Introduction
In breast cancer patients, bone is the most frequent
site of distant metastasis. Once the destruction of bone has
progressed, skeletal complications occur with increased pain,
immobility and deterioration of quality of life. The pathogenesis
of bone metastases is not fully understood, but it is thought that
breast cancer cells produce osteoclast-activating factors, which
induce the osteoclast resorption of bone, leading to the
development of lytic bone disease (1). Bisphosphonates strongly inhibit
osteoclast bone resorption and have beneficial effects on bone
metastases (2). They promote
apoptosis not only in osteoclasts, but also in tumor cells
(3,4). They also have direct cytotoxic effects
on breast cancer cell lines and fresh breast cancer tumor tissue
(5). Bisphosphonates activate
γδT-cell proliferation which contributes differently to the host
immune defense (6). They also
inhibit angiogenesis and matrix metalloproteinase activity which
are related to the processes of tumor growth, invasion and
metastasis (7). Based on these
mechanisms of action, bisphosphonates are expected to prevent the
development of bone metastases (8).
In an experimental in vivo study, risedronate
(third-generation bisphosphonate) reduced the development of bone
metastases in nude mice either by the simultaneous inoculation of
the human breast cancer cell line, MDA-231, or the prophylactic
administration prior to MDA-231 cell inoculation (9). Furthermore, in an in vitro
study, potent bisphosphonates (such as pamidronate and alendronate)
inhibited the adhesion of breast cancer cells to cortical and
trabecular bone (10). These
studies suggest that bisphosphonates may be useful not only in the
treatment of skeletal complications, but also in preventing the
development of bone metastases. If preventive therapy has
beneficial effects on the development of bone metastases, this will
significantly impact the patient quality of life.
Pamidronate, a second-generation bisphosphonate, is
a potent inhibitor of osteoclast activity. We initiated a
preliminary study to examine whether adjuvant pamidronate therapy
was able to prevent or delay the development of bone metastases in
breast cancer patients at high risk for bone metastasis.
Materials and methods
Patients
This preliminary study was carried out at the Itami
City Hospital and Hyogo Medical Center Hospital for Adults;
beginning in 1997 with the June enrollment of patients and ending
in November 2001. Ambulatory 20 years of age or older women with
breast cancer, who were histologically proven to have ≥4 positive
nodes, were enrolled. Patients were ineligible for the study if
they had received primary chemotherapy prior to surgery. This study
was in accordance with the Helsinki Declaration (1964, amended in
1975 and 1983). Fully informed consent was obtained from the
subjects enrolled. Although this study was designed as a randomized
controlled trial, at the time it was performed it was very
difficult to carry out a randomized trial in Japan. Japanese
patients were unwilling to be randomly assigned to treatment groups
and refused randomization. We therefore used the best available
design with non-randomized assignment based on patient preference.
A total of 90 patients were assigned either to treatment with
pamidronate (33 patients) or to the control group (57 patients) by
patient preference. Pamidronate (45 mg) was administered
intravenously every 2 weeks (standard use in Japan) for a total of
4 infusions. The infusion rate was consistent with the currently
recommended treatment schedule for patients with hypercalcemia.
Adjuvant therapy was performed in each hospital. Estrogen (ER) and
progesterone receptor (PgR) assays were performed on the cytosol of
each specimen by solid-phase enzyme immunoassay. Primary endpoints
of this study were: i) reduction of the incidence of bone
metastases and ii) the delay in the appearance of bone metastases
in breast cancer patients with ≥4 positive nodes. These patients
were at high risk for bone metastases. Patients were included in
the intent-to-treat analysis.
Treatment
The primary surgical treatment consisted of
breast-conserving surgery (plus 50 Gy radiation to the breast) or
mastectomy with axillary dissection. Pamidronate (45 mg) was
administered over a 45-min intravenous infusion every 2 weeks for a
total of 4 times. Adjuvant systemic therapy was based on the
protocols of each center and all 90 patients received chemotherapy.
The most frequently used regimens were anthracycline combinations
(60 patients; 66.7%). CAF therapy [doxorubicin (20
mg/m2, days 1 and 8), 5-fluorouracil (500
mg/m2, days 1 and 8) and cyclophosphamide (100 mg for 14
days orally)] was used in 55 patients (61.1%). CEF therapy
[epirubicin (60 mg/m2, day 1), 5-fluorouracil (500
mg/m2, days 1 and 8) and cyclophosphamide (500
mg/m2, days 1 and 8)] was used in 5 patients (5.6%). CMF
therapy [methotrexate (40 mg/m2, days 1 and 8),
5-fluorouracil (500 mg/m2, days 1 and 8) and
cyclophosphamide (100 mg for 14 days orally)] was used in 28
patients (31.1%). Uracil and tegafur (400 mg) were given orally
every day for 2 years. The use and type of chemotherapy were evenly
distributed between the pamidronate and control groups. Tamoxifen
(20 mg/day) was provided for 5 years to 37 patients (41.1%) who
were hormonal receptor-positive. If metastases were confirmed,
appropriate cytotoxic or hormonal therapies were used according to
the treatment protocols of each centre.
Follow-up
Follow-up investigations were carried out in each
centre. During the second year of treatment, patients continued to
be studied at 2-month intervals, with a physical examination
performed at every visit. Basic laboratory tests (blood counts,
liver enzymes, serum creatinine, calcium, electrolytes and tumor
antigen) were performed every 2 months. At entry into the study, it
was confirmed that patients had no distant metastases. Bone
scintigraphy was repeated every 6 months. Bone scintigraphy was
performed at any time bone metastases were clinically suspected. If
abnormal uptake was detected on bone scintigraphy, additional
computed tomography and/or magnetic resonance imaging was performed
to confirm bone metastases. At each center, radiological bone
surveys were received by radiologists who were unaware of the
patient’s treatment. Chest X-ray, ultrasonography of the liver and
chest, and abdominal computed tomography examinations were
performed every 6 months. Adverse events were recorded and graded
according to the National Cancer Institute Common Toxicity
Criteria.
Statistical analysis
The data were last updated in August 2004. The
Chi-square test was used to compare prognostic factors between the
groups. Fisher’s exact test was used for between-treatment
comparison of the incidence of the development of metastases
(distant, bone, visceral and non-osseous), as well as the incidence
of death. Time from surgery to the appearance of metastases was
estimated by the Kaplan-Meier method and compared with the log-rank
test and generalized Wilcoxon test. Tests were two-sided. P<0.05
was considered to be statistically significant.
Results
Thirty-three patients received adjuvant pamidronate
therapy and 57 patients comprised the control group. The
pamidronate and control groups were well-balanced for the
clinicopathological characteristics of age, tumor size, nodal
status, menopausal status, hormonal status and type of chemotherapy
(Table I).
 | Table IPatient characteristics in the
pamidronate and control groups. |
Table I
Patient characteristics in the
pamidronate and control groups.
| Pamidronate group
(n=33) | Control group
(n=57) | p-value |
|---|
| Age | | | 0.8233 |
| Median | 54 (34–71) | 50 (35–84) | |
| Tumor status | | | 0.1532 |
| T1 | 5 (15.2%) | 8 (14.0%) | |
| T2 | 14 (42.4%) | 25 (43.9%) | |
| T3 | 6 (18.2%) | 19 (33.3%) | |
| T4 | 6 (18.2%) | 3 (5.30%) | |
| Unknown | 2 (6.5%) | 2 (3.50%) | |
| Metastatic lymph
nodes | | | 0.2552 |
| Median | 16 (4–53) | 11 (4–47) | |
| Estrogen receptor
status | | | 0.3797 |
| Positive | 16 (48.5%) | 21 (36.8%) | |
| Negative | 17 (51.5%) | 33 (57.9%) | |
| Unknown | | 3 (5.30%) | |
| Progesterone receptor
status | | | 0.6928 |
| Positive | 13 (39.4%) | 19 (33.3%) | |
| Negative | 20 (60.6%) | 35 (61.4%) | |
| Unknown | | 3 (5.30%) | |
| Menopausal
status | | | 0.4632 |
| Premenopausal | 13 (39.4%) | 27 (47.4%) | |
| Postmenopausal | 20 (60.6%) | 30 (52.6%) | |
| Chemotherapy | | | 0.3534 |
| CAF or CEF | 20 (60.6%) | 40 (70.2%) | |
| Others | 13 (39.4%) | 17 (29.8%) | |
At the time of data cut-off, the median follow-up
period was 1834 days (890–2149 days) in the pamidronate group and
2489 days (927–3004 days) in the control group. Distant metastases
were detected in 12 patients (36.3%) in the pamidronate group and
in 32 patients (56.1%) in the control group (p=0.071; Table II). Bone metastases were detected
in 4 patients (12.1%) in the pamidronate group and in 18 patients
(31.6%) in the control group. The incidence of bone metastasis was
significantly lower in the pamidronate than in the control group
(p=0.005). Non-osseous (visceral and local) metastases were
detected in 11 patients (33.3%) in the pamidronate group and in 30
patients (52.6%) in the control group (p=0.077). Visceral
metastases were detected in 10 patients (30.3%) in the pamidronate
group and in 24 patients (42.1%) in the control group. Seven
patients (21.2%) in the pamidronate group, as well as 17 patients
(39.8%) overall succumbed to the disease (p=0.373).
 | Table IIIncidence of metastatic disease and
death in the pamidronate and control groups. |
Table II
Incidence of metastatic disease and
death in the pamidronate and control groups.
| Pamidronate group
(n=33) | Control group
(n=57) | p-value |
|---|
| Distant
metastases | 12 (36.3%) | 32 (56.1%) | 0.071 |
| Bone metastases | 4 (12.1%) | 23 (40.4%) | 0.005 |
| Non-osseous
metastases | 11 (33.3%) | 30 (52.6%) | 0.077 |
| Visceral
metastases | 10 (30.3%) | 24 (42.1%) | 0.266 |
| Death | 7 (21.2%) | 17 (29.8%) | 0.373 |
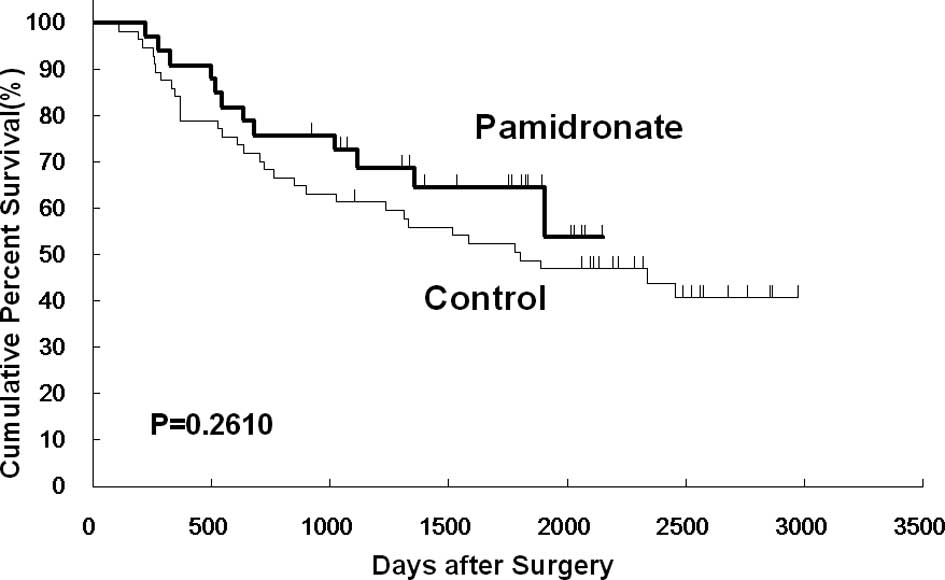
The Kaplan-Meier analysis showed that bone
metastasis-free survival at 5 years was 85.9% in the pamidronate
group and 64.0% in the control group. Bone metastasis-free survival
was significantly higher in the pamidronate group than in the
control group (p=0.023; Fig. 1).
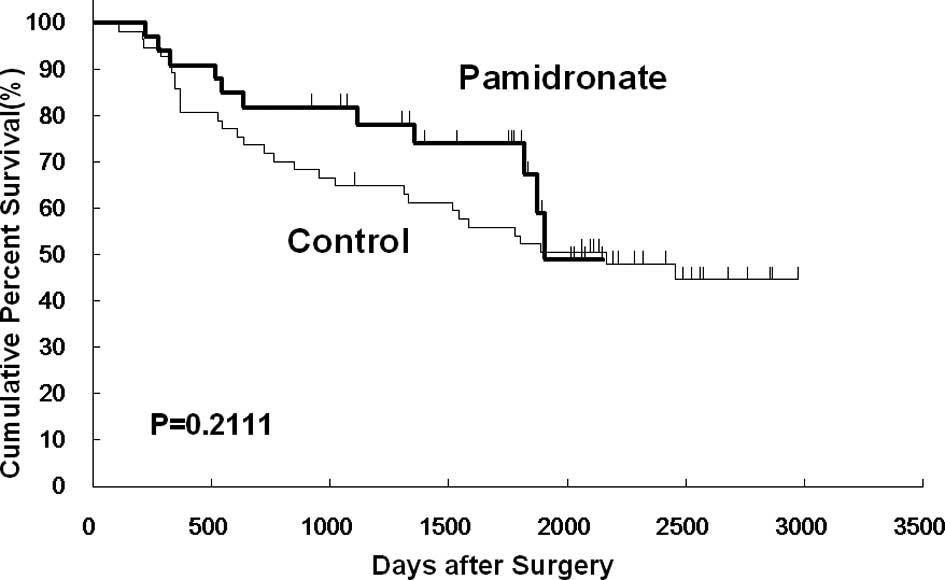
However, no significant differences were noted between the two
groups in overall, disease-free and non-osseous metastasis-free
survival (Figs. 2, 3 and 4).
Pamidronate was well-tolerated. No renal or
gastrointestinal side effects were observed. No serious adverse
events believed to be related to pamidronate occurred. The only
side effects were asymptomatic hypocalcemia (grade 1) in 2 patients
and transient fever (<38°C) in 3 patients.
Discussion
Bisphosphonates are very effective for bone
metastases in breast cancer patients. They relieve the pain of bone
metastases and improve quality of life for the patient. Prevention
of the development of bone metastases will have a great impact on
the course of disease in breast cancer patients. However, it is
unclear whether bisphosphonates have a beneficial effect on the
development of bone metastases in breast cancer. Three clodronate
adjuvant prevention trials have been reported. Diel et al
were the first to report on an adjuvant bisphosphonate study in 302
patients who had primary breast cancer and cancer cells in the bone
marrow, as identified by immunocytological studies (11). These patients were randomized to
receive clodronate at a dose of 1600 mg/day for 2 years, or not to
receive any clodronate. With a median follow-up of 36 months, the
incidences of distant (13 vs. 29%, p<0.001), bone (8 vs. 17%,
p=0.003) and visceral metastases (8 vs. 19%, p=0.003) were
significantly lower in the clodronate group than in the control
group. Metastasis-free and overall survival benefit were
significant in the clodronate group (p=0.001). Diel et al
updated their report at the May 2000 and June 2004 ASCO meetings
(both in New Orleans, LA) (12,13).
Incidences of bone and non-osseous metastases were similar in the
two groups. Survival was found to be significantly higher in the
clodronate group (p<0.049) at 103 months of follow-up. Powles
et al reported on a larger study that included 1069 primary
operable breast cancer patients who were randomized to receive
either clodronate 1600 mg/day or a placebo for 2 years (14). There was no significant reduction in
the occurrence of bone metastases for the total follow-up period.
However, during the medication period, a significant reduction in
this parameter in the clodronate group (2.3 vs. 5.2%, p=0.016) was
noted. The rate of occurrence of non-osseous metastases was similar
in the two groups, but there was a significant reduction in
survival in the clodronate group (83 vs. 79%, p=0.047). Powles
et al updated their report at the June 2004 ASCO meeting
(15). Clodronate significantly
reduced bone metastases not only during the medication period (2
years, p=0.031), but also during the entire study period (5 years,
p=0.043). Clodronate significantly improved survival at 10 years
(p=0.048). On the other hand, Saarto et al reported a
deleterious effect of adjuvant clodronate therapy. Two hundred and
ninety-nine women with node-positive breast cancer were randomized
to receive clodronate 1600 mg/day for 3 years, or no clodronate
with adjuvant therapy (16). With a
minimum follow-up of 5 years, bone metastases were equally detected
in the clodronate group (21 vs. 17%), and the rate of development
of non-skeletal metastases was significantly higher in the
clodronate group (43 vs. 25%, p=0.0007). Furthermore, overall and
disease-free survival were significantly lower in the clodronate
group (overall survival, 70 vs. 83%, p=0.009; disease-free
survival, 56 vs. 71%, p=0.007, respectively). However, there was no
significant difference in either overall or distant disease-free
survival with adjustment data by PgR status because of the present
imbalance between the two groups in PgR status. The report was
updated at the June 2004 ASCO meeting (17). Bone metastases were similar in the
two groups, but the incidence of non-skeletal metastasis was
significantly higher in the clodronate group (p=0.005). Ten-year
disease-free survival was lower in the clodronate group (p=0.01),
especially in ER-negative patients. No significant difference in
overall survival was found between the two groups. In the present
study, we demonstrated that adjuvant pamidronate therapy
significantly decreased the incidence of the development of bone
metastases, and prolonged bone metastasis-free survival in patients
with ≥4 positive nodes. There was a trend toward a decrease in the
incidence of distant and visceral metastases in the pamidronate
group, but no significant difference was noted between the two
groups with regard to the overall survival rate.
The discrepancy findings among the above-mentioned
studies appeared to result from the use of different patient
populations and regimens of adjuvant therapy. The study by Powles
et al consisted of broad stage I–III breast cancer patients,
including those who received only radiation or no axillary
dissection (~1/3 of all patients). Some of the patients appeared
not to require adjuvant therapy. This population appeared to be at
very low risk for bone metastases. It is possible that inclusion of
this low-risk population reduced the statistical power in the
detection of a beneficial effect of adjuvant clodronate therapy.
The candidates for inclusion in the present study were primary
breast cancer patients with ≥4 positive nodes. The Japanese Breast
Cancer Society classified ≥4 positive node as n1β and ≤3 positive
nodes as n1α prior to the initiation of this study. Patients with
≥4 positive nodes were considered to be a high-risk patient
population compared to those who were node-negative or had ≤3
positive nodes. The International (Ludwig) Breast Cancer Study
Group also demonstrated that patients with ≥4 positive nodes were
at high risk for bone metastasis and may thus benefit from
preventive treatment against bone metastasis with bisphosphonate
(18).
The St. Gallen expert consensus meeting classified
≥4 nodes in axilla as a high-risk group (19). With respect to the adjuvant therapy
regimen, in the study by Saarto, premenopausal patients received
chemotherapy with one regimen consisting of six cycles of CMF. On
the other hand, postmenopausal patients received only endocrine
therapy consisting of tamoxifen or tremifen, including hormonal
receptor-negative patients. In an in vivo model of a human
breast cancer cell line, bisphosphonate without anti-cancer drugs
decreased tumor burden in the bone, but increased tumor
accumulation in soft-tissue organs (20). Thus, an increase in non-osseous
metastases may occur in patients who do not receive effective
adjuvant therapy. In the present study, patients received standard
adjuvant chemotherapy. Hormonal receptor status was also taken into
account in decisions regarding endocrine therapy.
We used 45 mg of pamidronate every 2 weeks for a
total of 4 infusions in this prevention study. Pamidronate is a
potent bisphosphonate and has a long half-life in the bone (at
least 300 days) (21). Furthermore,
the administration of intravenous pamidronate is more effective
than that of oral bisphosphonates. The selection was based on the
findings, from the treatment of bone metastases, that almost all
clinical effects had been obtained with only 4 infusions of
pamidronate (45 mg). The possibility of adverse effects with
over-administration, especially the long-term suppression of bone
turnover in disease-free patients was also considered. It is not
known whether long-term prophylactic administration is effective in
the prevention of the development of bone metastases. Adjuvant
systemic chemotherapy is usually performed with short-term
administration. If 4 infusions of 45 mg pamidronate were to have a
beneficial effect, this would greatly impact the bisphosphonate
studies. Patients would therefore obtain not only direct cost
benefits, but also indirect effects in terms of quality of
life.
Gnant et al recently reported on an adjuvant
zoledronic acid study in 1801 premenopausal endocrine-positive
patients at the June 2008 ASCO meeting (Chicago, IL) (22). Zoledronic acid has the strongest
inhibitory activity against bone resorption and shows direct
anti-tumor activity and immune activation (γδT cell proliferation).
These patients were randomized to goserelin with tamoxifen or
anastrozole plus intravenous zoledronic acid (4 mg every 6 months)
for 3 years. With a median follow-up of 60 months, disease-free
survival was significantly higher in the zoledronic acid group than
in the group administered with endocrine therapy alone (hazard
ratio = 0.64; p=0.01). The addition of zoledronic acid
significantly reduced the risk of relapse-free survival events by
35% (p=0.015) compared with that of endocrine therapy alone. For
overall survival, there was a non-significant trend favoring
zoledronic acid treatment (hazard ratio = 0.60; p=0.10). These
results were considered to be similar to our results by mechanism
of potent bisphosphonate.
An ongoing definitive adjuvant clodronate study,
NSABP B-34, has randomized early breast cancer patients to a
clodronate (3 years) or placebo group. Adjuvant chemotherapy and/or
hormonal therapy have been applied. Other ongoing trials include
the AZURE and SWOG (0307)/intergroup trials. The AZURE trial aims
to evaluate the effect of adjuvant zoledronic acid (zoledronic acid
administration for 5 years). The SWOG (0307)/intergroup trial will
compare intravenous zoledronic acid with oral clodronate and
risedronate (3 years). The ASCO guideline states that an optimal
agent, dose, route of therapy, schedule and duration of therapy of
bisphosphonate for the prevention of bone metastases in breast
cancer patients remain unknown. Our study was a small and
non-randomized trial. However, its findings have encouraged further
investigation in a large population with a view to confirming these
results. At present, zoledronic acid is the most potent
bisphosphonate. Thus, we plan to perform a larger randomized study
of zoledronic acid for the prevention of the development of bone
metastases in breast cancer patients at high risk for bone
metastasis. Further investigations will confirm the significant
effects of adjuvant bisphosphonate therapy in patients at high risk
for bone metastasis.
References
|
1
|
Rodan GA and Fleisch HA: Bisphosphonates:
mechanisms of action. J Clin Invest. 97:2692–2696. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hortobagyi GN, Theriault RL, Porter L,
Blayney D, Lipton A, Sinoff C, Wheeler H, Simeone JF, Seaman J and
Knight RD: Efficacy of pamidronate in reducing skeletal
complications in patients with breast cancer and lytic bone
metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J
Med. 335:1785–1791. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hughes DE, Wright KR, Uy HL, Sasaki A,
Yoneda T, Roodman GD, Mundy GR and Boyce BF: Bisphosphonates
promote apoptosis in murine osteoclasts in vitro and in vivo. J
Bone Miner Res. 10:1478–1487. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jagdev SP, Coleman RE, Shipman CM, Rostami
HA and Croucher PI: The bisphosphonate, zoledronic acid induces
apoptosis of breast cancer cells: evidence for synergy with
paclitaxel. Br J Cancer. 84:1126–1134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crohns CA, Untch M and Konecny G:
Different bisphosphonates have direct cytotoxic effects on three
breast cancer cell lines and fresh breast cancer tumor tissue. Proc
Am Soc Clin Oncol. 20:abs. 20052001.
|
|
6
|
Santini D, Vespasiani Gentilucci U,
Vincenzi B, Picardi A, Vasaturo F, La Cesa A, Onori N, Scarpa S and
Tonini G: The antineoplastic role of bisphosphonates: from basic
research to clinical evidence. Ann Oncol. 14:1468–1476. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teronen O, Heikkila P, Konttinen YT,
Laitinen M, Salo T, Hanemaaijer R, Teronen A, Maisi P and Sorsa T:
MMP inhibition and down-regulation by bisphosphonates. Ann NY Acad
Sci. 878:453–465. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paterson AH: The potential role of
bisphosphonates as adjuvant therapy in the prevention of bone
metastases. Cancer. 88:3038–3046. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasaki A, Boyce BF, Story B, Wright KR,
Chapman M, Boyce R, Mundy GR and Yoneda T: Bisphosphonate
risedronate reduces metastatic human breast cancer burden in bone
in nude mice. Cancer Res. 55:3551–3557. 1995.PubMed/NCBI
|
|
10
|
Van der Pluijm G, Vloedgraven H, Van Beek
E, Van der Wee-Pals L, Lowik C and Papapoulos S: Bisphosphonates
inhibit the adhesion of breast cancer cells to bone matrices in
vitro. J Clin Invest. 98:698–705. 1996.
|
|
11
|
Diel IJ, Solomayer EF, Costa SD, Gollan C,
Goerner R, Wallwiener D, Kaufmann M and Bastert G: Reduction in new
metastases in breast cancer with adjuvant clodronate treatment. N
Engl J Med. 339:357–363. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diel IJ, Solomayer EF, Gollan C, Schutz F
and Bastert G: Bisphosphonates in the reduction of metastases in
breast cancer - results of the extended follow-up of the first
study population. Proc Am Soc Clin Oncol. 19:abs. 3142000.
|
|
13
|
Jaschke A, Bastert G, Solomayer EF, Costa
S, Schuetz F and Diel IJ: Adjuvant clodronate treatment improves
the overall survival of primary breast cancer patients with
micrometastases to bone marrow - a longtime follow-up. J Clin
Oncol. 22:abs. 5292004.
|
|
14
|
Powles T, Paterson S, Kanis JA, McCloskey
E, Ashley S, Tidy A, Rosenqvist K, Smith I, Ottestad L, Legault S,
Pajunen M, Nevantaus A, Mannisto E, Suovuori A, Atula S, Nevalainen
J and Pylkkanen L: Randomized placebo-controlled trial of
clodronate in patients with primary operable breast cancer. J Clin
Oncol. 20:3219–3224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Powles T, Paterson A, McCloskey E,
Kurkilahti M and Kanis J: Oral clodronate for adjuvant treatment of
operable breast cancer: results of a randomized, double-blind,
placebo-controlled multicenter trial. J Clin Oncol. 22:abs.
5282004.PubMed/NCBI
|
|
16
|
Saarto T, Blomqvist C, Virkkunen P and
Elomaa I: Adjuvant clodronate treatment does not reduce the
frequency of skeletal metastases in node-positive breast cancer
patients: 5-year results of a randomized controlled trial. J Clin
Oncol. 19:10–17. 2001.PubMed/NCBI
|
|
17
|
Saarto T, Vehmanen L, Blomqvist C and
Elomaa I: Ten-year follow-up of a randomized controlled trial of
adjuvant clodronate treatment in node-positive breast cancer
patients. J Clin Oncol. 22:abs. 5272004.PubMed/NCBI
|
|
18
|
Colleoni M, O’Neill A, Goldhirsch A,
Gelber RD, Bonetti M, Thurlimann B, Price KN, Castiglione-Gertsch
M, Coates AS, Lindtner J, Collins J, Senn HJ, Cavalli F, Forbes J,
Gudgeon A, Simoncini E, Cortes-Funes H, Veronesi A, Fey M and
Rudenstam CM: Identifying breast cancer patients at high risk for
bone metastases. J Clin Oncol. 18:3925–3935. 2000.PubMed/NCBI
|
|
19
|
Goldhirsch A, Wood WC, Gelber RD, Coates
AS, Thurlimann B and Senn HJ: Progress and promise: highlights of
the international expert consensus on the primary therapy of early
breast cancer 2007. Ann Oncol. 18:1133–1144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michiqami T, Wiliams P and Dallas M:
Bisphosphonate may increase metastases to soft tissue sites in
breast cancer and B-cell lymphoma. J Bone Miner Res. 12(Suppl 1):
s106abs 115. 1997.
|
|
21
|
Leyvraz S, Hess U, Flesch G, Bauer J,
Hauffe S, Ford JM and Burckhardt P: Pharmacokinetics of pamidronate
in patients with bone metastases. J Natl Cancer Inst. 84:788–792.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gnant M, Mlineritsch B, Schippinger W,
Luschin-Ebengreuth G, Poestlberger S, Menzel C, Jakesz R, Kubista
E, Marth C and Greil R; on behalf of the ABCSG. Adjuvant ovarian
suppression combined with tamoxifen or anastrozole, alone or in
combination with zoledronic acid, in premenopausal women with
hormone-responsive, stage I and II breast cancer: First efficacy
results from ABCSG-12. J Clin Oncol. 26:abs. LBA 42008.
|