Introduction
The anticancer drug cisplatin (CDDP), which contains
platinum, is widely used for the treatment of solid tumors such as
testicular, ovarian, cervical, bladder, head and neck, as well as
non-small cell lung cancer (NSCLC) (1). CDDP has been a key drug in
chemotherapy against NSCLC for more than 20 years. However, the
overall response rate to cisplatin as a single agent against NSCLC
is no more than 20% (2).
Furthermore, the development of resistance to CDDP is common during
treatment of NSCLC patients, and is an important concern for
clinical oncologists. Thus, exploring chemoresistance markers is
significant. Mechanisms of CDDP resistance include a decrease in
drug accumulation and enhanced detoxification, but an increase in
DNA repair efficiency. However, studies are mainly limited to in
vitro or in vivo experiments and none of the examined
markers have been validated as common contributors to clinical CDDP
resistance or the prognosis of patients with NSCLC (3).
Copper-transporting P-type adenosine triphosphatase
(ATP7B) plays a key role in copper distribution inside cells. ATP7B
is expressed in liver and kidney and, to a lesser extent, in the
brain in normal individuals (4,5). ATP7B
is responsible for the export of copper from the liver; thus,
mutations that disable the function of ATP7B may lead to excessive
hepatic copper accumulation, as suggested by the impairment of
biliary copper excretion in patients with Wilson disease (6). Previous studies suggested that the
copper export system functions as efflux transporters for platinum
drugs. Moreover, the immunohistochemical expression of ATP7B has
been shown to be associated with resistance to platinum drugs in
certain solid tumors (3,7–9).
However, the expression of ATP7B and its impact on CDDP resistance
in NSCLC have yet to be thoroughly investigated.
This study aimed to investigate the predictive value
of ATP7B gene expression on in vitro chemosensitivity to
CDDP, using surgically resected specimens from patients with
NSCLC.
Materials and methods
Study population
Eligible patients included those with a histological
diagnosis of NSCLC. These patients had not received chemotherapy
nor radiotherapy and had undergone surgical resection. Patient
specimens were tested using a chemosensitivity test. The study was
conducted between 2005 and 2007 at Teikyo University Hospital and
Tokai University Hospital. Eligible patients gave their informed
consent. The patients consisted of 16 men and 5 women, ranging in
age from 54 to 79 years (mean 68). Nine tumors were confirmed to be
adenocarcinomas; eight, squamous cell carcinomas; three, large-cell
carcinomas and one was confirmed to be poorly differentiated
NSCLC.
In vitro chemosensitivity test
In vitro chemosensitivity was examined using
the collagen gel-droplet embedded culture drug sensitivity test
(CD-DST), as previously described by Kobayashi et al, with
minor modifications (10–13). Briefly, surgically resected
specimens were finely minced using a scalpel and digested in a cell
dispersion enzyme solution EZ (Nitta Gelatin Inc.). The dispersed
cancer cells were washed and filtered through a nylon mesh with a
pore size of 200 mm, collected by centrifugation, suspended in
PCM-1 medium (Nitta Gelatin Inc.) and incubated in a CO2
incubator at 37°C for 24 h. Viable cells were collected and
re-suspended in reconstructed type I collagen solution with a final
density of 1×105 cells/ml. Three drops of the
collagen-cell mixture (30 ml/drop) were placed in each well of a
6-well multiplate and allowed to gel at 37°C overnight. Cisplatin
(Bristol-Myers Squibb Inc.) was then added at a final concentration
of 2 mg/ml and the plates were incubated for 24 h. After removal of
the medium containing the anticancer drugs, each well was rinsed
twice, overlaid with PCM-2 medium (Nitta Gelatin Inc.) and
incubated for 7 days. At the end of the incubation period, the
colonies were stained with neutral red (50 mg/m for 3 h). Each
collagen droplet was then fixed with 10% neutral formalin buffer,
washed in water, air dried and quantified using imaging analysis.
In vitro sensitivity was expressed as the growth inhibition
rate (GIR): (1 - T/C) × 100 (%), where T is the total volume of the
treated group and C is the total volume of the control group.
In this study, each tumor was judged as ‘resistant’
when GIR was ≤60% and ‘sensitive’ when GIR was >60%.
Cell line
A human colorectal cancer cell line (HCT8) was
obtained from Riken (Saitama, Japan). The cell line was cultured at
37°C in Dulbecco’s modified Eagle’s medium (containing 10% bovine
serum) under a 5% atmosphere.
Quantitative evaluation of ATP7B mRNA
expression
Total RNA was extracted from the tumor samples and a
cell line using the acid guanidinium thiocyanate-phenol-chloroform
extraction method. Complementary DNA (cDNA) was synthesized from 1
μg of total RNA, according to our previous reports (14–16).
Real-time PCR assays were run on an ABI PRISM 7000
Sequence Detection System (Perkin-Elmer Applied Biosystems)
according to the manufacturer’s recommendations, as well as the
recommendations of previously published studies. Briefly, a total
volume of 50 μl of reaction mixture containing 1 μl of cDNA
template, 25 μl of TaqMan Universal PCR Master Mix (Perkin-Elmer
Applied Biosystems) and 2.5 μl of primer probe mixture for ATP7B
and β-actin were amplified. The protocol used was: after the
initial denaturation (2 min at 95°C), amplification was performed
for 50 cycles for 15 sec at 95°C and 60 sec at 60°C. The primer
probe mixture for ATP7B (Taqman® Gene Expression Assays,
assay ID: Hs00163739_m1) and β-actin (human ACTB, 4310881E; both
from Perkin-Elmer Applied Biosystems) were purchased as part of the
commercial provider’s kit.
To quantify the ATP7B gene transcripts precisely, we
used the β-actin transcripts as a quantitative control and each
sample was normalized based on its β-actin transcript content.
Standard curves for ATP7B and β-actin mRNA were generated using
serially diluted solutions (1/5, 1/25, 1/125 and 1/625) of HCT8
cDNA. The threshold cycle (Ct) was defined as the PCR cycle number
at which point the fluorescent intensity exceeded the threshold.
Following the determination of Ct, the amount of target gene
expression was calculated using the standard curve. Quantitative
normalization of cDNA was performed in each sample using the
expression of the β-actin gene as an internal control. Finally, the
ATP7B mRNA levels were shown as a ratio of the β-actin mRNA levels.
Real-time PCR assays were conducted in duplicate on one dish for
each sample and the mean value was used to calculate the mRNA
expression levels.
Statistical analysis
Categorical variables were analyzed using Fisher’s
exact test. Continuous variables were compared using the
Mann-Whitney U test. Reported P-values were two-sided; P<0.05
was considered to be statistically significant.
Results
In vitro chemosensitivity test
GIR values ranged from 0 to 68.6% (mean ± SD,
38±18.4%). According to the protocol described in Materials and
methods, 12 tumors were judged to be ‘resistant (R)’ to CDDP, while
9 other tumors were judged to be ‘sensitive (S)’ to CDDP.
No significant correlation was observed between
in vitro CDDP sensitivity and any of the clinical parameters
including age, gender and histology (Table I).
 | Table IRelationship between in vitro
CDDP sensitivity and patient characteristics. |
Table I
Relationship between in vitro
CDDP sensitivity and patient characteristics.
| In vitro CDDP
sensitivitya | |
|---|
|
| |
|---|
| Characteristics | R | S | P-valueb |
|---|
| Age (mean ± SD,
years) | 69±7 | 67±8 | 0.60 |
| Gender |
| Male | 8 | 8 | 0.20 |
| Female | 4 | 1 | |
| Histology |
| Ad | 7 | 2 | 0.09 |
| Sq | 2 | 6 | |
| Other | 3 | 1 | |
ATP7B mRNA expression
The ATP7B mRNA expression levels varied from 0.02 to
0.57 (mean ± SD, 0.15±0.14). The relationship between the ATP7B
expression levels and clinical parameters, including age, gender
and histology, are summarized in Table
II. No significant correlation was observed between the ATP7B
mRNA expression levels and age or gender (P=0.7 and P=0.13,
respectively; Mann-Whitney U test).
 | Table IIRelationship between ATP7B mRNA
expression levels and patient characteristics. |
Table II
Relationship between ATP7B mRNA
expression levels and patient characteristics.
| Characteristics | Mean ATP7B gene
expression level ± SD | P-valuea |
|---|
| Age |
| >70 | 0.14±0.12 | 0.70 |
| ≤70 | 0.17±0.17 | |
| Gender |
| Male | 0.11±0.09 | 0.13 |
| Female | 0.30±0.18 | |
| Histology |
| Ad | 0.26±0.17 | 0.01 |
| Non-Ad | 0.09±0.08 | |
ATP7B gene expression in the adenocarcinoma group
was significantly higher than that in the non-adenocarcinoma group
(p=0.01; Mann-Whitney U test).
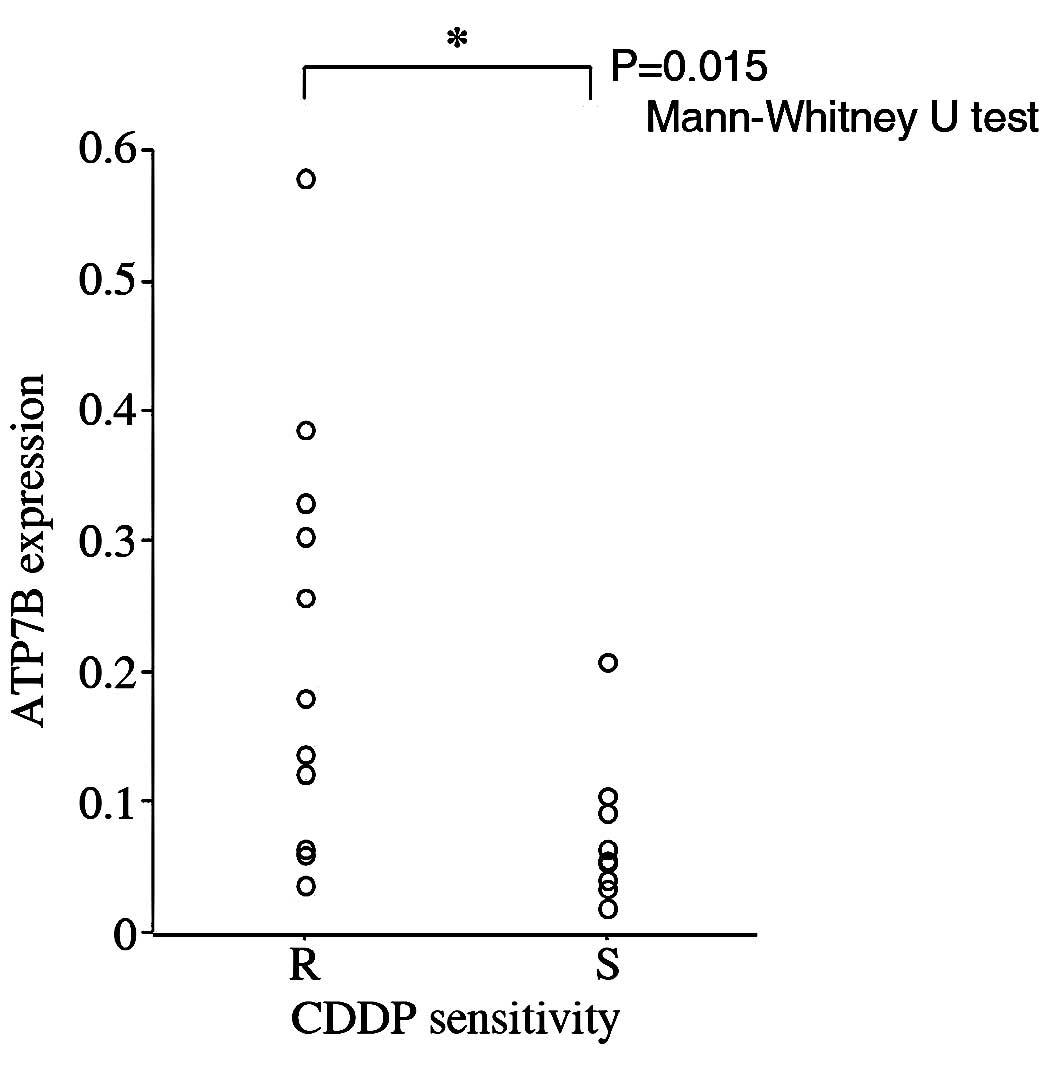
Relationship between ATP7B expression and
CDDP sensitivity
The difference in ATP7B expression between the
CDDP-resistant (R group, n=12) and CDDP-sensitive tumors (S group,
n=9) was evaluated. The ATP7B mRNA expression levels in the R group
were significantly higher than those in the S group (p=0.015;
Mann-Whitney U test; Fig. 1).
Discussion
This study investigated the association between
ATP7B mRNA expression and in vitro chemosensitivity to CDDP
using surgically resected specimens of NSCLC. As a result, we
observed that the CDDP-resistant tumors expressed a significantly
higher level of ATP7B mRNA than the CDDP-sensitive tumors.
ATP7B is a member of a class of heavy
metal-transporting P-type ATPases that pump copper, cadmium, zinc,
silver or lead (7,17,18).
Copper is an essential trace element and is transported to the
extracellular environment by an energy-dependent system.
Alterations in copper homeostasis can cause severe problems
(18). For example, Wilson disease,
an autosomal recessive disease of copper transport, is
characterized by chronic liver and/or neurological disorders,
occasionally accompanied by kidney damage (19,20).
ATP7B mRNA expression was reported to be associated
with in vitro cisplatin resistance in ovarian carcinoma cell
lines (21). The transfection of
epidermoid, head and neck and ovarian carcinoma cells with an ATP7B
expression vector rendered them resistant to platinum drugs, such
as cisplatin, carboplatin and oxaliplatin (7,8,22).
These ATP7B-transfected cells not only exhibited reduced
intracellular concentrations, but also an increased efflux of these
platinum drugs (7,8).
The possible clinical significance of ATP7B
expression was noted in various solid tumors. A higher expression
level of ATP7B is correlated with an unfavorable response to
platinum drug treatment in ovarian, esophageal and oral squamous
cell carcinoma (19,23,24).
We recently reported that ATP7B mRNA and protein expression levels
are associated with CDDP resistance in NSCLC xenografts (14). However, the expression of ATP7B in
NSCLC tissues and its clinical significance have yet to be
adequately studied.
Little is known about the differences in ATP7B
expression levels according to the histological subtypes of NSCLC.
In this study, the ATP7B expression level was found to be
significantly higher in the adenocarcinoma than in the
non-adenocarcinoma group. Further study is needed to confirm and
clarify the clinicopathological significance of this
observation.
In conclusion, although data presented were based
only on an in vitro assay, our results suggest that ATP7B
gene expression is correlated with CDDP resistance and that ATP7B
expression is a useful chemoresistance marker for cisplatin.
Abbreviations:
|
CDDP
|
cisplatin
|
|
ATP7B
|
copper-transporting P-type adenosine
triphosphatase
|
|
CD-DST
|
collagen gel-droplet embedded culture
drug sensitivity test
|
|
PCR
|
polymerase chain reaction
|
|
GIR
|
growth inhibition rate
|
References
|
1
|
Perez RP: Cellular and molecular
determinants of cisplatin resistance. Eur J Cancer. 34:1535–1542.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tiseo M, Franciosi V, Grossi F and
Ardizzoni A: Adjuvant chemotherapy for non-small cell lung cancer:
ready for clinical practice? Eur J Cancer. 42:8–16. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katoh R, Takebayashi Y and Takenoshita S:
Expression of copper-transporting P-type adenosine triphosphatase
(ATP7B) as a chemoresistance marker in human solid carcinomas. Ann
Thorac Cardiovasc Surg. 11:143–145. 2005.PubMed/NCBI
|
|
4
|
Safaei R: Role of copper transporters in
the uptake and efflux of platinum containing drugs. Cancer Lett.
234:34–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanzi RE, Petrukhin K, Chernov I, et al:
The Wilson disease gene is a copper transporting ATPase with
homology to the Menkes disease gene. Nat Genet. 5:344–350. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Safaei R, Holzer AK, Katano K, Samimi G
and Howell SB: The role of copper transporters in the development
of resistance to Pt drugs. J Inorg Biochem. 98:1607–1613. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Komatsu M, Sumizawa T, Mutoh M, Chen ZS,
Terada K, Furukawa T, Yang XL, Gao H, Miura N, Sugiyama T and
Akiyama S: Copper-transporting P-type adenosine triphosphatase
(ATP7B) is associated with cisplatin resistance. Cancer Res.
60:1312–1316. 2000.PubMed/NCBI
|
|
8
|
Katano K, Safaei R, Samimi G, Holzer A,
Tomioka M, Goodman M and Howell SB: Confocal microscopic analysis
of the interaction between cisplatin and the copper transporter
ATP7B in human ovarian carcinoma cells. Clin Cancer Res.
10:4578–4588. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakayama K, Kanzaki A, Ogawa K, Miyazaki
K, Neamati N and Takebayashi Y: Copper-transporting P-type
adenosine triphosphatase (ATP7B) as a cisplatin based
chemoresistance marker in ovarian carcinoma: comparative analysis
with expression of MDR1, MRP1, MRP2, LRP and BCRP. Int J Cancer.
101:488–495. 2002. View Article : Google Scholar
|
|
10
|
Kobayashi H: Collagen gel droplet culture
method to examine in vitro chemosensitivity. Methods Mol Med.
110:59–67. 2005.PubMed/NCBI
|
|
11
|
Kobayashi H: Development of a new in vitro
chemosensitivity test using collagen gel droplet embedded culture
and image analysis for clinical usefulness. Recent Results Cancer
Res. 161:48–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inoue Y, Gika M, Abiko T, Oyama T, Saitoh
Y, Yamazaki H, Nakamura M, Abe Y, Kawamura M and Kobayashi K: Bcl-2
overexpression enhances in vitro sensitivity against
docetaxel in non-small cell lung cancer. Oncol Rep. 13:259–264.
2005.PubMed/NCBI
|
|
13
|
Kawamura M, Gika M, Abiko T, Inoue Y,
Oyama T, Izumi Y, Kobayashi H and Kobayashi K: Clinical evaluation
of chemosensitivity testing for patients with unresectable
non-small cell lung cancer (NSCLC) using collagen gel droplet
embedded culture drug sensitivity test (CD-DST). Cancer Chemother
Pharmacol. 59:507–513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakagawa T, Inoue Y, Kodama H, Yamazaki H,
Kawai K, Suemizu H, Masuda R, Iwazaki M, Yamada S, Ueyama Y, Inoue
H and Nakamura M: Expression of copper-transporting P-type
adenosine triphosphatase (ATP7B) correlates with cisplatin
resistance in human non-small cell lung cancer xenografts. Oncol
Rep. 20:265–270. 2008.PubMed/NCBI
|
|
15
|
Nishi M, Abe Y, Fujimori S, Hamamoto A,
Inoue Y, Miyazaki N, Oida Y, Ikoma N, Ohnishi Y, Yamazaki H, Ueyama
Y and Nakamura M: The modifier subunit of glutamate cysteine ligase
relates to cisplatin resistance in human small-cell lung cancer
xenografts in vivo. Oncol Rep. 14:421–424. 2005.PubMed/NCBI
|
|
16
|
Fujimori S, Abe Y, Nishi M, Hamamoto A,
Inoue Y, Ohnishi Y, Nishime C, Matsumoto H, Yamazaki H, Kijima H,
Ueyama Y, Inoue H and Nakamura M: The subunits of glutamate
cysteine ligase enhance cisplatin resistance in human non-small
cell lung cancer xenografts in vivo. Int J Oncol.
25:413–418. 2004.PubMed/NCBI
|
|
17
|
Terada K, Schilsky ML, Miura N and
Sugiyama T: ATP7B (WND) protein. Int J Biochem Cell Biol.
30:1063–1067. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harris ED, Qian Y, Tiffany-Castiglioni E,
Lacy AR and Reddy MC: Functional analysis of copper homeostasis in
cell culture models: a new perspective on internal copper
transport. Am J Clin Nutr. 67:988–995. 1998.PubMed/NCBI
|
|
19
|
Higashimoto M, Kanzaki A, Shimakawa T,
Konno S, Naritaka Y, Nitta Y, Mori S, Shirata S, Yoshida A, Terada
K, Sugiyama T, Ogawa K and Takebayashi Y: Expression of
copper-transporting P-type adenosine triphosphatase in human
esophageal carcinoma. Int J Mol Med. 11:337–341. 2003.PubMed/NCBI
|
|
20
|
Petrukhin K, Lutsenko S, Chernov I, Ross
BM, Kaplan JH and Gilliam TC: Characterization of the Wilson
disease gene encoding a P-type copper transporting ATPase: genomic
organization, alternative splicing, and structure/function
predictions. Hum Mol Genet. 3:1647–1656. 1994. View Article : Google Scholar
|
|
21
|
Nakayama K, Miyazaki K, Kanzaki A,
Fukumoto M and Takebayashi Y: Expression and cisplatin sensitivity
of copper-transporting P-type adenosine triphosphatase (ATP7B) in
human solid carcinoma cell lines. Oncol Rep. 8:1285–1287.
2001.PubMed/NCBI
|
|
22
|
Katano K, Safaei R, Samimi G, Holzer A,
Rochdi M and Howell SB: The copper export pump ATP7B modulates the
cellular pharmacology of carboplatin in ovarian carcinoma cells.
Mol Pharmacol. 64:466–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakayama K, Kanzaki A, Terada K, Mutoh M,
Ogawa K, Sugiyama T, Takenoshita S, Itoh K, Yaegashi N, Miyazaki K,
Neamati N and Takebayashi Y: Prognostic value of the
Cu-transporting ATPase in ovarian carcinoma patients receiving
cisplatin-based chemotherapy. Clin Cancer Res. 10:2804–2811. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyashita H, Nitta Y, Mori S, Kanzaki A,
Nakayama K, Terada K, Sugiyama T, Kawamura H, Sato A, Morikawa H,
Motegi K and Takebayashi Y: Expression of copper-transporting
P-type adenosine triphosphatase (ATP7B) as a chemoresistance marker
in human oral squamous cell carcinoma treated with cisplatin. Oral
Oncol. 39:157–162. 2003. View Article : Google Scholar : PubMed/NCBI
|