Introduction
Bevacizumab is a humanized monoclonal antibody
against vascular endothelial growth factor (VEGF) (1,2). In
Japan, the use of bevacizumab alongside chemotherapy was approved
in 2007 for the treatment of unresectable advanced CRC patients.
Subsequently, clinical trials of bevacizumab have been undertaken
in combination with chemotherapy, such as 5-fluorouracil (5-FU) and
leucovorin (LV), 5-FU and LV (5-FU/LV) plus oxaliplatin (FOLFOX),
5-FU/LV plus ilinotecan (FOLFIRI) and capecitabine plus oxaliplatin
(XELOX) (3,4). For tailored individualized therapy,
many attempts have been made to identify predictive biomarkers to
help select those patients that will benefit from targeted agents
such as the association between the KRAS mutation status and
survival outcomes in patients with metastatic CRC treated with
cetuximab (5). For bevacizumab,
however, no established predictive biomarkers have been identified
which are associated with either treatment response or survival in
patients with advanced CRC (3,6).
VEGF and its receptors are essential for the
neovascularization of cancer. Numerous studies have indicated that
VEGF expression in tumor specimens is correlated with microvessel
density, metastasis, tumor growth and poor prognosis in a variety
of human solid cancer types including colorectal cancer (CRC)
(7,8). High preoperative serum or plasma VEGF
concentrations may predict poor prognosis in patients with CRC
(9,10). However, the values of VEGF levels as
biomarkers of anti-angiogenic therapy have yet to be established
and require further evaluation (5,11–13).
The VEGF family consists of related homodimeric glycoproteins,
including VEGF-A (also called VEGF), -B, -C, -D and -E. It is known
that VEGF-A binds to two types of cell membrane receptors: VEGF
(VEGFR)-1 and VEGFR-2, located in the endothelium. Moreover, VEGF-A
stimulates endothelial migration, proliferation, permeability and
survival (14,15). In addition to these receptors,
circulating soluble forms of VEGFR-1 (sVEGFR-1) and VEGFR-2
(sVEGFR-2) have attracted attention as potential biomarkers of
various malignancies. The sVEGFR-1 has been examined both as a
potential surrogate marker for disease progression and/or as a
potential inhibitor of tumor angiogenesis in colon, breast and
renal cell carcinoma (16–21). However, the clinical significance of
plasma sVEGFR-1 and sVEGFR-2 levels as biomarkers of
anti-angiogenic therapy combined with chemotherapy have yet to be
sufficiently investigated.
The present study aimed to evaluate the predictive
value of plasma VEGF-A, sVEGFR-1 and sVEGFR-2 levels as biomarkers
for clinical response and survival in unresectable advanced CRC
patients treated with bevacizumab and modified FOLFOX 6 (mFOLFOX6)
as a first-line therapy.
Materials and methods
Patients and study treatment blood
samples
Forty-six unresectable advanced CRC patients (TNM
stage IV) and 20 healthy controls were enrolled in this study. The
patients were treated with bevacizumab and mFOLFOX6 as a first-line
therapy between 2007 and 2009. Bevacizumab was administered at a
dosage of 5 mg/kg on day 1 of every two-week period. The regimen of
mFOLFOX6 was: oxaliplatin 85 mg/m2 on day 1 and 5-FU/LV
(LV 200 mg/m2 on day 1, 5-FU 400 mg/m2 on day
1 and 5-FU 2,400 mg/m2 continuous infusion on days 1 and
2). The median number of cycles of bevacizumab and mFOLFOX6 were
10. Patients were treated until disease progression, development of
unacceptable toxicity or patient refusal. Peripheral blood was
obtained from each patient before the treatment of bevacizumab
combined with mFOLFOX6. Informed written consent was obtained from
patients included in the study.
VEGFR-A, sVEGFR-1 and sVEGFR-2
measurements
Plasma samples were collected from the peripheral
blood of each patient by centrifugation and stored at −80°C until
use. VEGF-A, sVEGFR-1 and sVEGFR-2 plasma levels were measured
using the multiplex human immunoassay kit and the multiplex human
soluble cytokine receptor panel kit (both from Millipore Co., MA,
USA). Measurements were performed as follows: each 96-well filter
plate was washed with 200 μl wash buffer, followed by filtration
under a vacuum. The standard and control were added into
appropriate wells, followed by 25 μl assay buffer, 25 μl samples
and matrix solution. The bead mix was diluted in wash buffer, and
25 μl of the mix were added to each well. The plates were
maintained at 4°C overnight. The following day, the medium was
vacuum-filtered, and 25 μl detection antibody was added to each
well. The plates were incubated for 1 h at room temperature (RT).
Streptavidin-phycoerythin (25 μl) was added to each well and
incubated for 30 min at RT. The wells were washed twice with 200 μl
wash buffer, and 150 μl sheath fluid was added. The plates were
read on a Luminex 200™ (Millipore Co.), and data were analyzed by
xPONENT and Milliplex analyst software. The samples were examined
in duplicate.
Assessment of efficacy
Tumor responses were assessed every 4–6 weeks using
RECIST criteria and classified into four groups: complete response
(CR), partial response (PR), stable disease (SD) and progressive
disease (PD). Response rates were calculated by the number of
patients with CR or PR.
Statistical analysis
Plasma VEGF-A, sVEGFR-1 and sVEGFR-2 levels between
the advanced CRC patients and healthy controls were analyzed by the
Student’s t-test. Differences in the markers between the clinical
responses were examined with analysis of variance and
multi-comparison tests. The correlation between VEGF-A, sVEGFR-1
and sVEGFR-2 and the clinicopathological parameters was evaluated
using Fisher’s exact and the Chi-square tests. Progression-free
(PFS) and overall survival (OS) curves were analyzed using the
Kaplan-Meier method, and the differences were examined using
log-rank tests. Univariate and multivariate analyses were performed
using Cox proportional hazard regression analysis. The tests were
analyzed using JMP software (SAS Institute Inc., Cary, NC, USA).
Statistical significance was determined from two-sided tests as
p<0.05.
Results
Patient characteristics
Table I shows the
characteristics of the patients in this study. The median age was
63 years (range 49–77), with 33 male (71.7%) and 13 female (28.3%)
patients. The patients had a good performance status (ECOG PS0). As
primary tumor sites, 32 patients (69.6%) had colon cancer and 14
patients (30.4%) had rectal cancer. The evaluable tumor sites for
treatment were the liver (22 patients; 47.8%), lung (17 patients;
34.1%), lymph node (15 patients; 32.6%), omentum (14 patients;
30.4%), local (6 patients; 13.0%) and/or bone metastasis (1
patient; 2.2%). Serum CEA-positive patients numbered 35 (76.1%),
and there were 16 serum CA19-9-positive patients (34.8%).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| VEGF-A levels |
|---|
|
|
|---|
| Characteristics of
the patients (n=46) | No. of patients | Percent |
|---|
| Median age
(range) | 63 (49–77) | |
| Gender |
| Male | 33 | 71.7 |
| Female | 13 | 28.3 |
| Primary sites |
| Colon | 32 | 69.6 |
| Rectum | 14 | 30.4 |
| Site of
metastasisa |
| Liver | 22 | 47.8 |
| Lung | 17 | 34.1 |
| Lymph node | 15 | 32.6 |
| Omentum | 14 | 30.4 |
| Local | 6 | 13.0 |
| Bone | 1 | 2.2 |
| Serum CEA |
| CEA (+) | 35 | 76.1 |
| CEA (−) | 11 | 23.9 |
| Serum CA19-9 |
| CA19-9 (+) | 16 | 34.8 |
| CA19-9 (−) | 30 | 65.2 |
Comparison of VEGF-A, sVEGFR-1 and
sVEGFR-2 levels in CRC patients and healthy controls
Table II shows the
comparison of plasma VEGF-A, sVEGFR-1 and sVEGFR-2 levels in
patients with metastatic CRC and healthy controls. Plasma VEGF-A,
sVEGFR-1 and sVEGFR-2 levels were significantly higher in patients
with metastatic CRC than in the healthy controls.
 | Table IIVEGF-A, sVEGFR-1 and sVEGFR-2 levels
of CRC patients and healthy controls. |
Table II
VEGF-A, sVEGFR-1 and sVEGFR-2 levels
of CRC patients and healthy controls.
| No. | Median (pg/ml) | Average ± SD
(pg/ml) | P-value |
|---|
| VEGF-A | | | | 0.004 |
| Controls | 20 | 54.4 | 68.9±69.2 | |
| Patients | 46 | 158.0 | 194.0±178.6 | |
| sVEGFR-1 | | | | 0.048 |
| Controls | 20 | 334.9 | 375.6±200.5 | |
| Patients | 46 | 610.5 | 668.3±661.5 | |
| sVEGFR-2 | | | | <0.001 |
| Controls | 20 | 10938.8 | 10665.7±3207.4 | |
| Patients | 46 | 17800.5 | 17296.6±3987.0 | |
Plasma VEGF-A, sVEGFR-1 and sVEGFR-2
levels and clinical response
In this study, the patients received bevacizumab
plus mFOLFOX6 as a first-line therapy. After treatment, 1 patient
achieved CR, 10 patients had PR, 17 patients had SD and 18 patients
had PD. The overall response rate was 23.9% (11/46). Table III shows the association between
the plasma VEGF-A, sVEGFR-1 and sVEGFR-2 levels and clinical
responses. Regarding the VEGF-A levels, there were no significant
differences between the responder, SD and PD groups. In contrast,
the average level of sVEGFR-1 was 197.8±46.8 pg/ml in the responder
patients, 470.7±583.9 pg/ml in the SD patients and 906.2±694.7
pg/ml in the PD patients. Significant differences were noted
between the CR/PR vs. PD group (p=0.025), and the SD vs. PD group
(p=0.032). No significant differences were noted when sVEGFR-2
levels and clinical responses were compared. These results suggest
that sVEGFR-1 levels show a significant relationship with the
clinical response.
 | Table IIIPlasma VEGF-A, sVEGR-1 and sVEGFR-2
levels and clinical response. |
Table III
Plasma VEGF-A, sVEGR-1 and sVEGFR-2
levels and clinical response.
| No. of
patients | Median (pg/ml) | Average ± SD
(pg/ml) | P-value |
|---|
| VEGF-A |
| CR/PR | 11a | 186.0 | 156.4±71.5 | 0.300b |
| SD | 17 | 106.0 | 144.5±140.9 | 0.073c |
| PD | 18 | 214.2 | 249.5±214.9 | |
| sVEGFR-1 |
| CR/PR | 11a | 172.0 | 197.8±46.8 | 0.025b |
| SD | 17 | 250.0 | 470.7±583.9 | 0.032c |
| PD | 18 | 860.0 | 906.2±694.7 | |
| sVEGFR-2 |
| CR/PR | 11a | 16010.0 | 16148.9±2273.4 | 0.486b |
| SD | 17 | 17210.0 | 17276.8±5780.3 | 0.587c |
| PD | 18 | 18035.5 | 17549.7±2570.2 | |
Association of patient characteristics
and VEGF-A, sVEGFR-1 and sVEGFR-2 levels
To examine the association of patient
characteristics and VEGF-A, sVEGFR-1 and sVEGFR-2 levels, patients
were divided into two groups (a higher level and a lower level
group) by the setting of a cut-off based on median levels (Table IV). The median levels of VEGF-A,
sVEGFR-1 and sVEGFR-2 were 165.0 pg/ml, 327.5 pg/ml and 17800.5
pg/ml, respectively (data not shown). No statistically significant
differences were noted in the patient characteristics and VEGF-A,
sVEGFR-1 and sVEGFR-2 levels.
 | Table IVAssociation of patient
characteristics and VEGF-A, sVEGFR-1 and sVEGFR-2 levels. |
Table IV
Association of patient
characteristics and VEGF-A, sVEGFR-1 and sVEGFR-2 levels.
| VEGF-A levels | sVEGFR-1
levels | sVEGFR-2
levels |
|---|
|
|
|
|
|---|
| Characteristics of
the patients (n=46) | High (n=24) | Low (n=22) | P-value | High (n=23) | Low (n=23) | P-value | High (n=23) | Low (n=23) | P-value |
|---|
| Median age
(range) | 61.0 (46–77) | 64.5 (49–76) | 0.411 | 62.0 (46–77) | 64.0 (50–74) | 0.622 | 61.0 (46–74) | 65.0 (52–76) | 0.163 |
| Gender |
| Male | 20 (83.3) | 14 (63.6) | 0.129 | 17 (73.9) | 17 (73.9) | 1.000 | 19 (82.6) | 15 (65.2) | 0.179 |
| Female | 4 (16.7) | 8 (36.4) | | 6 (26.1) | 6 (26.1) | | 4 (17.4) | 8 (34.8) | |
| Primary sites |
| Colon | 19 (79.2) | 15 (68.2) | 0.397 | 19 (82.6) | 15 (65.2) | 0.179 | 19 (82.6) | 15 (65.2) | 0.179 |
| Rectum | 5 (20.8) | 7 (31.8) | | 4 (17.4) | 8 (34.8) | | 4 (17.4) | 8 (34.8) | |
| Serum CEA |
| CEA (+) | 19 (79.2) | 16 (72.7) | 0.609 | 17 (73.9) | 18 (78.3) | 0.730 | 17 (73.9) | 18 (78.3) | 0.730 |
| CEA (−) | 5 (20.8) | 6 (27.3) | | 6 (26.1) | 5 (21.7) | | 6 (26.1) | 5 (21.7) | |
| Serum CA19-9 |
| CA19-9 (+) | 6 (25.0) | 10 (45.5) | 0.146 | 7 (30.4) | 9 (39.1) | 0.536 | 8 (34.8) | 8 (34.8) | 1.000 |
| CA19-9 (−) | 18 (75.0) | 12 (54.5) | | 16 (69.6) | 14 (60.9) | | 15 (65.2) | 15 (65.2) | |
Plasma VEGF-A, sVEGFR-1 and sVEGFR-2
levels and survival
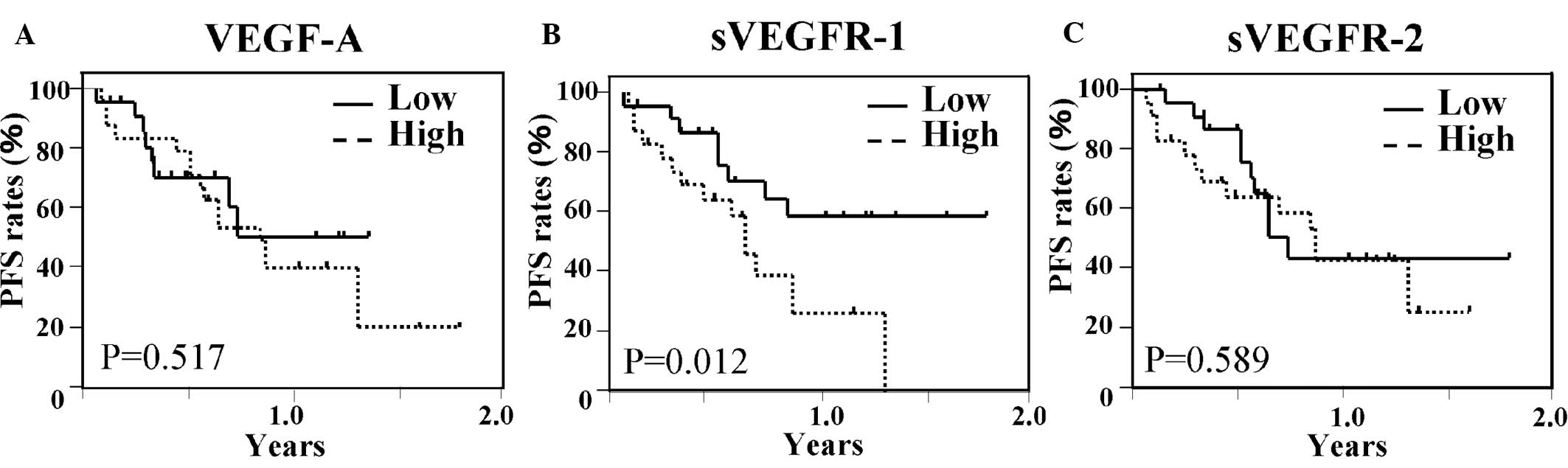
Fig. 1 shows the
Kaplan-Meier PFS curves according to the status of plasma VEGF-A,
sVEGFR-1 and sVEGFR-2 levels. In a comparative analysis based on
the levels of sVEGFR-1, significant differences were noted between
patients with higher and those with lower levels. In contrast, in
the analysis of VEGF-A and sVEGR-2, no significant differences were
found between patients with higher and those with lower levels. OS
curves according to the status of plasma VEGF-A, sVEGFR-1 and
sVEGFR-2 levels were then examined (Fig. 2). Patients with higher sVEGFR-1
levels showed a significantly poorer OS than those with lower
VEGFR-1 levels. An analysis of VEGF-A and sVEGFR-2 showed no
significant differences between patients with higher and those with
lower levels of sVEGFR-1. These results suggest that sVEGFR-1
levels are significantly associated with the PFS and OS of patients
treated with bevacizumab and mFOLFOX6.
Multivariate analysis of biological
factors for survival
Table V shows the
multivariate analysis of biological factors for PFS. In this
analysis, sVEGFR-1 levels showed a significant relationship with
PFS. Table VI shows the
multivariate analysis of biological factors for OS, and sVEGFR-1
levels showed a significant relationship with OS. In the analysis
of clinicopathological factors of patients and survival, there was
no significant relationship with PFS and OS (data not shown).
 | Table VMultivariate analysis of biological
factors for PFS. |
Table V
Multivariate analysis of biological
factors for PFS.
| Factors | Multivariate
analysis for PFS |
|---|
|
|
|---|
| Regression
coefficient | Hazard ratio (95%
CI) | P-value |
|---|
| VEGF-A | 0.364 | 1.439
(0.505–4.303) | 0.496 |
| sVEGFR-1 | 1.119 | 3.063
(1.189–8.520) | 0.021 |
| sVEGFR-2 | −0.143 | 0.866
(0.343–2.184) | 0.758 |
| Serum CEA | −0.222 | 0.861
(0.277–2.620) | 0.695 |
| Serum CA19-9 | 0.849 | 2.338
(0.736–8.105) | 0.151 |
 | Table VIMultivariate analysis of biological
factors for OS. |
Table VI
Multivariate analysis of biological
factors for OS.
| Factors | Multivariate
analysis for OS |
|---|
|
|
|---|
| Regression
coefficient | Hazard ratio (95%
CI) | P-value |
|---|
| VEGF-A | −0.291 | 0.747
(0.103–3.341) | 0.698 |
| sVEGFR-1 | 1.216 | 1.824
(0.654–5.604) | 0.040 |
| sVEGFR-2 | −0.356 | 0.701
(0.140–3.171) | 0.254 |
| Serum CEA | −0.165 | 0.848
(0.229–4.018) | 0.816 |
| Serum CA19-9 | −0.356 | 0.701
(0.140–3.171) | 0.647 |
These results suggest that plasma sVEGFR-1 levels
are a useful prognostic indicator for PFS and OS for advanced CRC
patients treated with bevacizumab and mFOLFOX6.
Discussion
This study showed that plasma sVEGFR-1 levels, but
not VEGF-A, and sVEGFR-2, are associated with clinical response and
survival in advanced CRC patients treated with bevacizumab and
mFOLFOX6.
It has been well established that VEGF is a key
mediator of tumor vascularization. There is growing recognition of
the central role that the VEGF family plays in angiogenesis, the
formation of new blood vessels, which is necessary for the growth
and spread of a tumor (22). The
VEGF family consists of seven members, VEGF-A (also called VEGF),
-B, -C, -D, -E, -F and placental growth factor (PIGF), which share
eight cytokine residues in a VEGF homology domain (23). VEGF-A, particularly the VEGF165 and
VEGF121 isoforms, plays an integral role in tumor angiogenesis both
as an activator and survival factor in endothelial cells.
Circulating plasma VEGF levels have been studied as a possible
surrogate marker of angiogenesis in numerous malignancies (24). However, the applicability of plasma
VEGF levels to predict the response and survival of patients
treated with bevacizumab and chemotherapy has yet to be
sufficiently proven. Burstein et al reported that lower
levels of plasma VEGF were associated with longer time to
progression in advanced breast cancer patients receiving
bevacizumab and vinorelbine chemotherapy (25). In contrast, Denduluri et al
reported that baseline levels of plasma VEGF in breast cancer
patients did not predict clinical response to bevacizumab (12). In our study, pretreatment plasma
VEGF-A levels in patients receiving bevacizumab with mFOLFOX6 did
not show any significant relationship between clinical response and
survival. Notably, Holden et al reported that in their
retrospective analysis of 398 metastatic CRC patients, the survival
benefit associated with bevacizumab was independent of pretreatment
plasma VEGF levels (26). Since
VEGF is only one of the markers of anti-angiogenesis, its
significance in clinical response and survival may be asserted
through several different pathways.
It is known that sVEGFR-1 and sVEGFR-2 are generated
either via proteolytic cleavage of the ectodomain from the cell
surface or via alternative mRNA splicing, which gives rise to a
secreted polypeptide lacking a transmembrane region and functioning
as a high-affinity receptor of VEGF. Expression of VEGFRs,
including VEGFR-1 and VEGFR-2 (both of which are expressed in a
number of tumor cell types in addition to endothelial cells), has
been correlated with various disease stages (27–29).
It has previously been shown that circulating levels of soluble
forms of these receptors, which are not capable of signal
transduction, bind VEGF in the bloodstream and reduce the levels of
free VEGF. This limits the pro-angiogenic effects of VEGF at the
endothelial cell level (1,30). In particular, sVEGFR-1 has been
studied, not only as a potential surrogate marker for disease
progression, but also as a potential inhibitor of tumor
angiogenesis in various types of cancers (5,31).
Previous studies have shown that sVEGFR-1 and/or sVEGFR-2 levels in
plasma were higher in cancer patients than in healthy volunteers
(21,32,33).
Our present data also demonstrated that plasma sVEGFR-1 and
sVEGFR-2 levels of advanced CRC patients are significantly higher
than those of healthy volunteers. Toi et al (18) and Yamaguchi et al (20) reported that sVEGFR-1 levels in tumor
tissue were an independent prognostic indicator of disease
progression in CRC patients. However, the predictive values of
plasma sVEGFR-1 and sVEGFR-2 levels for chemotherapy responses are
still controversial. Ustuner et al reported that no
significant differences were detected between the concentration of
serum sVEGFR-1 and sVEGR-2 and chemotherapy response in small cell
lung cancer patients (34). In
contrast, Wierzbowska et al reported correlations between
the pretreatment plasma VEGFR-1 concentration, tumor burden and
poor prognosis in acute myeloid leukemia (AML) patients (32). Additionally, the serum VEGFR-1/VEGF
ratio had a greater prognostic value than VEGF alone in their
study. Hu et al also reported that plasma sVEGFR-1, but not
sVEGFR-2, was an independent prognostic factor in AML and
myelodysplastic syndromes (21).
Little is currently known regarding the potential of plasma
sVEGFR-1 and sVEGFR-2 levels as a predictive biomarker for
treatment response and survival in CRC patients treated with
bevacizumab-based therapy. Our results in CRC patients indicated
that the pretreatment level of sVGEFR-2 showed no association with
clinical response and survival. This is in line with previous
reports that failed to detect a predictive marker for bevacizumab
with chemotherapy. Nevertheless, our data showed that plasma
sVEGFR-1 levels predicted the treatment response and survival in
advanced CRC patients treated with bevacizumab and mFOLFOX6. To the
best of our knowledge, the present study is the first to suggest
the predictive value of sVEGFR-1 for clinical response and survival
in advanced CRC patients treated with bevacizumab and mFOLFOX6. It
has been documented that the plasma sVEGFR-1 level is related to
tumor phenotype or prognosis, suggesting that sVEGFR-1 has a
significant biological function in tumor cells (30). Although it is difficult to elucidate
the correlation between the plasma sVEGFR-1 levels and clinical
response and survival, plasma sVEGFR-1 levels may reflect tumor
malignancy and predict tumor progression in the metastatic site.
Notably, Willett et al reported that pretreatment sVEGFR-1
levels in patients with rectal cancer were correlated with
post-treatment tumor stage after combination therapy with
bevacizumab, radiation and chemotherapy (11). These results support the possible
predictive value of plasma sVEGFR-1 levels in CRC patients treated
with bevacizumab-based chemotherapy. We await further studies that
may elucidate, in detail, the association between plasma sVEGFR-1
and clinical response and survival.
Although there were limitations to the present study
due to the small sample size and the fact that it was a single-arm
study, we believe that our findings warrant the further evaluation
of plasma sVEGFR-1 as a predictive marker for clinical response and
survival in metastatic CRC patients. Larger scale studies are
needed to further validate our results.
Acknowledgements
We thank Ms. J. Tamura for her excellent technical
support. This work was supported by a Grant-in-Aid for Scientific
Research (C) (21591734).
Abbreviations:
|
VEGF
|
vascular endothelial growth factor
|
|
sVEGFR-1
|
soluble vascular endothelial growth
factor receptor-1
|
|
sVEGFR-2
|
soluble vascular endothelial growth
factor receptor-2
|
|
CRC
|
colorectal cancer
|
References
|
1
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hurwits H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwasaki J and Nihira S: Anti-angiogenic
therapy against gastrointestinal tract cancers. Jpn J Clin Oncol.
16:1–9. 2009.
|
|
4
|
Yamaguchi K, Boku N, Kato K, Komatsu Y,
Muro K and Hamamoto Y: Preliminary efficacy, safety and operability
of bevacizumab plus capecitabine plus oxaliplatin (XELOX) as
first-line therapy in Japanese patients with initially unresectable
metastatic colorectal cancer. Ann Oncol. 19:viii1302008.
|
|
5
|
Karapetis CS, Khambata FS, Jonker DJ, et
al: K-ras mutations and benefit from cetuximab in advanced
colorectal cancer. N Engl J Med. 359:1757–1765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Willett CG, Boucher Y, Duda DG, et al:
Surrogate markers for antiangiogenic therapy and dose-limiting
toxicities for bevacizumab with radiation and chemotherapy:
continued experience of a Phase I trial and rectal cancer patients.
J Clin Oncol. 22:8136–8139. 2005. View Article : Google Scholar
|
|
7
|
Galizia G, Lieto E, Ferraraccio F, et al:
Determination of molecular marker expression can predict clinical
outcome in colon carcinoma. Clin Cancer Res. 10:3490–3499. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity metastasis and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
9
|
Werther K, Christensen IJ and Nielsen HJ:
Prognostic impact of matched preoperative plasma and serum VEGF in
patients with primary colorectal carcinoma. Br J Cancer.
86:417–423. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Broll R, Erdmann H, Duchrow M, et al:
Vascular endothelial growth factor (VEGF) – a valuable serum tumor
marker in patients with colorectal cancer? Eur J Surg Oncol.
27:37–42. 2001.
|
|
11
|
Willett CG, Boucher Y, Duda DG, et al:
Efficacy, safety and biomarkers of neoadjuvant bevacizumab,
radiation therapy and fluorouracil in rectal cancer: a
multidisciplinary phase II study. J Clin Oncol. 18:3020–3026. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Denduluri N, Yang SX, Berman AW, Nguyen D,
Liewehr DJ, Steinberg SM and Swalin SM: Circulating biomarkers of
bevacizumab activity in patients. Cancer Biol Ther. 7:15–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrara N and Alitalo K: Clinical
application of angiogenic growth factors and their inhibitors. Nat
Med. 5:1359–1364. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shibuya M: Structure and function of
VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct
Func. 26:25–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kendall RL and Thomas KA: Inhibition of
vascular endothelial cell growth factor activity by an endogenously
encoded soluble receptor. Proc Natl Acad Sci USA. 90:10705–10709.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kendall RL, Wang G and Thomas KA:
Identification of a natural soluble form of the vascular
endothelial growth factor receptor, FLT-1, and its
heterodimerization with KDR. Biochem Biophys Res Commun.
226:324–328. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harris AL, Reusch P, Barlenon B, Hang C,
Dobbs N and Marme D: Soluble Tie2 and Flt1 extracellular domains in
serum of patients with renal cancer and response to antiangiogenic
therapy. Clin cancer Res. 7:1992–1997. 2001.PubMed/NCBI
|
|
18
|
Toi M, Bando H, Ogawa T, Muta M, Horing C
and Weich HA: Significance of vascular endothelial growth factor
(VEGF)/soluble VEGF receptor-1 relationship in breast cancer. Int J
Cancer. 98:14–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tas F, Duranyildiz D, Oguz H, Camlica H,
Yasasever V and Topuz E: Circulating serum levels of angiogenic
factors and vascular endothelial growth factor receptors 1 and 2 in
melanoma patients. Melanoma Res. 16:405–411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamaguchi T, Nando H, Mori T, et al:
Overexpression of soluble vascular endothelial growth factor
receptor 1 in colorectal cancer: association with progression and
prognosis. Cancer Sci. 98:405–410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Q, Dey AL, Yang Y, et al: Soluble
vascular endothelial growth factor receptor 1, and not receptor 2,
is an independent prognostic factor in acute myeloid leukemia and
myeloidysplastic syndromes. Cancer. 100:1884–1891. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hormbrey E, Gillespie P, Turner K, Han C,
Roberts A, McGrouther D and Harris AL: A critical review of
vascular endothelial growth factor (VEGF) analysis in peripheral
blood: is the current literature meaningful? Clin Exp Metastasis.
19:651–663. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Otrock ZK, Makarem JA and Shamseddine AI:
Vascular endothelial growth factor family of ligands and receptors:
review. Blood Cells Mol Dis. 38:258–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Poon RT, Fan ST and Wong J: Clinical
implication of circulating angiogenic factors in cancer patients. J
Clin Oncol. 19:1207–1225. 2001.PubMed/NCBI
|
|
25
|
Burstein A, Chen Y-H and Parker LM: VEGF
as a marker for outcome among advanced breast cancer patients
receiving anti-VEGF therapy with bevacizumab and vinorelbine
chemotherapy. Clin Cancer Res. 14:7871–7877. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holden SN, Ryan E, Kearns A, Holmgren E
and Hurwitz H: Benefit from bevacizumab (BV) is independent of
pretreatment plasma vascular endothelial growth factor-A (pl-VEGF)
in patients (pts) with metastatic colorectal cancer (mCRC). J Clin
Oncol. 123:35552005.
|
|
27
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signaling – in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006.
|
|
28
|
Herold MC, Steiner HH, Andl T, et al:
Expression and functional significance of vascular endothelial
growth factor receptors in human tumor cells. Lab Invest.
79:1573–1582. 1999.PubMed/NCBI
|
|
29
|
Masood R, Cai J, Zheng T, Smith DL, Hinton
DR and Gill PS: Vascular endothelial growth factor (VEGF) is an
autocrine growth factor for VEGF receptor-positive human tumors.
Blood. 98:1904–1913. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holash J, Davis S, Papadopoulos N, et al:
VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl
Acad Sci USA. 99:11393–11398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wierzbowska A, Robak T, Wizesien-Kus A,
Krawczynska A, Maranda EL and Urbanska-Rys H: Circulating VEGF and
its soluble receptors sVEGFR-1 and sVEGFR-2 in patients with acute
leukemia. Eur Cytokine Netw. 14:149–153. 2003.PubMed/NCBI
|
|
33
|
AI-Moundhri M, Shukaili A, AI-Nabhani M,
AI-Bahrani B, Burney IA, Rozovo A and Ganguly SS: Measurement of
circulating levels of VEGF-A, -C and -D and their receptors,
VEGFR-1 and -2 in gastric adenocarcinoma. World Gastroenterol.
14:3879–3883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ustuner Z, Saip P, Yasasever V, et al:
Prognostic and predictive value of vascular endothelial growth
factor and its soluble reseptor VEGFR-1 and VEGFR-2 levels in the
sera of small cell lung cancer patients. Med Oncol. 25:394–399.
2008. View Article : Google Scholar : PubMed/NCBI
|