Introduction
Considerable advances have been made in the areas of
prevention, diagnosis and therapy. However, postoperative
infections following major surgery for gastro-intestinal cancer are
the most frequent complications that are noted. Complications due
to postoperative infection result in an increased cost of
treatment, longer hospital stay and ultimately, premature mortality
(1,2).
Many researchers have reported that postoperative
surgical and medical complications may contribute to a high rate of
recurrence and unfavorable long-term survival in various
malignancies such as cervical, colorectal and esophageal cancer
(3–5). Increasing evidence has shown that
postoperative complications due to infection, especially those
caused by anastomotic leakage after colorectal surgery, were
significantly associated with a negative long-term outcome
(3,6–8). In
contrast, beneficial effects of postoperative emphysema and
intrapleural infection have been reported after pneumonectomy for
lung cancer. Thus, whether or not infection and/or febrile
complication after surgery is associated with the long-term
survival of patients after curative resection for various types of
cancer is still controversial (8–11).
This study focused on the impact of postoperative
infection on long-term survival following resection with curative
intent for colorectal cancer.
Materials and methods
Patients
Between January 2002 and December 2007, 1669
consecutive patients underwent colorectal surgery at the National
Defense Medical College Hospital (Tokorozawa, Saitama, Japan).
Among the 1669 patients, 1083 patients (632 men, 451 women; mean
age 64.5±11.0 years, range 18–96 years) who underwent colorectal
surgery with resection with curative intent were enrolled in this
study. The population of 1083 patients was then divided into 2
groups based on the occurrence (65 patients, 6%) or absence (1018
patients, 94%) of complications due to postoperative infection. If
no gross residual disease was evident at the time of the operation
and the margins of resection were tumor-free on histological
examination, the surgery was considered to be curative. Resected
specimens were examined histopathologically and were staged
according to the International Union Against Cancer (UICC) TNM
classification of malignant tumors (12). Adjuvant therapy by oral anti-cancer
agents such as 5-fluorouracil (UFT) was recommended for patients
with disease stage III or IV, or those with a high potential of
recurrence based on the pathological findings. Adjuvant therapy was
then performed in 31 (47.7%) patients with postoperative infectious
complications and 466 (45.8%) patients without infectious
complication. These patients were retrospectively evaluated for
their pre- and post-operative status, pathological findings and
surgical procedure, according to our computer database or medical
and nursing charts.
Definition of infectious
complications
Complications due to postoperative infection were
defined by a combination of clinical findings and the results of
both laboratory and other tests recorded in medical records.
Clinical evidence was derived from direct observation of the
infection site or from reviewing patient charts. Laboratory
evidence included culture results, antigen or antibody detection
tests, or analysis by microscopic visualization. Supportive data
were derived from other diagnostic studies, such as X-ray,
ultrasonography (US) and computed tomography (CT). In our study,
complications from postoperative infection included anastomotic
leakage (identified by radiography or clinical suspicion), urinary
tract infection (pyrexia with microbiological evidence), bacteremia
(pyrexia with microbiological evidence), intraperitoneal abscess
(pyrexia with fluid collection diagnosed by US or CT, or identified
radiographically), pneumonia (pyrexia with infiltrate on chest
X-ray), pseudomembrane colitis (identified by colonoscopy and
microbiological evidence) and central venous catheter-related
infections (pyrexia with microbiological evidence). This study
included a wound infection involving deep soft tissues, e.g.,
facial and muscle layers, but excluded infection involving only
skin and subcutaneous tissue of the skin because of its minimal
effect on the systemic immune response.
Follow-up
Survival time was measured from the date of
resection to the date the patient succumbed due to any cause.
Patients who survived were censored in our survival analysis. The
patients were observed at our hospital or the outpatient clinic at
3- to 4-month intervals during the first 2 years of the study, and
then every 6 or 12 months for 3 years. After 5 years, an annual
follow-up was conducted through telephone conversations with the
patients, their family or practitioner.
Definition of recurrence patterns
Recurrences were identified by CT, positron emission
tomography (PET), US or colonoscopy. They were classified as
locoregional, liver, lung, brain, bone, distant lymph node and
peritoneal recurrence. Recurrence at cervical, celiac or paraaortic
lymph nodes was classified as distant lymph node recurrence. When
simultaneous recurrences were detected, the site of massive and/or
life-threatening recurrence was regarded as a main pattern of
recurrence.
Statistical analysis
Statistical calculations were performed using
StatView version 5.0 (SAS Institute, Inc., Cary, NC, USA). Data are
expressed as mean ± SD. Statistical analyses were performed using
either the Mann-Whitney U or Chi-square tests with Fisher’s exact
test, as appropriate. Survival rates were obtained using
Kaplan-Meier, and the significance of the difference in the
survival rate was determined by a log-rank test. Univariate and
multivariate analyses were performed using the Cox proportional
hazards model. P<0.05 was considered statistically
significant.
Results
Frequencies of complications due to postoperative
infection are shown in Table I.
Anastomotic leakage was the most frequent infectious complication
after colorectal cancer, followed by wound infection. No difference
was noted in the age, gender and incidence of hypertension between
patients with and without postoperative infectious complications
(Table II). Patients with
infectious complications had a significantly higher frequency of
diabetes mellitus and urgent surgery. We also found no statistical
difference in the tumor location, incidence of laparoscopic
surgery, inflammatory bowel disease, stoma construction, curability
and tumor stage between the two groups. Fourteen (21.5%) and 159
(15.6%) patients, with and without infectious complications,
respectively, had a relapse of cancer. Twelve (18.5%) and 122
patients (12%), with and without infectious complications,
respectively, succumbed to recurrence of the primary tumor. One
(1.5%) and 40 patients, respectively, succumbed to other causes
such as secondary malignancy, cerebral and heart infarction,
arrhythmia and accidents. Although there is no difference in
survival between patients with and without infectious
complications, we found statistical difference in cancer-specific
survival between the two groups (Fig.
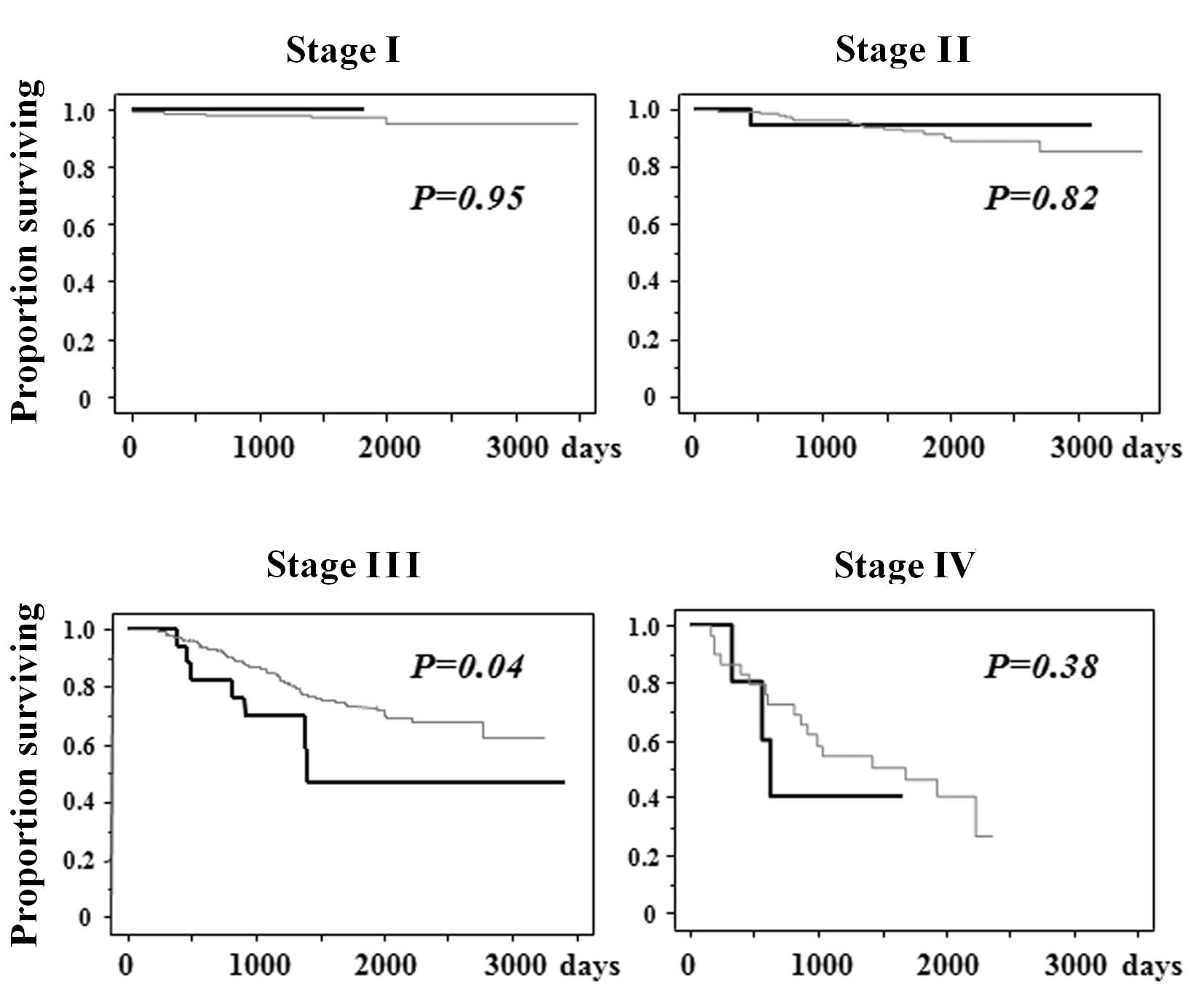
1). Specifically, in stage III of colorectal cancer, patients
with infectious complications had a poorer cancer-specific survival
than those without infectious complications (Fig. 2). This was not the case, however,
with patients in stages I, II and IV of colorectal cancer.
 | Table IFrequency of complications due to
postoperative infection. |
Table I
Frequency of complications due to
postoperative infection.
| Infectious
complications | No. | % |
|---|
| Anastomotic
leakage | 29 | 2.7 |
| Wound infection | 25 | 2.3 |
| Urinary tract
infection | 5 | 0.5 |
| Bacteremia | 3 | 0.3 |
| Intraperitoneal
abscess | 2 | 0.2 |
| Pneumonia | 2 | 0.2 |
| Pseudomembrane
colitis | 1 | 0.1 |
| Central venous
catheter-related infections | 1 | 0.1 |
| Total | 68 | |
 | Table IIDemographic and clinicopathological
data of patients with or without postoperative complications due to
infection. |
Table II
Demographic and clinicopathological
data of patients with or without postoperative complications due to
infection.
| Complications | No complications | P-value |
|---|
| Number | 65 | 1018 | |
| Age | 65.6±9.0 | 64.5±11.1 | 0.44 |
| Gender (M:F) | 44:21 | 588:430 | 0.11 |
| Co-morbidity |
| Hypertension |
| Yes | 10 (15.4) | 159 (15.6) | 0.99 |
| No | 50 (84.6) | 859 (84.4) | |
| Diabetes
mellitus |
| Yes | 13 (20.0) | 86 (8.5) | 0.01 |
| No | 52 (80.0) | 932 (91.5) | |
| Emergency
surgery |
| Yes | 2 (3.1) | 4 (0.4) | 0.04 |
| No | 63 (96.9) | 1014 (99.6) | |
| Location |
| Colon | 30 (46.2) | 532 (52.3) | 0.31 |
| Rectum | 35 (53.8) | 474 (57.7) | |
| Laparoscopic
surgery |
| Yes | 3 (4.6) | 30 (2.9) | 0.44 |
| No | 62 (95.4) | 988 (97.1) | |
| Inflammatory bowel
disease |
| Yes | 0 (0.0) | 2 (0.2) | 0.99 |
| No | 65 (100) | 1016 (99.8) | |
| Stoma
construction |
| Yes | 16 (24.6) | 159 (15.6) | 0.07 |
| No | 49 (75.4) | 859 (84.4) | |
| Curability |
| A | 6 (9.2) | 51 (5.0) | 0.15 |
| B | 59 (90.8) | 967 (95.0) | |
| Stage |
| 0 | 1 (1.5) | 33 (3.2) | 0.50 |
| I | 14 (21.5) | 222 (21.8) | |
| II | 23 (35.4) | 336 (33.0) | |
| III | 22 (33.8) | 388 (38.1) | |
| IV | 5 (7.7) | 39 (3.8) | |
| Outcome |
| Alive | 49 (75.4) | 826 (81.1) | 0.28 |
| Succumbed to tumor
relapse | 12 (18.5) | 122 (12.0) | |
| Succumbed to another
cause | 1 (1.5) | 40 (3.9) | |
| Unknown | 3 (4.6) | 30 (2.9) | |
In univariate analysis, age, tumor location,
curability and tumor stage, but not postoperative infectious
complications, were significantly associated with the overall
survival rate (Table III).
Multivariate analysis demonstrated that age, tumor location and
tumor stage were significantly associated with overall
survival.
 | Table IIIUnivariate and multivariate analysis
of factors that affect survival in patients with colorectal
cancer. |
Table III
Univariate and multivariate analysis
of factors that affect survival in patients with colorectal
cancer.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age, 1-year
increment | 1.03 | 1.02–1.05 | <0.0001 | 1.05 | 1.03–1.06 | <0.0001 |
| Gender (male) | 1.04 | 0.76–1.42 | 0.8000 | - | - | - |
| Urgent operation
(Yes) | 2.84 | 0.70–11.5 | 0.1400 | - | - | - |
| Laparoscopic
operation (Yes) | 0.40 | 0.10–1.62 | 0.2000 | - | - | - |
| Tumor location
(rectum) | 1.42 | 1.04–1.92 | 0.0300 | 1.51 | 1.04–2.00 | 0.0100 |
| Infectious
complications (Yes) | 1.58 | 0.88–2.85 | 0.1300 | - | - | - |
| Curability (A) | 0.21 | 0.14–0.32 | 0.0010 | 0.50 | 0.31–1.29 | 0.0600 |
| Stage (compared
with Stage I) |
| II | 2.92 | 1.41–6.06 | 0.0040 | 2.65 | 1.27–5.52 | 0.0100 |
| III | 8.08 | 4.09–16.0 | <0.0001 | 7.78 | 3.92–15.4 | <0.0001 |
| IV | 20.20 | 9.19–44.4 | <0.0001 | 12.5 | 4.41–35.4 | <0.0001 |
Of the 134 patients who succumbed due to a
recurrence of colorectal cancer, 52 (38.8%), 95 (70.9%) and 128
patients (95.5%) had a recurrence of cancer before 12, 24 and 60
months, respectively. In order to identify which factors are
responsible for the time interval until patients succumbed to the
disease due to cancer recurrence, univariate and multivariate
analyses were performed (Table
IV). Univariate analysis revealed that age, gender and
infectious complications were associated with a shorter time
interval of survival due to cancer recurrence, but this was not the
case for tumor location, curability and tumor stage. Multivariate
analysis revealed that infectious complications, as well as gender,
were related with a shorter time interval of survival due to cancer
recurrence. In patients with infectious complications, the mean
time interval until death due to cancer recurrence was 708.8±114.4
days, while that of patients without infectious complications was
1046.0±54.9 days. The accumulative hazard ratio for time interval
until death due to cancer recurrence was significantly higher in
patients with infectious complications, than that in patients
without infectious complications (Fig.
3). The univariate analysis performed with regard to the
influence of infectious complications for the time interval for
poor survival due to cancer recurrence showed that only an
anastomotic leakage affects the time interval in patients with
infectious complications (Table V).
Patients with infectious complications had a more frequent
locoregional relapse as compared to those without infectious
complications as a main pattern of recurrence (Table VI).
 | Table IVUnivariate and multivariate analysis
for time interval until patients succumbed due to recurrence from
colorectal cancer. |
Table IV
Univariate and multivariate analysis
for time interval until patients succumbed due to recurrence from
colorectal cancer.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age, 1-year
increment | 1.03 | 1.01–1.05 | 0.001 | 1.01 | 0.99–1.03 | 0.14 |
| Gender (male) | 0.57 | 0.39–0.84 | 0.005 | 0.60 | 0.41–0.88 | 0.01 |
| Emergency operation
(Yes) | 2.00 | 0.28–14.5 | 0.490 | - | - | - |
| Laparoscopic
operation (Yes) | 0.39 | 0.05–2.80 | 0.350 | - | - | - |
| Tumor location
(rectum) | 1.27 | 0.87–1.84 | 0.220 | - | - | - |
| Infectious
complications (Yes) | 2.10 | 1.09–4.07 | 0.030 | 2.16 | 1.11–4.24 | 0.03 |
| Curability (A) | 0.78 | 0.48–1.25 | 0.290 | - | - | - |
| Stage (compared
with stage I) |
| II | 1.01 | 0.34–2.94 | 0.990 | - | - | - |
| III | 1.14 | 0.42–3.13 | 0.800 | - | - | - |
| IV | 1.55 | 0.53–4.54 | 0.430 | - | - | - |
 | Table VThe influence of infectious
complications for the time interval until patients succumbed due to
cancer recurrence. |
Table V
The influence of infectious
complications for the time interval until patients succumbed due to
cancer recurrence.
| Infections | No. | Hazard ratio | 95% CI | P-value |
|---|
| Anastomotic
leakage | 29 | 3.48 | 1.49–8.14 | 0.004 |
| Wound
infection | 25 | 1.54 | 0.49–4.85 | 0.470 |
| Urinary tract
infection | 5 | 0.93 | 0.23–3.77 | 0.920 |
 | Table VIMain recurrence pattern of colorectal
cancer. |
Table VI
Main recurrence pattern of colorectal
cancer.
| Site of
reccurence | Complications | No
complications | P-value |
|---|
|
|
| |
|---|
| Total (%) | Colon | Rectum | Total (%) | Colon | Rectum | |
|---|
| Locoregional | 8 (57.1) | 2 | 6 | 46 (28.9) | 16 | 30 | |
| Liver | 5 (35.7) | 1 | 4 | 58 (36.5) | 28 | 30 | |
| Lung | 0 (0.0) | 0 | 0 | 42 (26.4) | 17 | 25 | 0.04 |
| Distant lymph
nodes | 0 (0.0) | 0 | 0 | 11 (6.9) | 2 | 9 | |
| Brain | 0 (0.0) | 0 | 0 | 1 (0.6) | 0 | 1 | |
| Bone | 1 (7.1) | 1 | 0 | 1 (0.6) | 0 1 | | |
| Total | 14 (100.0) | 4 | 10 | 159 (100.0) | 63 | 96 | |
Discussion
We demonstrated that complications due to
postoperative infection are important prognostic factors after
resection with curative intent for colorectal cancer, especially in
patients with stage III disease. In addition, infectious
complications were associated with time interval until patients
succumbed due to the recurrence of colorectal cancer after
resection for curative intent.
Although many previous reports demonstrated that
postoperative infectious complications contribute to a high rate of
recurrence and an unfavorable long-term survival in various
malignancies (5,7,8,13), the
precise mechanism(s) relating long-term survival and postoperative
infection remains unclear.
We also demonstrated that patients with infectious
complications had more frequent locoregional relapse as compared to
those without infectious complications. Substantial evidence exists
indicating the presence of viable cancer cells in the bowel lumen
of patients with colorectal cancer at the time of operation
(14–16), which can be detected on suture or
staple lines of anastomosis (17).
Anastomotic leakage, the most frequent infectious complication
found in this study, may lead to extraluminal implantation, which
has the effect of upstaging the disease and increasing the
incidence of locoregional relapse (13). Fujita et al demonstrated that
the incidence of local recurrence in patients with anastomotic
leakage was significantly higher than that in patients without
leakage in colorectal cancer (18),
which is consistent with our findings.
Another possible mechanism relating diminished
survival and postoperative infection is a deregulated host immune
response during infection that may contribute to tumorigenesis. It
is known that inflammation caused by bacterial infection could
develop systemic inflammatory response syndrome and lead to a shift
towards a Th2-type lymphocyte pattern (19,20),
which is especially enhanced after surgical trauma (21–23).
Th-2 cytokines, such as IL-10, were shown to down-regulate
tumor-specific immune responses by directly suppressing IFNγ and
IL-12 production. This caused a reduction in MHC expression on the
surface of tumor cells and inhibited tumor antigen presentation by
antigen-presenting cells (24–26).
Taken together, these findings suggest that development of a
postoperative Th-2 response during infectious complications
following major surgical trauma likely contributes to the
proliferation of occult or dormant cancer cells, resulting in
decreased survival (27).
In conclusion, our study indicates that
complications due to postoperative infection are a favorable
predictor of adverse clinical outcome in patients with colorectal
cancer. Further immunological study, however, is essential to
substantiate our current data and to provide an assessment of their
overall biological effects. Nonetheless, more effort is required to
prevent such postoperative infections to improve long-term as well
as short-term survival in colorectal cancer patients.
References
|
1
|
Collins TC, Daley J, Henderson WH, et al:
Risk factors for prolonged length of stay after major elective
surgery. Ann Surg. 230:251–259. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsutsui S, Moriguchi S, Morita M, et al:
Multivariate analysis of postoperative complications after
esophageal resection. Ann Thorac Surg. 53:1052–1056. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nowacki MP and Szymendera JJ: The
strongest prognostic factors in colorectal carcinoma.
Surgicopathologic stage of disease and postoperative fever. Dis
Colon Rectum. 26:263–268. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirai T, Yamashita Y, Mukaida H, et al:
Poor prognosis in esophageal cancer patients with postoperative
complications. Surg Today. 28:576–579. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lagarde SM, De Boer JD, Ten Kate FJ, et
al: Postoperative complications after esophagectomy for
adenocarcinoma of the esophagus are related to timing of death due
to recurrence. Ann Surg. 247:71–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fucini C, Bandettini L, D’Elia M, et al:
Are postoperative fever and/or septic complications prognostic
factors in colorectal cancer resected for cure? Dis Colon Rectum.
28:94–95. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akyol AM, McGregor JR, Galloway DJ, et al:
Anastomotic leaks in colorectal cancer surgery: a risk factor for
recurrence? Int J Colorectal Dis. 6:179–183. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nespoli A, Gianotti L, Totis M, et al:
Correlation between postoperative infections and long-term survival
after colorectal resection for cancer. Tumori. 90:485–490.
2004.PubMed/NCBI
|
|
9
|
Dionigi R, Dominioni L and Campani M:
Infections in cancer patients. Surg Clin North Am. 60:145–159.
1980.
|
|
10
|
Wigmore SJ, McMahon AJ, Sturgeon CM, et
al: Acute-phase protein response, survival and tumour recurrence in
patients with colorectal cancer. Br J Surg. 88:255–260. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Varty PP, Linehan IP and Boulos PB:
Intra-abdominal sepsis and survival after surgery for colorectal
cancer. Br J Surg. 81:915–918. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sobin LH and Fleming ID: TNM
Classification of Malignant Tumors. 5th edition (1997) Union
Internationale Contre le Cancer and the American Joint Committee on
Cancer. Cancer. 80:1803–1804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walker KG, Bell SW, Rickard MJ, et al:
Anastomotic leakage is predictive of diminished survival after
potentially curative resection for colorectal cancer. Ann Surg.
240:255–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Keynes WM: Implantation from the bowel
lumen in cancer of the large intestine. Ann Surg. 153:357–364.
1961. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fermor B, Umpleby HC, Lever JV, et al:
Proliferative and metastatic potential of exfoliated colorectal
cancer cells. J Natl Cancer Inst. 76:347–349. 1986.PubMed/NCBI
|
|
16
|
Skipper D, Cooper AJ, Marston JE, et al:
Exfoliated cells and in vitro growth in colorectal cancer. Br J
Surg. 74:1049–1052. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gertsch P, Baer HU, Kraft R, et al:
Malignant cells are collected on circular staplers. Dis Colon
Rectum. 35:238–241. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujita S, Teramoto T, Watanabe M, et al:
Anastomotic leakage after colorectal cancer surgery: a risk factor
for recurrence and poor prognosis. Jpn J Clin Oncol. 23:299–302.
1993.PubMed/NCBI
|
|
19
|
Ramer-Quinn DS, Baker RA and Sanders VM:
Activated T helper 1 and T helper 2 cells differentially express
the beta-2-adrenergic receptor: a mechanism for selective
modulation of T helper 1 cell cytokine production. J Immunol.
159:4857–4867. 1997.
|
|
20
|
Spolarics Z, Siddiqi M, Siegel JH, et al:
Depressed interleukin-12-producing activity by monocytes correlates
with adverse clinical course and a shift toward Th2-type lymphocyte
pattern in severely injured male trauma patients. Crit Care Med.
31:1722–1729. 2003. View Article : Google Scholar
|
|
21
|
Aosasa S, Ono S, Mochizuki H, et al:
Activation of monocytes and endothelial cells depends on the
severity of surgical stress. World J Surg. 24:10–16. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mokart D, Capo C, Blache JL, et al: Early
postoperative compensatory anti-inflammatory response syndrome is
associated with septic complications after major surgical trauma in
patients with cancer. Br J Surg. 89:1450–1456. 2002. View Article : Google Scholar
|
|
23
|
Tsujimoto H, Ono S, Majima T, et al:
Differential toll-like receptor expression after ex vivo
lipopolysaccharide exposure in patients with sepsis and following
surgical stress. Clin Immunol. 119:180–187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clerici M, Shearer GM and Clerici E:
Cytokine dysregulation in invasive cervical carcinoma and other
human neoplasias: time to consider the TH1/TH2 paradigm. J Natl
Cancer Inst. 90:261–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moore KW, O’Garra A, De Waal Malefyt R, et
al: Interleukin-10. Annu Rev Immunol. 11:165–190. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beissert S, Hosoi J, Grabbe S, et al:
IL-10 inhibits tumor antigen presentation by epidermal
antigen-presenting cells. J Immunol. 154:1280–1286. 1995.PubMed/NCBI
|
|
27
|
Mynster T, Christensen IJ, Moesgaard F, et
al: Effects of the combination of blood transfusion and
postoperative infectious complications on prognosis after surgery
for colorectal cancer. Danish RANX05 Colorectal Cancer Study Group.
Br J Surg. 87:1553–1562. 2000. View Article : Google Scholar
|