Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide and non-small cell lung cancer (NSCLC) accounts
for approximately 80% of lung cancer. At presentation, only 30% of
NSCLCs are resectable, while the remaining 70% of patients with
NSCLC have advanced disease. Subsequently, these patients are
potential candidates for systemic treatment only.
Platinum-based chemotherapy is the recommended
first-line treatment for advanced NSCLC. This type of chemotherapy
prolongs survival, improves symptom control and results in a
superior quality of life compared to best supportive care. Benefits
of second-line chemotherapy have been substantiated by randomized
trials using docetaxel (1,2), pemetrexed (3) and erlotinib (4). However, the response rate was low, and
it is well known that almost all patients with advanced NSCLC
ultimately progress or relapse. Thus, more treatment is needed to
control the disease and improve quality of life for patients
suffering from NSCLC.
S-1 is a novel oral fluoropyrimidine derivative
consisting of tegafur (FT), which is a prodrug of 5-fluorouracil
(5-FU) and the modulators 5-chloro-2,4-dihydroxypyridine (CDHP) and
potassium oxonate (Oxo), at a molar ratio of 1:0.4:1 (5). CDHP is a reversible competitive
inhibitor of dihydropyrimidine dehydrogenase, an enzyme involved in
the degradation of 5-FU. The degradation of FT-derived 5-FU is
inhibited by CDHP, resulting in enhancement of the antitumor effect
(6,7). Oxo reduces the gastrointestinal
toxicity of 5-FU. After its oral administration, Oxo is distributed
selectively to the small and large intestines. In the intestines,
Oxo inhibits the phosphorylation of 5-FU to fluoropyrimidine
monophosphate which is catalyzed by orotate
phosphoribosyltransferase within the gastrointestinal mucosal
cells, thereby reducing the incidence of diarrhea (8).
S-1 is reportedly effective in the treatment of
certain types of solid cancer, such as gastric (9), breast (10), colorectal (11) and pancreatic (12). As regards the efficacy of S-1
monotherapy for NSCLC, phase II trials showed an overall response
rate of 22% and a median survival time (MST) of 10.2 months
(13). Furthermore, Furuse et
al reported that there was a low frequency of irreversible,
severe or unexpected toxicities (14). S-1 monotherapy may be a treatment
regimen suitable for NSCLC patients after the failure of two or
more prior chemotherapy regimens. However, at present, there is no
defined role for S-1 chemotherapy after second-line chemotherapy
for NSCLC patients.
To investigate the efficacy of S-1 monotherapy,
records of patients with advanced or recurrent NSCLC who received
S-1 following a previously failed response to chemotherapy were
retrospectively analyzed.
Patients and methods
Patient selection
The study included patients with advanced or
recurrent NSCLC who received S-1 monotherapy following the failure
of previous chemotherapy at Nagoya City University Hospital and
Gifu Prefectural Tajimi Hospital between January 2005 and December
2008. Patients who fulfilled the following selection criteria were
included: failure of two or more chemotherapy regimens, Eastern
Cooperative Oncology Group (ECOG) performance status (PS) of 2 or
better, no other active malignancies and adequate bone marrow,
renal and hepatic function. These patients were observed until
March 15, 2009.
Treatment method
S-1 was administered orally, twice daily, after
meals, on days 1–28 every six weeks. The dose of S-1 was determined
to be 80 mg/day (body surface area <1.25 m2), 100
mg/day (body surface area ≥1.25 and <1.50 m2) or 120
mg/day (body surface area ≥1.50 m2). The schedule and
dose for each patient were modified by the physician according to
the medical condition of the patient, as well as toxicity observed
in the previous chemotherapy regimen or courses.
Evaluation and statistical analysis
Patient medical records were retrospectively
analyzed. Responses were assessed with the use of the Response
Evaluation Criteria in Solid Tumors (15). Progression-free (PFS) and overall
survival were measured from the start of S-1 therapy until
progressive disease and death, respectively. Survival results were
analyzed using the Kaplan-Meier method. Toxicity was assessed
according to the common toxicity criteria of the National Cancer
Institute version 2.0.
Results
Patient numbers and characteristics
Table I shows
patient characteristics. The median age was 68 years (range 41–76).
There were 27 male (75%) and 9 female (25%) patients of whom the
majority (33 patients, 91.6%) had an ECOG PS of 1 or 0. Nineteen
patients (52.7%) had adenocarcinoma and 14 patients (38.9%) had
squamous cell carcinoma. Six patients (16.7%) had clinical stage
IIIB and 28 (77.8%) had stage IV disease. Two regimens were given
to 10 patients (27.8%), 3 regimens were given to 13 patients
(36.1%) and ≥4 to 13 patients (36.1%). Of a total of 36 patients,
31 (86.1%) had previously received platinum-based chemotherapy and
27 patients (75%) had previously received both platinum-based
chemotherapy and docetaxel. Three female patients with
adenocarcinoma had already received epidermal growth factor
receptor tyrosine kinase inhibitor (gefitinib or erlotinib).
Twenty-one patients (58.3%) received subsequent chemotherapy
following the termination of S-1 monotherapy.
 | Table ICharacteristics of the 36 treated
patients. |
Table I
Characteristics of the 36 treated
patients.
| Characteristic | No. of patients
(%) |
|---|
| Median age, years
(range) | 68 (41–76) |
| Gender |
| Male | 27 (75.0) |
| Female | 9 (25.0) |
| Performance status
(ECOG) |
| 0 | 12 (33.3) |
| 1 | 21 (58.3) |
| 2 | 3 (8.3) |
| Smoking history |
| Current smoker or
ever smoker | 22 (61.1) |
| Never smoked | 11 (30.6) |
| Unknown | 3 (8.3) |
| Histology |
| Adenocarcinoma | 19 (52.8) |
| Squamous cell
carcinoma | 14 (38.9) |
| NSCLC not
specified | 3 (8.3) |
| Stage of disease |
| IIIA | 2 (5.6) |
| IIIB | 6 (16.7) |
| IV | 28 (77.8) |
| Number of prior
regimens |
| 2 | 10 (27.8) |
| 3 | 13 (36.1) |
| 4 | 10 (27.8) |
| 5 | 3 (8.3) |
Treatment delivery
In total, 102 courses were given (2 median courses
per patient; range 1–10). The 27 male patients (75%) started S-1
monotherapy at the established treatment dose, while the 9 female
patients (25%) were started at a reduced dose. During S-1
monotherapy, 5 patients (13.9%) required a change in the treatment
method: the period of S-1 administration was shortened in 4
patients, while the S-1 dose was reduced in 1 patient. In 3
patients, S-1 monotherapy was still continued at the end of the
observation period. Thirty-three patients stopped S-1 treatment due
to disease progression (27 patients), adverse events (5 patients)
and refusal of treatment (1 patient).
Toxicity
The adverse events observed in the 36 patients are
shown in Table II. Grade 3 anemia
was observed in 1 patient, while 2 patients had grade 3
neutropenia. Grade 4 hematological toxicities did not occur.
Non-hematological toxicities of grade ≥3 were observed in 5
patients (13.9%): elevation of the serum amylase level in 2
patients, interstitial pneumonia in 1 patient and pneumonitis
caused by infection in 2 patients. Interstitial pneumonia of any
grade was observed in 3 patients, two of whom were diagnosed as
having acute exacerbation of idiopathic pulmonary fibrosis (IPF) by
their physician. No deaths related to S-1 monotherapy occurred.
 | Table IIToxicities for the 36 treated
patients. |
Table II
Toxicities for the 36 treated
patients.
| Toxicity | Grade | |
|---|
|
| |
|---|
| 1 | 2 | 3 | 4 | ≥3 (%) |
|---|
| Leukopenia | 1 | 5 | 1 | 0 | 2.8 |
| Neutropenia | 1 | 1 | 2 | 0 | 5.6 |
| Anemia | 3 | 10 | 1 | 0 | 2.8 |
| Thrombocytopenia | 12 | 3 | 0 | 0 | 0 |
| Anorexia | 9 | 5 | 0 | 0 | 0 |
| Fatigue | 6 | 1 | 0 | 0 | 0 |
| Diarrhea | 3 | 0 | 0 | 0 | 0 |
| Nausea | 6 | 2 | 0 | 0 | 0 |
| Vomiting | 1 | 1 | 0 | 0 | 0 |
| Stomatitis | 2 | 0 | 0 | 0 | 0 |
| Rash | 5 | 1 | 0 | 0 | 0 |
|
Hyperbilirubinemia | 4 | 0 | 0 | 0 | 0 |
| ASTa | 7 | 1 | 0 | 0 | 0 |
| ALTa | 7 | 1 | 0 | 0 | 0 |
| Creatininea | 0 | 1 | 0 | 0 | 0 |
| Amylasea | 0 | 0 | 1 | 1 | 5.6 |
| Interstitial
pneumonitis | 1 | 1 | 0 | 1 | 2.8 |
| Pneumonitis | 0 | 0 | 2 | 0 | 5.6 |
Antitumor activity
Treatment response was not assessed in 4 patients
because treatment was terminated during the first course due to
toxicity or refusal. In the remaining 32 patients, no patient
achieved complete response (CR), 4 patients (11.1%) achieved
partial response (PR), 10 patients (27.8%) had stable disease (SD)
and 18 patients (50%) had progressive disease. The disease control
rate (DCR), as well as the rate of patients who had achieved CR, PR
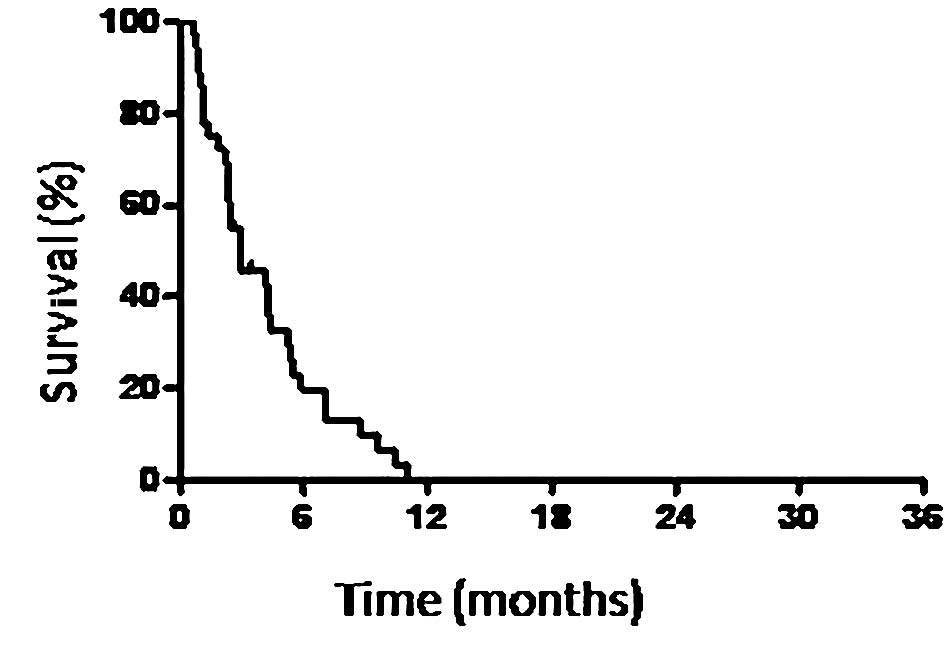
or SD was 38.9%. The 36 patients exhibited a median PFS time of 3
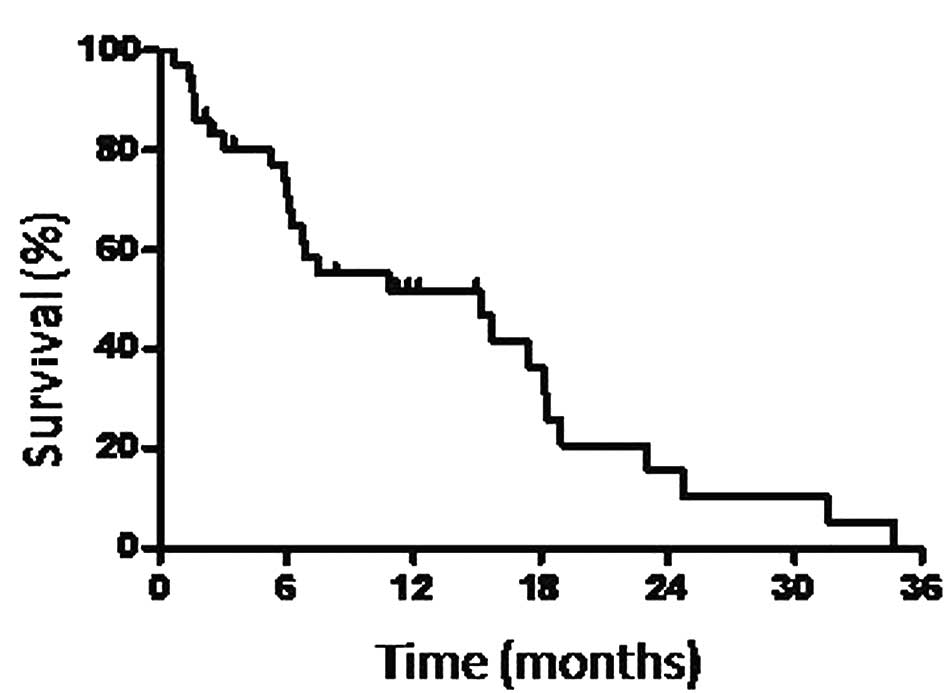
months (Fig. 1) and the overall MST
was 15.2 months (Fig. 2).
Discussion
This study retrospectively analyzed the efficacy and
safety of S-1 monotherapy in patients with advanced or recurrent
NSCLC who received S-1 after previous chemotherapy. S-1 monotherapy
thus exhibited activity with acceptable toxicities.
Concerning the outcome of patients who received two
chemotherapy regimens, including platinum and docetaxel for
recurrent NSCLC, Massarelli et al reported that response
rates decreased with each line of treatment (third-line, 2.3%;
fourth line, 0%). DCR also decreased (third-line, 30.2%;
fourth-line, 21.4%), while MST from the last treatment was 4 months
(16). Shepherd et al
reported that the overall response rate was 9.9% in advanced NSCLC
patients treated with erlotinib after two or three prior regimens
(4). Igawa et al reported
the efficacy of amrubicin for NSCLC patients after the failure of
≥2 prior chemotherapy regimens; the overall response rate was 10.2%
and MST was 4.8 months (17). The
results of the present study indicate that S-1 monotherapy had a
variable clinical outcome for patients with advanced or recurrent
NSCLC who received S-1 after previous chemotherapy.
As regards the toxicities of S-1 monotherapy for
NSCLC patients, previous phase II studies showed that grade 3 or 4
neutropenia was observed in 5.4–6.8% of patients. Grade ≥3
non-hematological toxicities, such as anorexia, fatigue or diarrhea
were observed in approximately 5% of patients (13,14).
In the current study, grade 3 leukopenia and anemia were observed
in only 1 patient (2.8%) each. Grade 3 neutropenia was observed in
2 patients (5.6%). Grade 4 hematological toxicity was not observed.
Grade ≥3 non-hematological toxicities were observed in 5 patients
(13.9%). Given that patients in the present study received S-1 as
third-line or later chemotherapy, the toxicities observed were
sufficiently acceptable.
Interstitial pneumonia was observed in 3 patients
(8.3%), two of whom were diagnosed as having an acute exacerbation
of IPF by their physician. Interstitial pneumonia-related S-1
toxicity was reported to be relatively rare (18). It is unclear whether the
interstitial pneumonia noted in patients was a direct result of S-1
treatment, but careful consideration is needed when S-1 is given to
patients with IPF.
It is important to avoid severe toxicity and make an
effort to obtain a reasonable quality of life for advanced NSCLC
patients previously treated with chemotherapy. Oral chemotherapy is
beneficial in terms of patient quality of life and
cost-effectiveness (19). In the
present study, 15 patients (41.7%) received S-1 chemotherapy at a
reduced dose or with a shortened length of administration because
of toxicity. A prospective study aimed at elucidating the efficacy
of this agent, and investigating the appropriate treatment setting
for NSCLC following the failure of prior chemotherapy regimens is
warranted.
References
|
1
|
Shepherd FA, Dancey J, Ramlau R, et al:
Prospective randomized trial of docetaxel versus best supportive
care in patients with non-small-cell lung cancer previously treated
with platinum-based chemotherapy. J Clin Oncol. 18:2095–2103.
2000.
|
|
2
|
Fossella FV, DeVore R, Kerr RN, et al:
Randomized phase III trial of docetaxel versus vinorelbine or
ifosfamide in patients with advanced non-small cell lung cancer
previously treated with platinum-containing chemotherapy regimens.
The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol.
18:2354–2362. 2000.
|
|
3
|
Hanna N, Shepherd FA, Fossella FV, et al:
Randomized phase III trial of pemetrexed versus docetaxel in
patients with non-small cell lung cancer previously treated with
chemotherapy. J Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, et al: Erlotinib in previously treated non-small cell lung
cancer. N Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shirasaka T, Nakano K, Takechi T, et al:
Antitumor activity of 1 M tegafur-0.4 M
5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against
human colon carcinoma orthotopically implanted into nude rats.
Cancer Res. 56:2602–2606. 1996.PubMed/NCBI
|
|
6
|
Tatsumi K, Fukushima M, Shirasaka T and
Fujii S: Inhibitory effects of pyrimidine, barbituric acid and
pyridine derivatives on 5-fluorouracil degradation in rat liver
extracts. Jpn J Cancer Res. 78:748–755. 1987.PubMed/NCBI
|
|
7
|
Oguri T, Achiwa H, Bessho Y, et al: The
role of thymidylate synthase and dihydropyrimidine dehydrogenase in
resistance to 5-fluorouracil in human lung cancer cells. Lung
Cancer. 49:345–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shirasaka T, Shimamoto Y and Fukushima M:
Inhibition by oxonic acid of gastrointestinal toxicity of
5-fluorouracil without loss of its anti-tumor activity in rats.
Cancer Res. 53:4004–4009. 1993.PubMed/NCBI
|
|
9
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saeki T, Takashima S, Sano M, et al: A
phase II study of S-1 in patients with metastatic breast cancer - a
Japanese trial by the S-1 Cooperative Study Group, Breast Cancer
Working Group. Breast Cancer. 11:194–202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goto A, Yamada Y, Yasui H, et al: Phase II
study of combination therapy with S-1 and irinotecan in patients
with advanced colorectal cancer. Ann Oncol. 17:968–973. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okusaka T, Funakoshi A, Furuse J, Boku N,
Yamao K, Ohkawa S and Saito H: A late phase II study of S-1 for
metastatic pancreatic cancer. Cancer Chemother Phamacol.
61:615–621. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawahara M, Furuse K, Segawa Y, et al:
Phase II study of S-1, a novel oral fluorouracil, in advanced
non-small cell lung cancer. Br J Cancer. 85:939–943. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Furuse K, Kawahara M, Hasegawa K, et al:
Early phase II study of S-1, a new oral fluoropyrimidine, for
advanced non-small cell lung cancer. Int J Clin Oncol. 6:236–241.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
16
|
Massarelli E, Andre F, Liu DD, et al: A
retrospective analysis of the outcome of patients who have received
two prior chemotherapy regimens including platinum and docetaxel
for recurrent non-small cell lung cancer. Lung Cancer. 39:55–61.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Igawa S, Takahashi T, Nakamura Y, et al:
Efficacy of amrubicin for non-small cell lung cancer after failure
of two or more prior chemotherapy regimens. Anticancer Res.
28:3855–3858. 2008.PubMed/NCBI
|
|
18
|
Kurakawa E, Kasuga I, Ishizuka S, et al:
Interstitial pneumonia possibly due to a novel anticancer drug,
TS-1: first case report. Jpn J Clin Oncol. 31:284–286. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
DeMario MD and Ratain MJ: Oral
chemotherapy: rational and future directions. J Clin Oncol.
16:2557–2567. 1998.PubMed/NCBI
|