormone therapy to the start of DXM therapy, age at
diagnosis of prostate cancer and the administration of DXM therapy
and PSA velocity during the 3 months prior to DXM therapy (Table IIA) (10).
 | Table IIParameters for prostate-specific
antigen response and survival in the androgen-independent prostate
cancer patients undergoing low-dose dexamethasone therapy. |
Table II
Parameters for prostate-specific
antigen response and survival in the androgen-independent prostate
cancer patients undergoing low-dose dexamethasone therapy.
| A, Clinical
parameters for PSA response |
|---|
|
|---|
| | PSA response | |
|---|
| |
| |
|---|
| Clinical
parameters | | PR+CR | NC+PD | P-value |
|---|
| Clinical stage
(n=99) |
| C | | 9 | 10 | n.s. |
| D | | 31 | 49 | |
| Gleason score
(n=94) |
| 2–7 | | 17 | 20 | n.s. |
| 8–10 | | 19 | 37 | |
| Interval to DXM
(years) (n=99) |
| ≥3 | | 22 | 20 | 0.0370 |
| <3 | | 18 | 39 | |
| Anti-androgen
withdrawal syndrome (n=92) |
| + | | 7 | 14 | n.s. |
| − | | 30 | 41 | |
| Age at diagnosis
(n=99) |
| Mean ± SD | | 67.8±7.7 | 71.3±6.9 | 0.0196 |
| Range | | 46–85 | 53–86 | |
| Median | | 68 | 72 | |
| PSA at diagnosis
(ng/ml) (n=99) |
| Mean ± SD | | 1392.3±436.6 | 1288.8±543.6 | n.s. |
| Range | | 27.6–12490.0 | 8.2–29600.0 | |
| Median | | 400.0 | 211.0 | |
| Age at
administration of DXM (n=99) |
| Mean ± SD | | 71.2±8.2 | 74.6±7.5 | 0.0349 |
| Range | | 47–88 | 54–89 | |
| Median | | 72 | 75 | |
| PSA at
administration of DXM (n=99) |
| Mean ± SD | | 271.7±452.4 | 419.6±849.9 | n.s. |
| Range | | 10.7–2110.0 | 0.8–5911.0 | |
| Median | | 106.0 | 140.0 | |
| PSA velocity
(ng/ml/month) in 3 months before start of DXM (n=66) |
| Mean ± SD | | 35.8±76.4 | 117.3±262.5 | 0.0272 |
| Range | | 1.8–382.3 | −2.0–1496.6 | |
| Median | | 12.0 | 23.0 | |
| PSA doubling time
(month) 3 months before start of DXM (n=66) |
| Mean ± SD | | 2.5±1.9 | 2.5±2.9 | n.s. |
| Range | | 0.6–8.7 | −0.8–14.1 | |
| Median | | 2.0 | 1.5 | |
| EOD (n=98) |
| 0–2 | | 25 | 34 | n.s. |
| 3–4 | | 15 | 24 | |
| Bone pain |
| Improved | | 20 | 23 | 0.0552 |
| Unimproved | | 1 | 7 | |
| Hemoglobin before
start of DXM (g/dl) (n=99) |
| Mean ± SD | | 11.9±1.71 | 11.7±1.7 | n.s. |
| Range | | 7.7–14.8 | 7.2–16.3 | |
| Median | | 12.3 | 12.0. | |
|
| B, Prognostic
parameters for survival |
|
| Prognostic
parameters | Improved in | 95% confidence
interval | Relative hazards
ratio | P-value |
|
| PSA response (≥50%,
<50% decrease) | ≥50% decrease |
0.16006–0.75542 | 0.34770 | 0.0076 |
| PSA velocity
(ng/ml/month) | Lower |
0.99784–0.99993 | 0.99889 | 0.0373 |
| Interval to
DXMa (mean 38.2 months) | >38.2
months |
0.19571–0.95283 | 0.43183 | 0.0375 |
| EOD | Lower |
0.47123–0.83882 | 0.62900 | 0.0016 |
Improvement of pain
Low-dose DXM therapy was associated with improvement
of cancer-related pain. Before administration of DXM therapy, 51
patients (51.5%) had bone pain due to bone metastases. Thirty-four
of these 51 used oral analgesics regularly for pain control. After
the initiation of DXM therapy, 23 of these 34 patients (67.6%)
showed pain relief. Eight out of 11 were able to discontinue opioid
analgesics. Pain relief was achieved irrespective of the PSA
response (Table III).
 | Table IIIPain relief after DXM therapy. |
Table III
Pain relief after DXM therapy.
| Analgesics | PSA response | n | Discontinuation of
analgesics | Pain relief
(%) |
|---|
| Non-steroidal
anti-inflammatory drugs | ≥50% decrease | 12 | 9 | 75 |
| <50%
decrease | 11 | 6 | 55 |
| Total | 23 | 15 | 65 |
| Opioid analgesics ±
non-steroidal anti-inflammatory drugs | ≥50% decrease | 4 | 3 | 75 |
| <50%
decrease | 7 | 5 | 71 |
| Total | 11 | 8 | 73 |
| Total | | 34 | 23 | 68 |
Improvement of hemoglobin levels
Hemoglobin levels were compared before and after the
administration of DXM, and levels increased significantly in PSA
responders (median, average ± SD) (0.9, 0.8±1.0 g/dl; P<0.0001),
whereas no change was observed in the PSA non-responders (0.2,
−0.16±1.57 g/dl; not significant).
Survival
The median progression-free survival was 3 months
for the entire cohort, as well as for patients with a PSA decline
of <50%. In addition, the median progression-free survival was 7
months for patients with a PSA decline of ≥50% (but not <4.0
ng/ml), and 8.5 months for those with a PSA of <4.0 ng/ml
(P<0.0001).
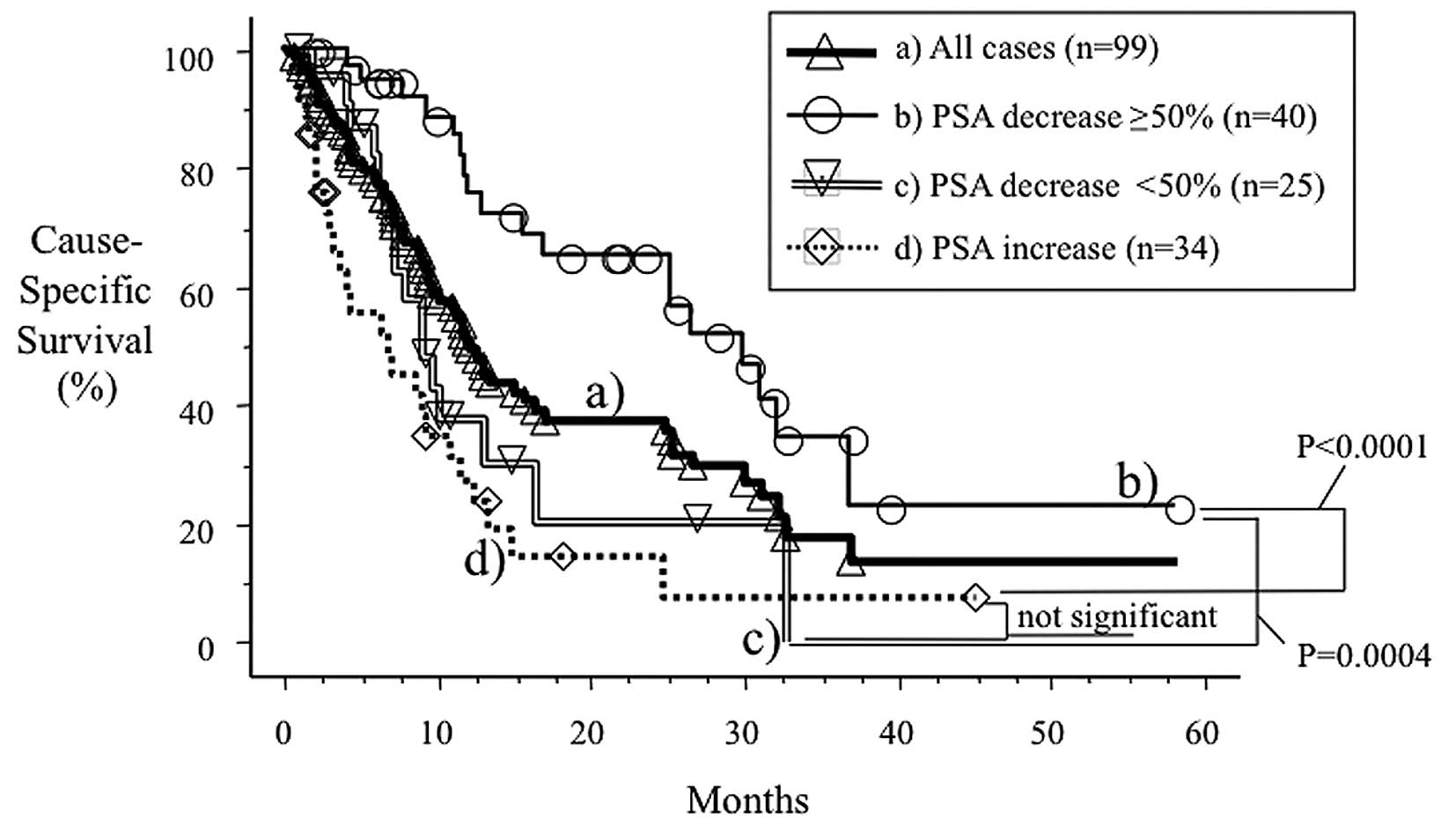
In Fig. 1, curve (a)
shows the cause-specific survival of the 99 cases who underwent
oral low-dose DXM therapy. Median survival was 12.0 months (range
0.7–58.2). Curves (b–d) show the cause-specific survival according
to the response to low-dose DXM therapy. When patients were
stratified by response, a significant difference in survival was
observed among patient groups (P=0.0004, PSA decrease ≥50% versus
PSA decrease <50%; P<0.0001, PSA decrease ≥50% versus PSA
increase). The median cause-specific survival was 30.1, 9.0 and 6.9
months in patients with a PSA decrease of ≥50%, <50% and a PSA
increase, respectively. Differences in survival between the latter
two groups were not significant. When the latter two groups (PSA
non-responders) were combined, the median cause-specific survival
was 8.8 months, which was poorer than that of the PSA responders
(P<0.001).
Table IIB shows the
multivariate analysis of cause-specific survival in patients who
underwent DXM therapy. Clinical parameters shown in Table IIA were assessed in the prognostic
model. The PSA response to DXM, the PSA velocity 3 months before
the administration of DXM, the interval from the initiation of
hormone therapy to the administration of DXM, and the extent of
disease (EOD) score of bone metastasis at initial diagnosis were
independent prognostic factors. Alkaline phosphatase was not
assessed in this study. In addition, neither the hemoglobin levels
nor the number of prior therapies were considered to be prognostic
variables (data not shown).
Changes in serum interleukin-6
levels
We measured the serum levels of IL-6 before and
after DXM in 18 patients. The patients had elevated serum IL-6
levels (>4.0 pg/ml) (cut-off). As shown in Table IV, changes in serum IL-6 levels
were significantly associated with response to DXM in AIPC
patients.
 | Table IVRelationship between response to
dexamethasone therapy and change in serum interleukin-6 levels in
18 patients. |
Table IV
Relationship between response to
dexamethasone therapy and change in serum interleukin-6 levels in
18 patients.
| IL-6 normalized
(n) | IL-6 not normalized
(n) | Total (n) | P-value |
|---|
| DXM responder | 10 | 3 | 13 | 0.0065 |
| DXM
non-responder | 0 | 5 | 5 | |
Adverse events
A total of 68 of the 99 (68.7%) cases did not
experience any adverse effects due to DXM. Although a routine
assessment of osteoporosis, steroid myopathy or skin ecchymosis was
not performed, these adverse events did not warrant discontinuation
of the DXM treatment. The most frequent adverse events were moon
face (n=10, 11.1%), gastric ulcer (n=6, 6.1%), and fracture (n=5,
5.1%). We found 2 cases of body weight gain and hot flash.
Cataracts were present in 1 case, hypertension in 1, edema in 1 and
depression in 1. These cases were grades 1–2. Only one grade 3
gastric ulcer occurred; based on the National Cancer Institute -
Common Toxicity Criteria, version 2.0. Neither grade 4 events nor
treatment-related deaths occurred in this cohort.
Discussion
In the present study, we retrospectively examined
the efficacy of low-dose DXM therapy in the management of AIPC in a
larger number of patients as compared with previous studies.
Important characteristics of low-dose DXM include i) a relatively
high response rate, ii) efficacy in the improvement of subjective
symptoms, iii) the ability of DXM to be administered orally (helps
maintain quality of life) and iv) lack of severe adverse effects.
The PSA response to DXM was a strong independent prognostic factor.
Younger patients with a longer history of hormone therapy and with
slow-growing cancer appear to be favourable candidates for DXM
therapy.
In previously reported outcomes of glucocorticoids
for AIPC (3,11), the PSA response rate (≥50% decrease
in PSA) ranged from 44 to 68%, and the median time to progression
was approximately 9 months. Our results were comparable to these
findings. The response rate itself was not significantly lower than
that noted with other cytotoxic agents used for AIPC. Safety was
achieved, although 5 patients (5.1%) experienced a fracture, which,
in addition to DXM, may have been due to a relatively longer period
of androgen deprivation therapy known to cause osteoporosis.
Routine assessment of osteoporosis and its treatment using
bisphosphonate such as zoledronic acid are recommended, although
neither were performed in this study (12). Prophylactic anti-ulcerative agents
should be prescribed simultaneously with DXM to prevent gastric
ulcers as this adverse effect occurred relatively frequently in
this study (6/99 patients, 6.1%), albeit in only one grade 3
case.
Even though docetaxel has become one of the most
frequently used options for the management of AIPC in Western
countries, our findings indicate that low-dose DXM is an important
treatment option for urologists. This treatment is suitable
particularly for patients who are not favourable candidates for
cytotoxic agents.
Suppression of adrenal androgen secretion by DXM is
believed to have a significant anti-tumor effect against prostate
cancer. Glucocorticoids can inhibit prostate cancer cell growth by
modulating cellular growth factors (11). Our previous report (13) demonstrated that serum levels of
adrenal androgens such as dehydroepiandrosterone,
dehydroepiandrosterone sulfate or androstendione were suppressed by
DXM therapy irrespective of the response to this treatment.
Akakura et al (13) suggested that the significant
suppression of IL-6 is another possible mechanism of dexamethasone
action. IL-6 is known to be suppressed by glucocorticoids (6) and to stimulate the growth of prostate
cancer cell lines through glucocorticoid receptors in an
androgen-independent manner (3,14–17).
In addition, IL-6 has been shown to activate the androgen receptor
through a STAT3-dependent pathway (17–19).
In this study, changes in serum IL-6 levels were significantly
associated with the response to DXM in AIPC patients. Therefore,
suppression of IL-6 has been accepted as a significant mechanism of
DXM activity in AIPC.
The recent position of glucocorticoids in the
management of prostate cancer is mainly found in the control arms
or is for prophylactic use for adverse events in clinical trials
for AIPC, in which prednisone is most frequently administered
(20,21). On the other hand, results for DXM
monotherapy have been less documented. DXM is thought to be a more
potent agent than prednisone/prednisolone or hydrocortisone because
of its stronger glucocorticoid activity and lower mineralocorticoid
activity (3). In clinical practice,
the PSA response rate for AIPC was 14–20% for hydrocortisone (40
mg/day), 27–38% for prednisone/prednisolone (7.5–10 mg/day) and
40–62% for dexamethasone (0.5–1.5 mg/day) (Table V) (3,11,13).
Relatively higher doses of dexamethasone are currently used in an
intermittent manner to reduce the hypersensitivity reactions and
fluid retention associated with docetaxel (20,21).
It was reported that DXM does not significantly contribute to the
response rate of docetaxel and estramustine in AIPC (5), and that intermittent DXM results in a
less profound suppression of adrenal androgens. Glucocorticoid
receptors are generally down-regulated by glucocorticoids in a
dose-dependent manner (22).
Therefore, low-dose DXM is a reasonable choice of therapy for a
longer duration of response as well as for reducing adverse
events.
 | Table VComparison of efficacy of
glucocorticoids for androgen-independent prostate cancer. |
Table V
Comparison of efficacy of
glucocorticoids for androgen-independent prostate cancer.
| Authors, year
(reference) | n |
Glucocorticoids | Dose (mg) | Median time to
progression (months) | Response (%) | Response (%) |
|---|
| Kelly et al,
1995 (23) | 30 | Hydrocortisone | 40 | 4 | 20 | 17/111 (15) |
| Kantoff et
al, 1999 (24) | 81 | Hydrocortisone | 40 | 2 | 14 | |
| Tannock et
al, 1989 (25) | 37 | Prednisone | 7.5–10 | Not indicated | 38 | 28/81 (35) |
| Sartor et
al, 1998 (26) | 29 | Prednisone | 10 | 2 | 34 | |
| Fuse et al,
2006 (27) | 15 | Prednisolone | 10 | 3.5 (mean) | 27 | |
| Nishiyama et
al, 1998 (28) | 7 | Dexamethasone | 1.5→0.5 | 9 | 57 | 71/143 (50) |
| Nishimura et
al, 2000 (3) | 37 | Dexamethasone | 1.0→2.0 | 9 | 62 | |
| Present study,
2008 | 99 | Dexamethasone | 1.5→0.5–1.0 | 7 | 40 | |
In conclusion, low-dose DXM therapy in the treatment
of AIPC is well tolerated and safe, and may be one of the
acceptable options in the management of AIPC, particularly for
patients who are not favourable candidates for cytotoxic agents or
in countries where docetaxel is not available.
References
|
1
|
Scher HI, Steineck G and Kelly WK:
Hormone-refractory (D3) prostate cancer: refining the concept.
Urology. 46:142–148. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fakih M, Johnson CS and Trump DL:
Glucocorticoids and treatment of prostate cancer: a preclinical and
clinical review. Urology. 60:553–561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishimura K, Nonomura N, Yasunaga Y, et
al: Low doses of oral dexamethasone for hormone-refractory prostate
carcinoma. Cancer. 89:2570–2576. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang C, Mattern J, Haferkamp A, et al:
Corticosteroid-induced chemotherapy resistance in urological
cancers. Cancer Biol Ther. 5:59–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weitzman AL, Shelton G, Zuech N, et al:
Dexamethasone does not significantly contribute to the response
rate of docetaxel and estramustine in androgen-independent prostate
cancer. J Urol. 163:834–837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang CY, Walther PJ and McDonnell DP:
Glucocorticoids manifest androgenic activity in a cell line derived
from a metastatic prostate cancer. Cancer Res. 61:8712–8717.
2001.PubMed/NCBI
|
|
7
|
Reyes-Moreno C, Frenette G, Boulanger J,
Lavergne E, Govindan MV and Koutsilieris M: Mediation of
glucocorticoid receptor function by transforming growth factor beta
I expression in human PC-3 prostate cancer cells. Prostate.
26:260–269. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao QZ, Lu JJ, Liu ZD, Zhang H, Wang SM
and Xu H: Dexamethasone suppresses DU145 cell proliferation and
cell cycle through inhibition of the extracellular signal-regulated
kinase 1/2 pathway and cyclin D1 expression. Asian J Androl.
10:635–641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akakura K, Akimoto S, Ohki T and Shimazaki
J: Antiandrogen withdrawal syndrome in prostate cancer after
treatment with steroidal antiandrogen chlormadinone acetate.
Urology. 45:700–705. 1995. View Article : Google Scholar
|
|
10
|
Soloway MS, Hardeman SW, Hickey D, et al:
Stratification of patients with metastatic prostate cancer based on
extent of disease on initial bone scan. Cancer. 61:195–202. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lam JS, Leppert JT, Vemulapalli SN,
Shvarts O and Belldegrun AS: Secondary hormonal therapy for
advanced prostate cancer. J Urol. 175:27–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saad F, Gleason DM, Murray R, et al:
Long-term efficacy of zoledronic acid for the prevention of
skeletal complications in patients with metastatic
hormone-refractory prostate cancer. J Natl Cancer Inst. 96:879–882.
2004. View Article : Google Scholar
|
|
13
|
Akakura K, Suzuki H, Ueda T, et al:
Possible mechanism of dexamethasone therapy for prostate cancer:
suppression of circulating level of interleukin-6. Prostate.
56:106–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okamoto M, Lee C and Oyasu R:
Interleukin-6 as a paracrine and autocrine growth factor in human
prostatic carcinoma cells in vitro. Cancer Res. 57:141–146.
1997.PubMed/NCBI
|
|
15
|
Chung TD, Yu JJ, Spiotto MT, Bartkowski M
and Simons JW: Characterization of the role of IL-6 in the
progression of prostate cancer. Prostate. 38:199–207. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lou W, Ni Z, Dyer K, Tweardy DJ and Gao
AC: Interleukin-6 induces prostate cancer cell growth accompanied
by activation of stat3 signaling pathway. Prostate. 42:239–242.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hobisch A, Eder IE, Putz T, et al:
Interleukin-6 regulates prostate-specific protein expression in
prostate carcinoma cells by activation of the androgen receptor.
Cancer Res. 58:4640–4645. 1998.PubMed/NCBI
|
|
18
|
Chen T, Wang LH and Farrar WL: Interleukin
6 activates androgen receptor-mediated gene expression through a
signal transducer and activator of transcription 3-dependent
pathway in LNCaP prostate cancer cells. Cancer Res. 60:2132–2135.
2000.
|
|
19
|
Ueda T, Bruchovsky N and Sadar MD:
Activation of the androgen receptor N-terminal domain by
interleukin-6 via MAPK and STAT3 signal transduction pathways. J
Biol Chem. 277:7076–7085. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tannock IF, de Wit R, Berry WR, et al:
Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Petrylak DP, Tangen CM, Hussain MH, et al:
Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimojo M, Hiroi N, Yakushiji F, Ueshiba
H, Yamaguchi N and Miyachi Y: Differences in down-regulation of
glucocorticoid receptor mRNA by cortisol, prednisolone and
dexamethasone in HeLa cells. Endocr J. 42:629–636. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kelly WK, Curley T, Leibretz C, Dnistrian
A, Schwartz M and Scher HI: Prospective evaluation of
hydrocortisone and suramin in patients with androgen-independent
prostate cancer. J Clin Oncol. 13:2208–2213. 1995.PubMed/NCBI
|
|
24
|
Kantoff PW, Halabi S, Conaway M, et al:
Hydrocortisone with or without mitoxantrone in men with
hormone-refractory prostate cancer: results of the cancer and
leukemia group B 9182 study. J Clin Oncol. 17:2506–2513.
1999.PubMed/NCBI
|
|
25
|
Tannock I, Gospodarowicz M, Meakin W,
Panzarella T, Stewart L and Rider W: Treatment of metastatic
prostatic cancer with low-dose prednisone: evaluation of pain and
quality of life as pragmatic indices of response. J Clin Oncol.
7:590–597. 1989.PubMed/NCBI
|
|
26
|
Sartor O, Weinberger M, Moore A, Li A and
Figg WD: Effect of prednisone on prostate-specific antigen in
patients with hormone-refractory prostate cancer. Urology.
52:252–256. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fuse H, Nozaki T, Fujiuchi Y, Mizuno I,
Nagakawa O and Okumura A: Treatment with prednisolone of
hormone-refractory prostate cancer. Arch Androl. 52:35–38. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishiyama T and Terunuma M:
Hormone/antihormone withdrawal and dexamethasone for
hormone-refractory prostate cancer. Int J Urol. 5:44–47. 1998.
View Article : Google Scholar : PubMed/NCBI
|