Introduction
Epidemiological and experimental studies have
demonstrated that heterocyclic amines generated in meat and fish
cooked at high temperature are highly mutagenic and rodent
carcinogenic (1–3). For example, case control studies have
provided evidence that high-temperature cooked meat is associated
with risk of colon (4,5), breast (6,7),
gastric (8) and lung (9,10)
cancer. As a heterocyclic amine,
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), is
associated with human lung cancer risk, whereas
2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQx)
and 2-amino-1-methyl-6-phenylimidazo-[4,5-b]pyridine (PhIP) are not
(11). MeIQx has been reported to
induce liver and lung tumors, lymphomas and leukemias in
CDF1 mice (12).
Although the incidence of MeIQx-induced liver tumors was high,
values for lung and hematopoetic system tumors were much lower, and
in the case of lung only female patients were affected. Therefore,
whether the lung is actually a target organ for MeIQx is somewhat
controversial.
Since epidemiological and experimental studies have
demonstrated that fat consumption is associated with cancer risk in
several organs (13–17) including the lung (10,14,18–20),
this study investigated whether this correlation was able to modify
risk in the MeIQx mouse model. A high-fat diet was previously shown
to enhance the 4-nitroquinoline 1-oxide (4NQO)-induction of lung
tumorigenesis in mice (21). Female
A/J mice which are highly susceptible to lung carcinogens were
selected to investigate the relationship between the lung
carcinogenic potential of MeIQx and a high-fat diet in a
medium-term test.
MeIQx is thought to be metabolically activated to
genotoxic intermediates by CYP1A2-mediated N-hydroxylation in the
liver (22,23). MeIQx then undergoes
O-esterification catalyzed by arylamine
N-acetyltransferase (NAT) (24). The rat and human CYP1A2 share a 75%
identity in their amino acid sequences (25) and levels of CYP1A2 mRNA are
increased approximately 1.5–2-fold by treatment with MeIQx in the
rat liver (26). However, CYP1A2
levels in the lung have not been assessed and were thus
investigated.
Materials and methods
Chemicals
MeIQx was purchased from the Nard Institute (Osaka,
Japan).
Animals
Female A/J mice (5 weeks of age), purchased from
Shizuoka Laboratory Animal Center (Shizuoka, Japan), were
maintained in the Kagawa University Animal Facility according to
the institutional animal care guidelines. The animals were housed
in polycarbonate cages with white wood chips for bedding and given
free access to drinking water. Starting at 7 weeks of age, groups 1
and 2 were fed a diet supplemented with MeIQx at a concentration of
600 ppm. For groups 1 and 3 the diets contained 20% corn oil. Group
4 was fed the basal diet, CE-2 (Clea Japan Inc., Tokyo, Japan),
without supplement. The mice were maintained on their respective
diets and under controlled conditions of humidity (60±10%),
lighting (12-h light/dark cycle) and temperature (24±2°C) until
termination at week 32. Surviving mice were then sacrificed under
ether anesthesia and their lungs were excised, weighted, inflated
with 10% neutral-buffered formalin and carefully inspected grossly.
Macroscopically detected lung nodules were counted under a
stereomicroscope and each lung lobe was examined
histopathologically.
Subjects for mRNA quantitation
For mRNA studies, groups of 10 mice were sacrificed
after 1 week of study for lung and liver RNA isolation and
quantitative analysis of CYP1A2.
RNA isolation
Total RNA was isolated from the 30 mg of whole lung
and liver tissues using RNAlater RNA Stabilization Reagent and an
RNeasy Mini Kit (both from Qiagen Corp., Hilden, Germany). The RNA
concentration was measured at an absorbance of 260 nm. First-strand
cDNA was synthesized from 400 ng of total RNA using TaqMan Reverse
Transcription Reagents (Applied Biosystems, Foster City, CA, USA),
according to the manufacturer’s instructions.
Quantitative real-time RT-PCR
Optimal primers and probes were purchased from the
Assays-on-demand system of Applied Biosystems (ABI). The TaqMan
rodent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control
reagent (ABI) was used for the PCR of GAPDH mRNA as an internal
control. Primer sequences and TaqMan probes for CYP1A2 and GAPDH
mRNA were closed because of purchasing from the Assays-on-demand
system of ABI.
Quantitative real-time RT-PCR was performed with the
ABI PRISM 7000 Sequence Detection System using specific primers and
a TaqMan probe for CYP1A2. PCR was carried out in 50 μl reaction
mixtures containing 25 μl of 2X TaqMan Universal PCR Master Mix, 50
ng of cDNA, 100 nM of each primer and 200 nM of TaqMan probe.
Cycling conditions were: 2 min at 50°C, 10 min at 95°C and then 40
cycles of 15 sec at 95°C, followed by 1 min at 60°C. PCR
amplification of GAPDH mRNA was carried out as above. TaqMan PCR
products were detected as an increase in fluorescence from cycle to
cycle. The amplification plots of the PCR reaction were used to
determine the threshold cycle (Ct). The Ct value represented the
PCR cycle at which an increase in reporter fluorescence (ΔRn) above
the line of the optimal value was first detected. The initial copy
number of the target mRNA was calculated from plots of the Ct
against the input target quantity.
The precise amount and quality of total RNA are
difficult to assess. Therefore, we quantified transcripts of the
GAPDH gene as an internal control according to a quantitative
RT-PCR assay. Normalization of the data was achieved by
quantitating the cycle number at an arbitrary fluorescence
intensity in the linear exponential phase by calculating the ratio
of the cycle number of each enzyme relative to that of GAPDH.
Statistical analysis
The data for final body and relative organ weights
were analyzed by Student’s t-test. The incidences of lung
proliferative lesions were analyzed by Fisher’s exact probability
test and data for multiplicity by Student’s t-test. CYP1A2 mRNA
levels were analyzed by the Mann-Whitney U test.
Results
The results for final body and relative organ
weights are shown in Table I. Final
body weights increased significantly in the high-fat group (group
3) compared to the control (group 4). However, no increase was
noted in group 1 which received both MeIQx and high fat. The
relative organ weights in the high fat-treated group (group 3)
decreased significantly, while the relative liver weights
significantly increased in the groups treated with MeIQx (groups 1
and 2).
 | Table IFinal body and relative organ weights
for A/J mice fed diets containing MeIQx and/or high fat. |
Table I
Final body and relative organ weights
for A/J mice fed diets containing MeIQx and/or high fat.
| | Final | Organ weight |
|---|
| |
|
|
|---|
| Group | Treatment | No.a | Body weight (g) | Lung (g%) | Liver (g%) | Kidneys (g%) |
|---|
| 1 | MeIQx 600 ppm +
high-fat diet | 20 | 32.11±3.69b,c | 0.61±0.08 | 4.24±0.33d | 1.06±0.09 |
| 2 | MeIQx 600 ppm + basal
diet | 20 | 29.34±2.90 | 0.63±0.08 | 4.56±0.32 | 1.13±0.07 |
| 3 | High-fat diet | 18 | 45.47±5.41e | 0.48±0.06e | 3.36±0.30f | 0.87±0.11f |
| 4 | Basal diet | 19 | 30.27±5.05 | 0.63±0.09 | 3.84±0.38f | 1.05±0.14 |
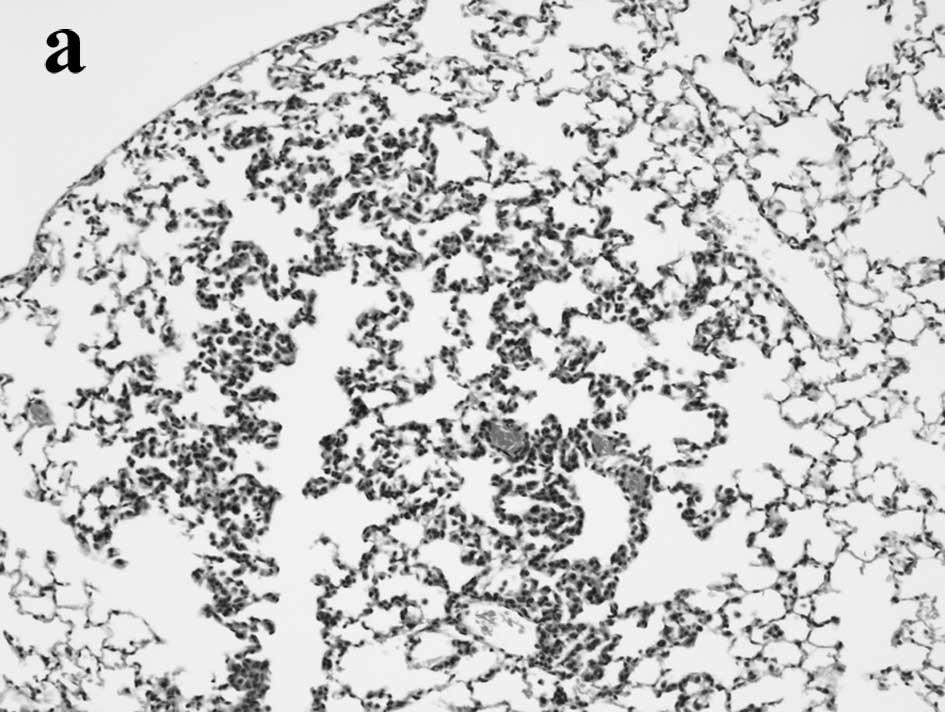
Lung whitish nodules were detected macroscopically
in all of the groups. Lung proliferative lesions, hyperplasias
(Fig. 1a), adenomas (Fig. 1b) and carcinomas (Fig. 1c and d) were diagnosed according to
the criteria of the ‘International Classification of Rodent Tumors:
The Mouse’ (27), and their numbers
were counted under a microscope. Incidences and multiplicities of
lung proliferative lesions are summarized in Tables II and III. Values for hyperplasias and adenomas
in the MeIQx-treated groups (groups 1 and 2) were significantly
higher than the respective data for the groups without MeIQx
treatment. Both incidences and multiplicities of adenoma +
carcinoma, as well as hyperplasia + adenoma + carcinoma (lung
proliferative lesions; Table III)
were also significantly elevated. Lung carcinomas were observed in
1 mouse in each of the groups (groups 1 and 2) treated with MeIQx.
However, the high-fat diet (groups 1 and 3) did not affect the
incidences or multiplicities of lung proliferative lesions.
Although liver tumors diagnosed as adenomas were observed only in
animals treated with MeIQx (groups 1 and 2), their incidences were
not significant.
 | Table IIIncidences and multiplicities of lung
proliferative lesions in A/J mice fed diets containing MeIQx and/or
high fat. |
Table II
Incidences and multiplicities of lung
proliferative lesions in A/J mice fed diets containing MeIQx and/or
high fat.
| | | Hyperplasia | Adenoma | Carcinoma |
|---|
| | |
|
|
|
|---|
| Group | Treatment | No.a | Incidence
(%)b |
Tumors/mousec | Incidence (%) | Tumors/mouse | Incidence (%) | Tumors/mouse |
|---|
| 1 | MeIQx + high-fat
diet | 20 | 13/20
(65.0)d | 0.95±0.94d | 12/20
(60.0)e | 0.85±0.81e | 1/20 (5.0) | 0.05±0.22 |
| 2 | MeIQx | 20 | 7/20 (35.0)f | 0.45±0.69f | 14/20
(70.0)g | 1.40±1.27f | 1/20 (5.0) | 0.05±0.22 |
| 3 | High-fat diet | 18 | 3/18 (16.7) | 0.17±0.38 | 4/18 (22.2) | 0.33±0.69 | 0/18 (0.0) | 0 |
| 4 | Basal diet | 19 | 0/19 (0.00) | 0 | 6/19 (31.6) | 0.32±0.48 | 0/19 (0.0) | 0 |
 | Table IIIIncidences and multiplicities of lung
and liver proliferative lesions in A/J mice fed diets containing
MeIQx and/or high fat. |
Table III
Incidences and multiplicities of lung
and liver proliferative lesions in A/J mice fed diets containing
MeIQx and/or high fat.
| | | Adenoma +
carcinoma | Lung proliferative
lesiona | Liver adenoma |
|---|
| | |
|
|
|
|---|
| Group | Treatment | No.b | Incidence
(%)c |
Tumors/moused | Incidence (%) | Tumors/mouse | Incidence (%) | Tumors/mouse |
|---|
| 1 | MeIQx + high-fat
diet | 20 | 12/20
(60.0)e | 0.90±0.85e | 18/20
(90.0)f | 1.85±1.27f | 0/20 (0.0) | 0 |
| 2 | MeIQx | 20 | 15/20
(75.0)g | 1.45±1.23h | 17/20
(85.0)i | 1.90±1.45j | 1/20 (5.0) | 0.05±0.22 |
| 3 | High-fat diet | 18 | 4/18 (22.2) | 0.33±0.69 | 6/18 (33.3) | 0.50±0.86 | 0/18 (0.0) | 0 |
| 4 | Basal diet | 19 | 6/19 (31.6) | 0.32±0.48 | 6/19 (31.6) | 0.32±0.48 | 0/19 (0.0) | 0 |
The data for the relative quantification of CYP1A2
mRNAs in the livers and lungs of A/J mice are summarized in
Table IV. Expression levels in the
livers of mice significantly increased by 3.56- and 3.59-fold with
MeIQx treatment and significantly decreased with the high-fat diet.
However, the expression of CYP1A2 mRNA in the lungs of the
MeIQx-treated groups was only 1/210 and 1/923 of that noted in the
livers. In the MeIQx-untreated groups, the values were 1/146 in the
lungs and 1/133 in the livers, with no inter-group differences in
lung CYP1A2 mRNA expression.
 | Table IVData for CYP1A2 mRNA expression of
A/J mice fed diets containing MeIQx and/or high fat. |
Table IV
Data for CYP1A2 mRNA expression of
A/J mice fed diets containing MeIQx and/or high fat.
| | | Liver | Lung |
|---|
| | |
|
|
|---|
| Group | Treatment | No.a | CYP1A2b | CYP1A2 |
|---|
| 1 | MeIQx 600 ppm +
high-fat diet | 10 | 480.3±105.2c | 2.3±2.8 |
| 2 | MeIQx 600 ppm +
basal diet | 10 | 872.0±159.6d | 0.9±1.2 |
| 3 | High-fat diet | 10 | 134.8±34.8e | 0.9±1.3 |
| 4 | Basal diet | 10 | 233.9±65.0 | 1.8±2.2 |
Discussion
Both the previous and current studies showed that
MeIQx induces lung tumors in mice (12), supporting the epidemiological
finding that MeIQx is associated with lung cancer risk, whereas
DiMeIQx and PhIP are not (11). In
this study, lung tumors were observed in 1 mouse in each of the
groups treated with MeIQx (groups 1 and 2). The experimental
duration for a large yield of carcinomas was limited. However, a
relatively shorter duration showed that the A/J mouse model appears
to have advantages in terms of detecting lung the tumorigenic
potential of a test compound, such as MeIQx (28).
One epidemiological study has linked a high-fat diet
to an increased risk of human lung adenocarcinomas, but specific
fats were no longer considered to be significant following
adjustment for total fat intake (10). Experimental studies in rodents have
shown that ω-3 unsaturated fatty acids may enhance the development
of cancer in colon (29), breast
(30) and liver (31). We previously noted the promotion of
4NQO-induced pulmonary tumorigenesis in male ICR mice due to a
high-fat diet (21). The lack of
any enhancing effect of a high concentration of corn oil in the
diet in the present study is therefore noteworthy. However, the
final body weights in group 1 which was fed both MeIQx and high fat
significantly decreased in comparison with group 3 which was fed a
diet containing high fat alone, suggesting a complicated influence
of the toxicity of MeIQx.
Ohgaki et al (12) previously reported on the high
incidence of liver tumor development in mice given a diet
containing MeIQx for 84 weeks (12). Liver tumors were also observed in
groups receiving MeIQx only, despite the fact that the A/J mouse
strain is resistant in terms of hepatocarcinogenesis (32).
It is well known that MeIQx is converted to
genotoxic metabolites by liver CYP1A2 (33,34). A
previous study showed an increase of about 1.5–2-fold in the
expression levels of CYP1A2 mRNA in livers treated with MeIQx
(26). Similarly, results of the
present study showed a significant increase of 3.56- and 3.59-fold
in expression levels of livers when treated with MeIQx. In humans,
CYP1A2 has only been detected in the liver (35), and since lung expression was found
to be very low, we concluded that the lung tumorigenic potential of
MeIQx is mainly due to proximate carcinogens generated in the
liver. Following the feeding of mice with a high-fat diet, levels
of CYP1A2 mRNA were found to have decreased rather than
increased.
In conclusion, we successfully confirmed the lung
tumorigenic potential of MeIQx in A/J mice over a shorter period of
time than the time period used in Ohgaki’s experiment (12), although no enhancement by a high-fat
diet was evident. Further investigation with more limited exposure
to MeIQx may therefore be warranted.
Acknowledgements
We thank Ms. Kyoko Hosokawa for her expert technical
assistance. This study was supported in part by Grants-in-Aid for
Cancer Research from the Ministry of Health, Labour and Welfare of
Japan.
Abbreviations:
|
MeIQx
|
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline
|
|
CYP
|
cytochrome P450
|
|
DiMeIQx
|
2-amino
-3,4,8-trimethylimidazo[4,5-f]quinoxaline
|
|
PhIP
|
2-amino-1-methyl-6-phenylimidazo-[4,5-b]pyridine
|
|
4-NQO
|
4-nitroquinoline 1-oxide
|
|
NAT
|
arylamine N-acetyltransferase
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
RT-PCR
|
reverse-transcriptase polymerase chain
reaction
|
References
|
1
|
Sugimura T: Studies on environmental
chemical carcinogenesis in Japan. Science. 233:312–318. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wakabayashi K, Nagao M, Esumi H and
Sugimura T: Food-derived mutagens and carcinogens. Cancer Res.
52:2092–2098. 1992.PubMed/NCBI
|
|
3
|
Layton DW, Bogen KT, Knize MG, Hatch FT,
Johnson VM and Felton JS: Cancer risk of heterocyclic amines in
cooked foods: an analysis and implications for research.
Carcinogenesis. 16:39–52. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sinha R, Chow WH, Kulldorff M, Denobile J,
Butler J, Garcia-Closas M, Weil R, Hoover RN and Rothman N:
Well-done, grilled red meat increases the risk of colorectal
adenomas. Cancer Res. 59:4320–4324. 1999.PubMed/NCBI
|
|
5
|
Probst-Hensch NM, Sinha R, Longnecker MP,
Witte JS, Ingles SA, Frankl HD, Lee ER and Haile RW: Meat
preparation and colorectal adenomas in a large sigmoidoscopy-based
case-control study in California (United States). Cancer Causes
Control. 8:175–183. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng W, Gustafson DR, Sinha R, Cerhan JR,
Moore D, Hong CP, Anderson KE, Kushi LH, Sellers TA and Folsom AR:
Well-done meat intake and the risk of breast cancer. J Natl Cancer
Inst. 90:1724–1729. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sinha R, Gustafson DR, Kulldorff M, Wen
WQ, Cerhan JR and Zheng W:
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, a carcinogen in
high-temperature-cooked meat and breast cancer risk. J Natl Cancer
Inst. 92:1352–1354. 2000.
|
|
8
|
De Stefani E, Boffetta P, Mendilaharsu M,
Carzoglio J and Deneo-Pellegrini H: Dietary nitrosamines,
heterocyclic amines and risk of gastric cancer: a case-control
study in Uruguay. Nutr Cancer. 30:158–162. 1998.PubMed/NCBI
|
|
9
|
Sinha R, Kulldorff M, Curtin J, Brown CC,
Alavanja MC and Swanson CA: Fried, well-done red meat and risk of
lung cancer in women (United States). Cancer Causes Control.
9:621–630. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Stefani E, Brennan P, Boffetta P,
Mendilaharsu M, Deneo-Pellegrini H, Ronco A, Olivera L and Kasdorf
H: Diet and adenocarcinoma of the lung: a case-control study in
Uruguay. Lung Cancer. 35:43–51. 2002.
|
|
11
|
Sinha R, Kulldorff M, Swanson CA, Curtin
J, Brownson RC and Alavanja MC: Dietary heterocyclic amines and the
risk of lung cancer among Missouri women. Cancer Res. 60:3753–3756.
2000.PubMed/NCBI
|
|
12
|
Ohgaki H, Hasegawa H, Suenaga M, Sato S,
Takayama S and Sugimura T: Carcinogenicity in mice of a mutagenic
compound, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx)
from cooked foods. Carcinogenesis. 8:665–668. 1987.
|
|
13
|
Sugimura T: Nutrition and dietary
carcinogens. Carcinogenesis. 21:387–395. 2000. View Article : Google Scholar
|
|
14
|
Hinds MW, Kolonel LN, Lee J and Hankin JH:
Dietary cholesterol and lung cancer risk among men in Hawaii. Am J
Clin Nutr. 37:192–193. 1983.PubMed/NCBI
|
|
15
|
Reddy BS, Cohen LA, McCoy GD, Hill P,
Weisburger JH and Wynder EL: Nutrition and its relationship to
cancer. Adv Cancer Res. 32:237–345. 1980. View Article : Google Scholar
|
|
16
|
Wynder EL, Kajitani T, Ishikawa S, Dodo H
and Takano A: Environmental factors of cancer of the colon and
rectum. II Japanese epidemiological data. Cancer. 23:1210–1220.
1969. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugimura T, Wakabayashi K, Nakagama H and
Nagao M: Heterocyclic amines: mutagens/carcinogens produced during
cooking of meat and fish. Cancer Sci. 95:290–299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Knekt P, Seppanen R, Jarvinen R, Virtamo
J, Hyvonen L, Pukkala E and Teppo L: Dietary cholesterol, fatty
acids and the risk of lung cancer among men. Nutr Cancer.
16:267–275. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Stefani E, Deneo-Pellegrini H,
Mendilaharsu M, Carzoglio JC and Ronco A: Dietary fat and lung
cancer: a case-control study in Uruguay. Cancer Causes Control.
8:913–921. 1997.
|
|
20
|
Alavanja MC, Brown CC, Swanson C and
Brownson RC: Saturated fat intake and lung cancer risk among
non-smoking women in Missouri. J Natl Cancer Inst. 85:1906–1916.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Imaida K, Sato H, Okamiya H, Takahashi M
and Hayashi Y: Enhancing effect of high fat diet on
4-nitroquinoline 1-oxide-induced pulmonary tumorigenesis in ICR
male mice. Jpn J Cancer Res. 80:499–502. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamazoe Y, Abu-Zeid M, Manabe S, Toyama S
and Kato R: Metabolic activation of a protein pyrolysate promutagen
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline by rat liver
microsomes and purified cytochrome P-450. Carcinogenesis.
9:105–109. 1988.PubMed/NCBI
|
|
23
|
Turesky RJ, Constable A, Richoz J, Varga
N, Markovic J, Martin MV and Guengerich FP: Activation of
heterocyclic aromatic amines by rat and human liver microsomes and
by purified rat and human cytochrome P450 1A2. Chem Res Toxicol.
11:925–936. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davis CD, Schut HA and Snyderwine EG:
Enzymatic phase II activation of the N-hydroxylamines of IQ, MeIQx
and PhIP by various organs of monkeys and rats. Carcinogenesis.
14:2091–2096. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jaiswal AK, Nebert DW, McBride OW and
Gonzalez FJ: Human P(3)450: cDNA and complete protein sequence,
repetitive Alu sequences in the 3′ nontranslated region and
localization of gene to chromosome 15. J Exp Pathol. 3:1–17.
1987.PubMed/NCBI
|
|
26
|
Fujita K, Ohnishi T, Sekine K, Iigo M and
Tsuda H: Down-regulation of
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx)-induced
CYP1A2 expression is associated with bovine lactoferrin inhibition
of MeIQx-induced liver and colon carcinogenesis in rats. Jpn J
Cancer Res. 93:616–625. 2002.
|
|
27
|
Dungworth DL, Rittinghausen S, Schwartz L,
Harkema JR, Hayashi Y, Kittel B, Lewis D, Miller RA, Mohr U, Morgan
KT, Rehm S and Slayter MV: Respiratory System and Mesothelium
International Classification of Rodent Tumors: The Mouse WHO.
International Agency for Research on Cancer; New York: 2001
|
|
28
|
Shimkin MB and Stoner GD: Lung tumors in
mice: application to carcinogenesis bioassay. Adv Cancer Res.
21:1–58. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reddy BS: Dietary fat and colon cancer:
animal model studies. Lipids. 27:807–813. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cohen LA, Thompson DO, Maeura Y, Choi K,
Blank ME and Rose DP: Dietary fat and mammary cancer. I Promoting
effects of different dietary fats on N-nitrosomethylurea-induced
rat mammary tumorigenesis. J Natl Cancer Inst. 77:33–42.
1986.PubMed/NCBI
|
|
31
|
Rahman KM, Sugie S, Okamoto K, Watanabe T,
Tanaka T and Mori H: Modulating effects of diets high in omega-3
and omega-6 fatty acids in initiation and postinitiation stages of
diethylnitrosamine-induced hepatocarcinogenesis in rats. Jpn J
Cancer Res. 90:31–39. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dragani TA, Manenti G and Della Porta G:
Quantitative analysis of genetic susceptibility to liver and lung
carcinogenesis in mice. Cancer Res. 51:6299–6303. 1991.PubMed/NCBI
|
|
33
|
Boobis AR, Lynch AM, Murray S, De la Torre
R, Solans A, Farre M, Segura J, Gooderham NJ and Davies DS:
CYP1A2-catalyzed conversion of dietary heterocyclic amines to their
proximate carcinogens is their major route of metabolism in humans.
Cancer Res. 54:89–94. 1994.PubMed/NCBI
|
|
34
|
Rich KJ, Murray BP, Lewis I, Rendell NB,
Davies DS, Gooderham NJ and Boobis AR: N-hydroxy-MeIQx is the major
microsomal oxidation product of the dietary carcinogen MeIQx with
human liver. Carcinogenesis. 13:2221–2226. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Landi MT, Sinha R, Lang NP and Kadlubar
FF: Human cytochrome P450 1A2. IARC Sci Publ. 173–195. 1999.
|