Introduction
One of the most well-known and lethal gynecological
malignancies is that of ovarian cancer. The molecular pathology of
ovarian cancer is not well understood and various pathways of
development are currently under investigation. p53 as well
as BRCA1 and BRCA2 gene (proteins) dysfunctions are
broadly taken into consideration (1,2). It is
accepted that ovarian cancer as well as other malignancies begin
growth in the primary site of transformation and then spread to the
pelvic and abdominal cavities before metastasizing to distant
organs. Peritoneal dissemination of cancer cells is characteristic
of advanced stages of ovarian, breast and lung cancers, and is
associated with poor patient survival (2). The presence of cancer cells in
effusions complicates treatment protocols. Cell eradication
involving the serosal cavities by a standard oncological approach
is limited. One of the alternative therapeutic options available is
cancer gene therapy.
Gene therapy represents a novel method of therapy of
various diseases based on the introduction of genetic material in
cells in order to correct their biological functions. The first
gene therapy clinical trial was conducted by Blaese and co-workers
in 1990 (3). However, many other
studies are ongoing (4). Due to
promising reports already published, the focus has been on cancer
gene therapy trials (4).
Nevertheless, it is obvious that future trials require new
experiments to be conducted with a view to evaluating the
mechanisms of cellular gene uptake. Currently, genes encoding
therapeutic factors can be delivered into cells by viral and
non-viral methods. The viral vectors are used worldwide in gene
therapy clinical trials because of their efficiency of gene
introduction in human cells (4).
Among them, retrovirus- and adenovirus-derived genetic constructs
are mainly used in the clinic (4).
Well-known advantages of retroviral vectors include the ability of
these vectors to integrate into host cell genomes and to show a
long-term expression of transgenes. A serious limitation, however,
involves the possibility of inducing oncogenesis following
insertional mutagenesis (5,6). A significant advantage of adenoviral
constructs is their ability to infect both dividing and
non-dividing cells, while an important disadvantage is the immune
response against recombinant viruses (6).
Improving the biosafety and infection efficiency of
viral vectors has been emphasized (6). Initial clinical applications used
adeno-associated virus-derived vectors (rAAV) (4,7).
Approximately 50 rAAV-based gene therapy clinical trials have been
reported worldwide (4). AAV belong
to the Parvoviridae family, genus Dependovirus and represent
small, ~20 nm in diameter, icosahedral, non-enveloped particles
containing single-stranded 4.7-kb DNA genomes (7). AAV were discovered in the 1960s as
small adenovirus subunits or adenovirus-contaminating viruses
(8). Finally, it was established
that AAV represent small, defective, DNA-containing viruses which
require a helper adenovirus or herpesvirus to replicate in host
cells (7,9). Significant advantages of AAV as
genetic vehicles include the ability to infect a broad range of
dividing and non-dividing cells and the lack of pathogenicity in
humans. Additionally, AAV can integrate into a defined region of
the human genome, and the observed transgene expression occurs over
an extensive period of time (7,9).
Recombinant AAV vectors have been shown to infect normal tissues
such as liver, muscle, skin, the nervous system and cancer cells
(4,7,10). The
ability of rAAV vectors to introduce therapeutic genes to cancer
cells disseminated into the peritoneal cavity has also been
reported (11–13).
In this study, we considered viral vectors to be
vehicles for gene transfer to cells disseminated in the serosal
cavities. We showed a utility of recombinant adeno-associated virus
vector serotype 2 (rAAV2) for the intraperitoneal administration of
genes to cancer cells. rAAV2 vectors encoding reporter genes, green
fluorescent protein (GFP) and β-galactosidase (LacZ)
were also investigated. The aim of the experiments was to evaluate
the infection efficiency of L1 cancer cells by rAAV serotype 2
vectors in mouse peritoneal cavity. The results appear to be
directly useful for the planning of cancer gene therapy clinical
trials for ovarian cancer.
Materials and methods
Production of recombinant
adeno-associated virus serotype 2
Packaging cell culture
The AAV-293 cell line (Stratagene) was used for the
production of recombinant infectious AAV particles. AAV2 was the
only serotype produced and used in the studies. The AAV-293 cell
line represents stable transformed by adenovirus type 5 DNA human
embryonic kidney cells that have an adenovirus E1 gene required for
rAAV production in vitro. For rAAV production, AAV-293 cells
were maintained in Dulbecco’s modified Eagle’s medium (DMEM),
supplemented with 10% fetal bovine serum (FBS), 4 mM L-glutamine
and 4.5 g/l glucose at 37°C in a humidified atmosphere of 5%
CO2. The cells were cultured to a confluence of 60–70%
and then the triple co-transfection procedure with AAV plasmids was
performed.
Plasmid constructs
The expression plasmid vectors pRC, pHelper, pGFP
and pLacZ (Stratagene) were used in the experiments. The constructs
contain AAV and adenovirus genes that are required for the
production of infectious AAV particles. The pRC vector supplies
rep and cap genes encoding AAV replication and capsid
proteins, respectively. The pHelper vector contains the adenovirus
E2A, E4, VA genes, and the pGFP or pLacZ vectors contain the
GFP and β-GAL reporter genes, respectively. The
reporter vectors represent the ITR-containing plasmids with
cmv promoters. The AAV plasmid vectors were amplified in
transformed bacteria Escherichia coli and isolated by an
alkaline lysis method using Endofree chromatographic columns
(Qiagen). The quality of the isolated plasmids was confirmed
spectrophotometrically and by restriction digestion mapping.
rAAV isolation and purification
rAAV infectious particles (rAAV vectors) were
produced in a helper-free system, without using a helper adenovirus
or herpesvirus for productive infection. The AAV-293 packaging
cells were triple transient co-transfected with rAAV plasmid
vectors pRC, pHelper and pGFP/pLacZ at a molar ratio of 1:1:1 using
25 kDa polyethylenimine (PEI 25 kDa; Sigma) cationic polymer as a
DNA carrier agent. The transfected cells were harvested after 48–72
h. To release rAAV virions, the transfected cells were lysed by the
freeze-thaw method. The isolation and purification procedure was
performed according to the Zolotukhin et al protocol
(14). Briefly, after the
temperature-dependent cell lysis, the crude lysate
(virus-containing supernatant) was purified by iodixanol gradient
ultracentrifugation, followed by heparin agarose affinity
chromatography (Sigma). The rAAV crude lysate was placed into an
iodixanol gradient column (comprising 15, 25, 40 and 60% fractions)
and ultracentrifugated (85 min, 18°C, 255,000 × g). Immediately
after centrifugation, the fraction between 40 and 60% was
transferred into the heparin agarose column (Sigma). The
purification was performed at room temperature conditions. The
eluted primary virus stock was concentrated by centrifugation
through the 100 MVCO filter columns (Millipore) and stored at −80°C
for further studies.
rAAV quantitative analysis
The number of genome copies of rAAV particles in
obtained vector stocks (genome copies per ml) was determined by
quantitative real-time PCR (SYBR® Green PCR Master Mix,
Applied Biosystems). Assessment was performed using primers within
the cmv promoter sequence (forward: CACCAAAATCAACGGGACTT and
reverse: GAGGTCAAAACAGCGTGGAT; 156-bp product; Tm=50°C).
The qPCR curve of each AAV stock sample was related to the standard
curve prepared using a 6 log spanning serial dilution of pLacZ
plasmid construct containing one cmv promoter sequence per
molecule. Each dilution step was measured in duplicate per run. The
standard curve was calculated by the ABI Prism 7000 SDS software
(Version 1.1; Applied Biosystems) by regression of the crossing
points of the PCR curves from the dilution series of the vector
plasmid. Melting curve analysis and agarose gel electrophoresis
were performed, demonstrating specificity of the qPCR products.
Intraperitoneal infection of L1 cancer
cells
The L1 cancer cells (mouse fibrosarcoma) were grown
in vitro to a confluence of 70–80% in standard cell culture
conditions. The cells were maintained in DMEM, supplemented with
10% FBS at 37°C in a humidified atmosphere of 5% CO2.
The cells were then harvested and intraperitoneally (i.p.) injected
to Balb/c mice at a dose of 2–3×105 cells per mouse. Ten
days later, the mice were i.p. injected with the rAAV preparations
at different doses (1.2×105-4.2×106 gc/ml;
MOI of 0.01–200). After three days, the mice were sacrified and the
intraperitoneal effusions containing infected cancer L1 cells were
taken for further studies. The presence of virus DNA sequences as
well as the expression of reporter genes in the studied cells were
evaluated. The total DNA from the infected cells was isolated using
the Qiamp mini kit (Qiagen) according to the manufacturer’s
protocol. The amplification of virus sequences was performed by a
standard PCR method using specific starters (GAPDH:
GAGTACGTCGTGGAGTCCACTGGCGTC3′ and 5′CTTGATGTCATCATATTTGGCAGGTTT3′;
GFP: 5′AGCTGGAC GGCGACGTAAAC3′ and 5′GTCGGCCATGATATAGACGT3′;
LacZ: 5′CATCTGCTGCACGCGGAAGAA3′ and 5′TACATG
GTTGCTTTGACGT).
Reporter gene expression
To determine the intraperitoneal infection
efficiency of cancer cells with a rAAV/LacZ vector the
β-galactosidase test was performed. The β-galactosidase enzyme
assay was used to study β-galactosidase activity in L1/rAAV/LacZ
cell lysates prepared from L1 cells i.p. infected with viral
particles. The test was performed according to the Promega
β-galactosidase Assay System protocol with ONPG
(O-nitrophenyl-β-D-galactopyranoside; Sigma) as a substrate.
β-galactosidase derived from rAAV/LacZ-infected L1 cells hydrolyzes
the colorless ONPG substrate to yellow o-nitrophenol, the content
of which is defined with a spectrophotometer at an absorbance of
420 nm. Additionally, the total protein content was determined by
the Lowry method (absorbance was read at 750 nm). The relative
β-galactosidase activity was calibrated as the ratio of β-gal 420
nm absorbance to the total protein 750 nm absorbance (ABS 420/750
nm). The GFP-positive L1 cancer cells were also evaluated. The
i.p.-infected L1 cancer cells with rAAV/GFP vectors were observed
and counted under an inverted fluorescence microscope (Olympus
IX51).
Results
Recombinant adeno-associated viral vectors (rAAV2)
were produced in adenovirus-free conditions by a transient
transfection of AAV-293 packaging cells with AAV plasmid constructs
encoding essential viral genes. Based on a determination of virus
genome copies performed by quantitative real-time PCR, it was
estimated that produced virus stocks contain 0.3–0.7×108
gc/ml. Experiments evaluating the infectious activity of obtained
recombinant adeno-associated virus vectors encoding reporter genes
(GFP and β-GAL) were performed in vivo on
Balb/c mice i.p. injected with L1 cancer cells. The cells were i.p.
infected for three days with rAAV vectors encoding reporter genes.
rAAV preparations were injected directly into the mouse peritoneal
cavity at a multiplicity of infection (MOI) ranging from 0.01 to
200. The transgene expression was tested on L1-infected cells
involving the peritoneal effusions.
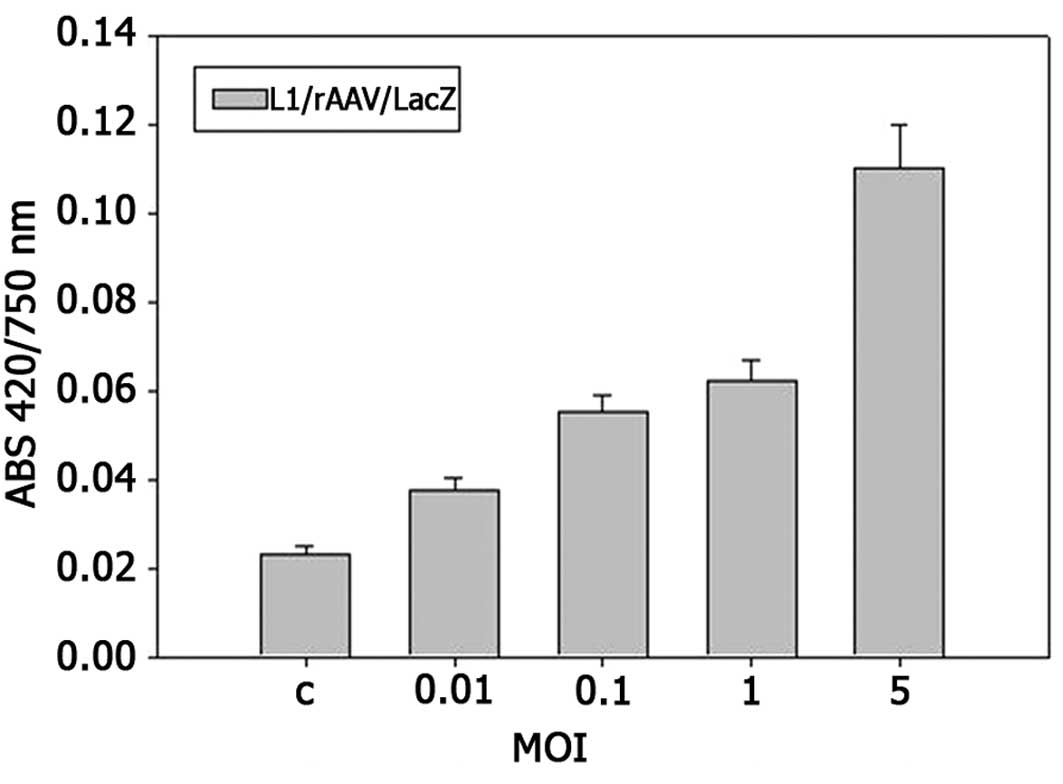
Figs. 1 and 2 show that recombinant AAV effectively
infect L1 cells disseminated in the peritoneal cavity. The cells
revealed an ability to uptake the rAAV vectors and express the
reporter β-GAL gene. Figs. 1
and 2 show that the infection
efficiency depends on the virus dose used in the experiments. The
infection, calculated as β-galactosidase activity vs. total protein
content (ABS420/750) increases with a MOI ratio. The highest
expression of the LacZ reporter gene was observed at a MOI of 50
and 200. Studies performed with rAAV/GFP vectors also revealed that
the i.p. infection is closely dependent on the virus particle
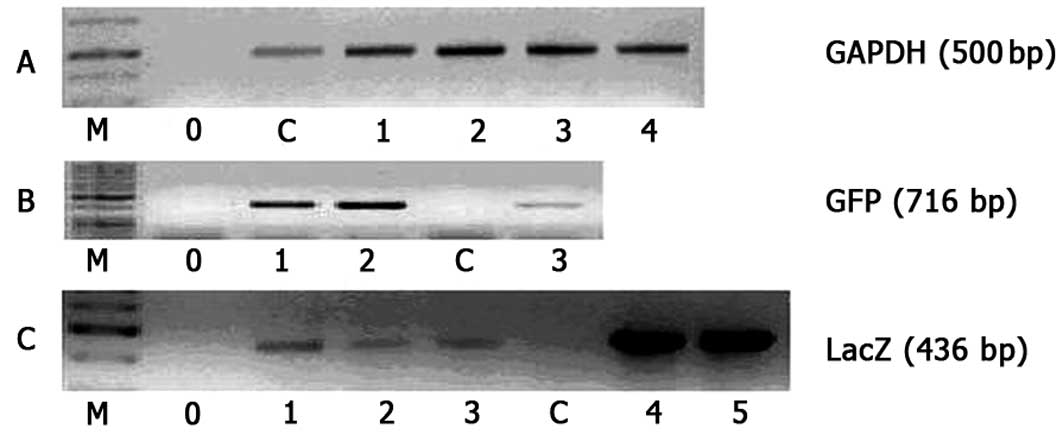
number injected into the peritoneal cavity (Table I). As shown in Fig. 3, L1 cells infected with rAAV
displayed presence of the virus sequence in their genome.
Amplification of the fragment of the rAAV β-GAL and
GFP genes by PCR confirmed the intraperitoneal infection of
L1 cells by recombinant adeno-associated viruses.
 | Table INumber of GFP-positive L1 cells found
in mouse intraperitoneal effusion.a |
Table I
Number of GFP-positive L1 cells found
in mouse intraperitoneal effusion.a
| Multiplicity of
infection (MOI) |
|
| C | 0.001 | 1 | 2 | 3 | 10 |
|
| No. of L1/rAAV/GFP
cells |
|
| 0.0 | 0.0 | 0.0 | 3.5±1.7 | 9.0±5.7 | 17±2.3 |
| n=3 | n=3 | n=4 | n=3 | n=3 | n=5 |
Discussion
The presence of cancer cells in the effusion fluid
involving the serosal cavities is a frequent clinical finding. As
described, cancer cells disseminated into the peritoneal, pleural
or pericardial cavities have a unique biology. The expression
pattern of various cell proteins may vary between cancer types at
the primary site (solid tumors) and their effusion counterparts
(2,15). The accumulation of cancer cells in
the effusions has clinical prognostic importance and directly
indicates efficiency of the treatment of serious malignancies. It
is known that the eradication of cancer cells and effusion fluids
from serosal cavities is very difficult because of surgical
limitations. Additionally, the usefulness of chemotherapy is poor,
since cancer cells in effusions often reveal a resistance to
apoptosis (15). Gene therapy and
gene preparations represent a novel therapeutic approach in
contemporary medicine. The focus lies on engineering gene transfer
vectors that effectively deliver to cancers genes, thereby encoding
therapeutic proteins such as antiangiogenic (soluble receptors of
vascular endothelial growth factors), proapoptotic (caspases,
tissue inhibitors of matrix metalloproteinases) or immunomodulating
(interleukins) (16). Cancer gene
therapy is currently one of the most advanced gene therapy
strategies found in the clinic (4).
Preliminary outcomes underline the clinical significance of
recombinant virus vectors for cancer cell infection (4). Thus, rAAV vectors are one of the more
promising vehicles for gene delivery. Experimental observations
confirm their biosafety and infection efficiency (7,10).
rAAV is able to transfer genes to cancer cells involving the
serosal cavities as previously described (11–13).
In this study, we showed the ability of rAAV2 vector encoding
reporter genes, GFP and β-GAL, to infect fibrosarcoma
cells disseminated into the mouse peritoneal cavity. We observed
that rAAV2 was ingested by L1 cancer cells. The efficiency of
infection was correlated to a MOI ratio. These experiments showed
that rAAV has delivered the gene of interest to cancer cells in the
peritoneal cavity. Notably, no toxicity was observed (data not
shown). The utility of recombinant virus vectors for gene transfer
to cells involving the serosal cavities is tentatively reported by
others. Studies published by Isayeva et al revealed that
recombinant AAV vectors encoding antiangiogenic factors
(angiostatin and endostatin) may be useful for the treatment of
intraperitoneal ovarian cancer dissemination (11,12).
The possibility of preventing ovarian cancer metastasis through
intraperitoneal rAAV-mediated gene transfer was also described by
Li et al (13). The authors
underlined the benefit of the rAAV-dependent long-term expression
of the nm23H1 gene in vivo.
Gene therapy and gene-based preparations generate a
new strategy for cancer treatment. Preliminary observations
demonstrate the ability of recombinant adeno-associated viruses to
facilitate therapeutic gene delivery to cancer cells disseminated
in the serosal cavities. Our study illustrates that rAAV 2 vectors
are useful for the intraperitoneal infection of cancer cells. The
findings appear to be important for cancer gene therapy clinical
trials for ovarian, breast or pancreas cancers.
Acknowledgements
This work was supported by a grant from the Polish
Committee for Scientific Research (KBN 2P05E03328).
References
|
1
|
Reibenwein J and Krainer M: Targeting
signaling pathways in ovarian cancer. Expert Opin Ther Targets.
12:353–365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cannistra SA: Cancer of ovary. N Engl J
Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blaese RM, Culver KW, Miller AD, et al: T
lymphocyte-directed gene therapy for ADA-SCID: initial trial
results after 4 years. Science. 270:475–480. 1995.PubMed/NCBI
|
|
4
|
|
|
5
|
Porteus MH, Connelly JP and Pruett SM: A
look to future directions in gene therapy research for monogenic
diseases. PLoS Genet. 2:1285–1292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raty JK, Lesch HP, Wirth T and
Ylä-Herttuala S: Improving safety of gene therapy. Curr Drug Saf.
3:46–53. 2008. View Article : Google Scholar
|
|
7
|
Le Bec C and Douar A: Gene therapy
progress and prospects-vectorology: design and production of
expression cassettes in AAV vectors. Gene Ther. 13:805–813.
2006.PubMed/NCBI
|
|
8
|
Atchison RW, Casto BC and Hammon WM:
Adenovirus-associated defective virus particles. Science.
149:754–756. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Małecki M, WoŸniak A and Janik P: Wirusy
związane z adenowirusami (AAV). Postępy Biochem. 54:57–63.
2008.
|
|
10
|
Flotte TR: Gene therapy progress and
prospects: recombinant adeno-associated virus (rAAV) vectors. Gene
Ther. 11:805–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Isayeva T, Ren C and Ponnazhagan S:
Recombinant adeno-associated virus 2-mediated antiangiogenic
prevention in a mouse model of intraperitoneal ovarian cancer. Clin
Cancer Res. 11:1342–1347. 2005.
|
|
12
|
Isayeva T, Ren C and Ponnazhagan S:
Intraperitoneal gene therapy by rAAV provides long-term survival
against epithelial ovarian cancer independently of survivin
pathway. Gene Ther. 14:138–146. 2007.PubMed/NCBI
|
|
13
|
Li J, Zhou J, Chen G, et al: Inhibition of
ovarian cancer metastasis by adeno-associated virus-mediated gene
transfer of nm23H1 in an orthotopic implantation model. Cancer Gene
Ther. 13:266–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zolotukhin S, Byrne BJ, Mason E, et al:
Recombinant adeno-associated virus purification using novel methods
improves infectious titer and yield. Gene Ther. 6:973–985. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davidson B: Biological characteristics of
cancers involving the serosal cavities. Crit Rev Oncog. 13:189–227.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malecki M, Kolsut P and Proczka P:
Angiogenic and antiangiogenic gene therapy. Gene Ther. 12:159–169.
2005. View Article : Google Scholar
|