Introduction
Malignant transformation of teratoma (MTT) is the
transformation of a somatic teratomatous component of a germ cell
tumor to an aggressive non-germ cell tumor phenotype (1). There have been many reports of
secondary MTT after chemotherapy or radiotherapy in metastatic
lesions and primary MTT in the ovary; however, primary MTT of the
testis without chemotherapy or radiotherapy has rarely been
reported. Due to the rarity of this entity, clinical features and
prognosis have not yet been identified. However, three cases of MTT
of the testis have been reported; one revealed colon type
adenocarcinoma, and the other two did not describe the specific
histology. Therefore, this is the first case report of MTT of the
testis with gastric adenocarcinoma differentiation. We were
presented with a patient with MTT of the testis associated with
signet ring cell-type adenocarcinoma who had a symptomless
testicular mass for several decades.
Case report
A 48-year-old male visited our facility, presenting
with a large, intermittent painful mass in his right scrotum. The
fist-sized mass showed a firm consistency, but without inguinal
lymphadenopathy. The patient reported that the right testis had
been slightly larger than the left one since childhood, but there
were no symptoms. The mass had been growing very slowly without
pain until recently when the patient experienced intermittent pain
in the right scrotum.
We received the ethics committee’s approval and the
patient’s written informed consent. Ultrasonography showed a right
hydrocele with the heterogeneous mass. Computed tomography (CT)
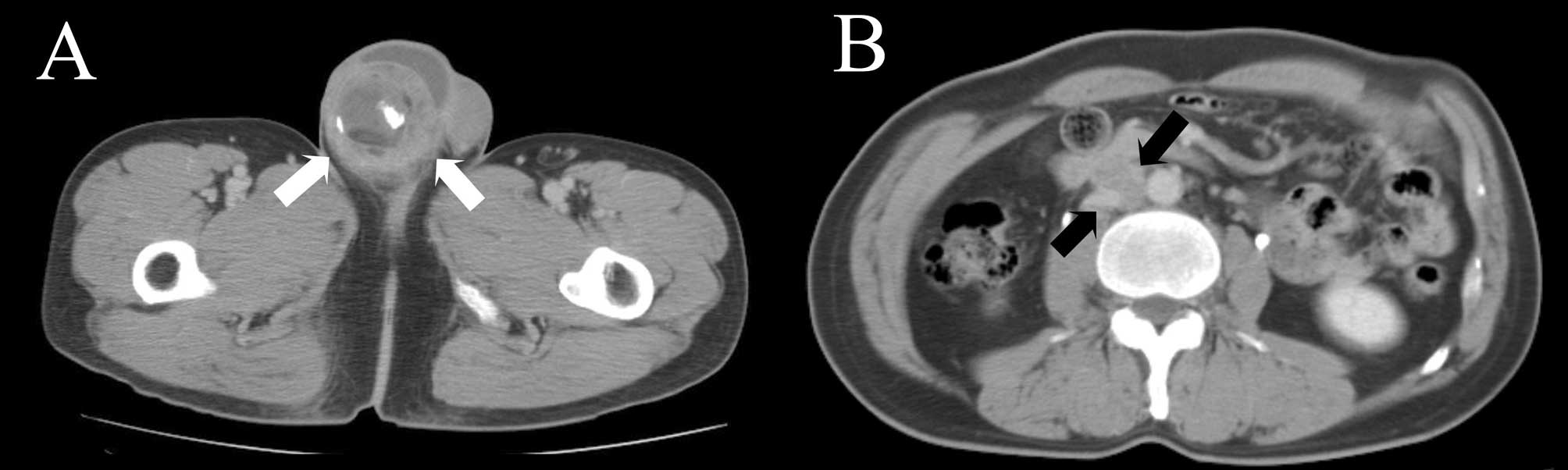
revealed a right testicular mass measuring 5 cm and multiple
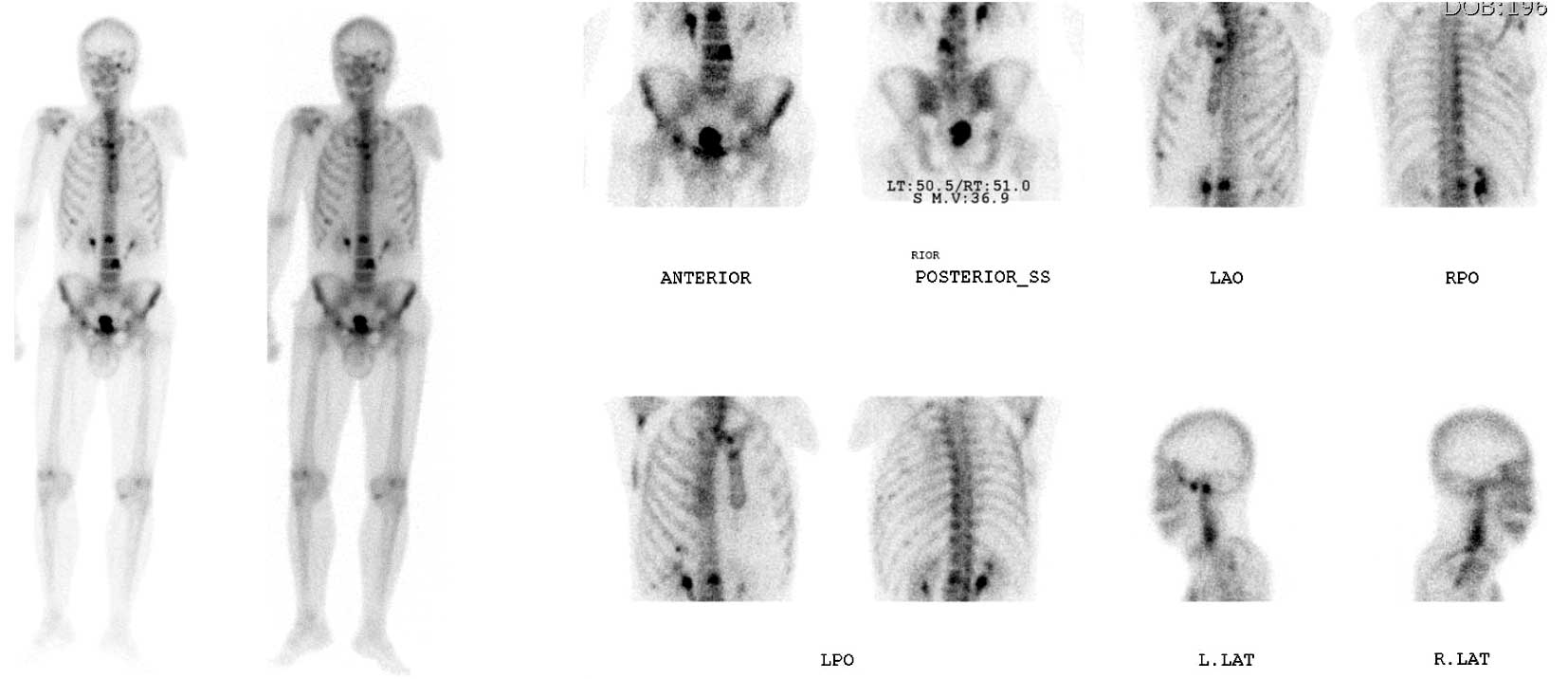
aortocaval and paraaortic lymphadenopathies (Fig. 1). A bone scan showed increased
radioisotope uptake at the 2nd and 4th lumbar vertebrae, sternum,
right scapula and ribs (Fig.
2).
Serum α-fetoprotein, β-human chorionic gonadotropin,
carcinoembrionic antigen and other laboratory results were within
normal limits. Radiologic and clinical evaluations found no other
primary malignant tumors.
Following diagnosis of a primary testicular tumor
with multiple metastases, right orchiectomy with high inguinal
incision was performed. Pathologically, the tumor measured 10.5 ×
8.3 × 7.0 cm and was classified as a mature teratoma associated
with adenocarcinoma showing signet ring cell-type adenocarcinoma
differentiation and presenting invasion into the spermatic cord
with involvement of the epididymis (Fig. 3). There was perineural invasion, but
no evidence of lymphovascular tumor emboli was found. The spermatic
cord resection and specimen margins were free of tumors. Results
from immunohistochemical staining showed that tumor cells were
positive to CDX-2, CK20 and focal-positive to CK7, but negative to
TTF-1.
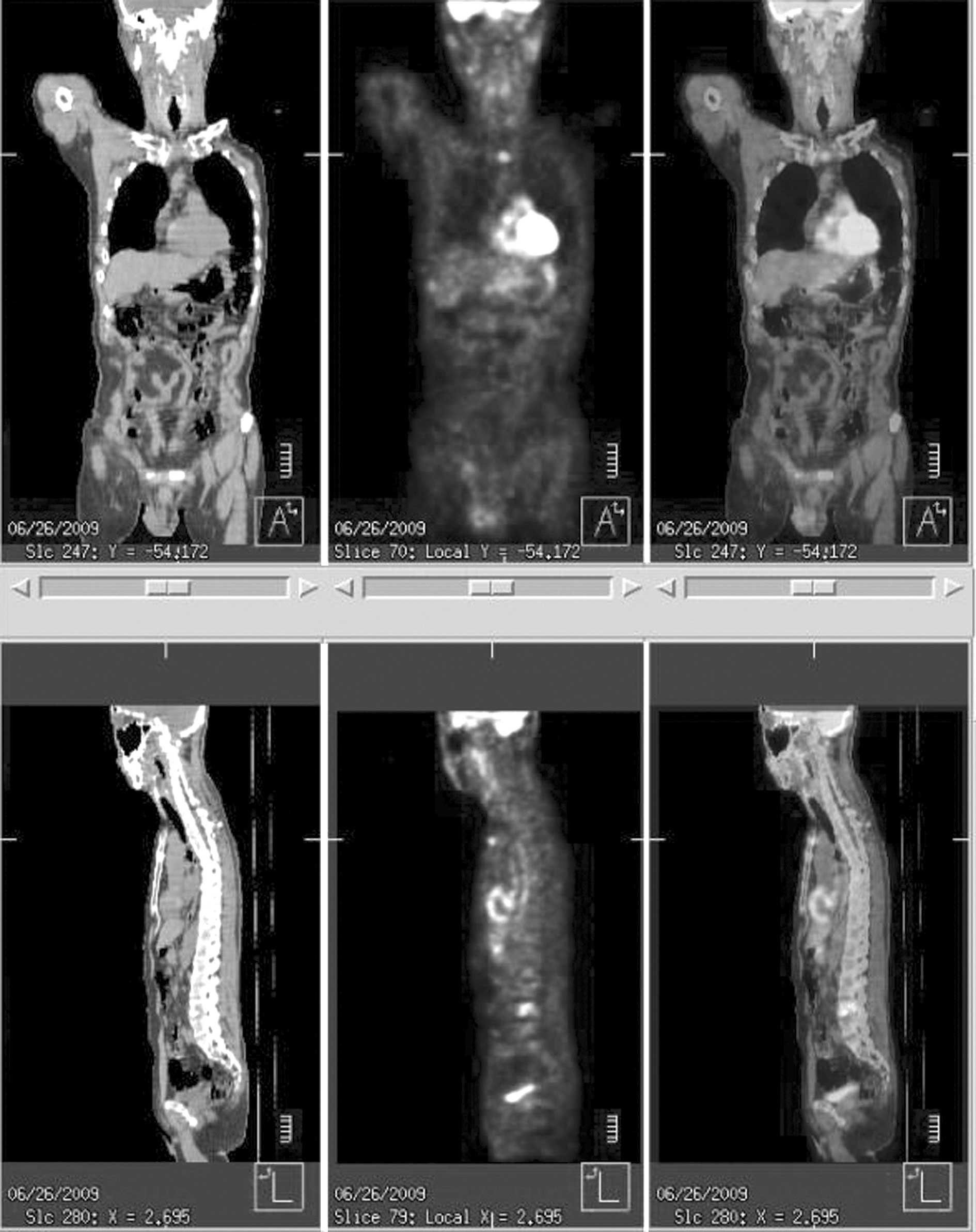
Positron emission tomography showed an increased
uptake at the sternum, lumbar vertebrae, rib and retroperitoneal
lymph node, with no abnormal uptake in the gastrointestinal tract
(Fig. 4). The patient complained of
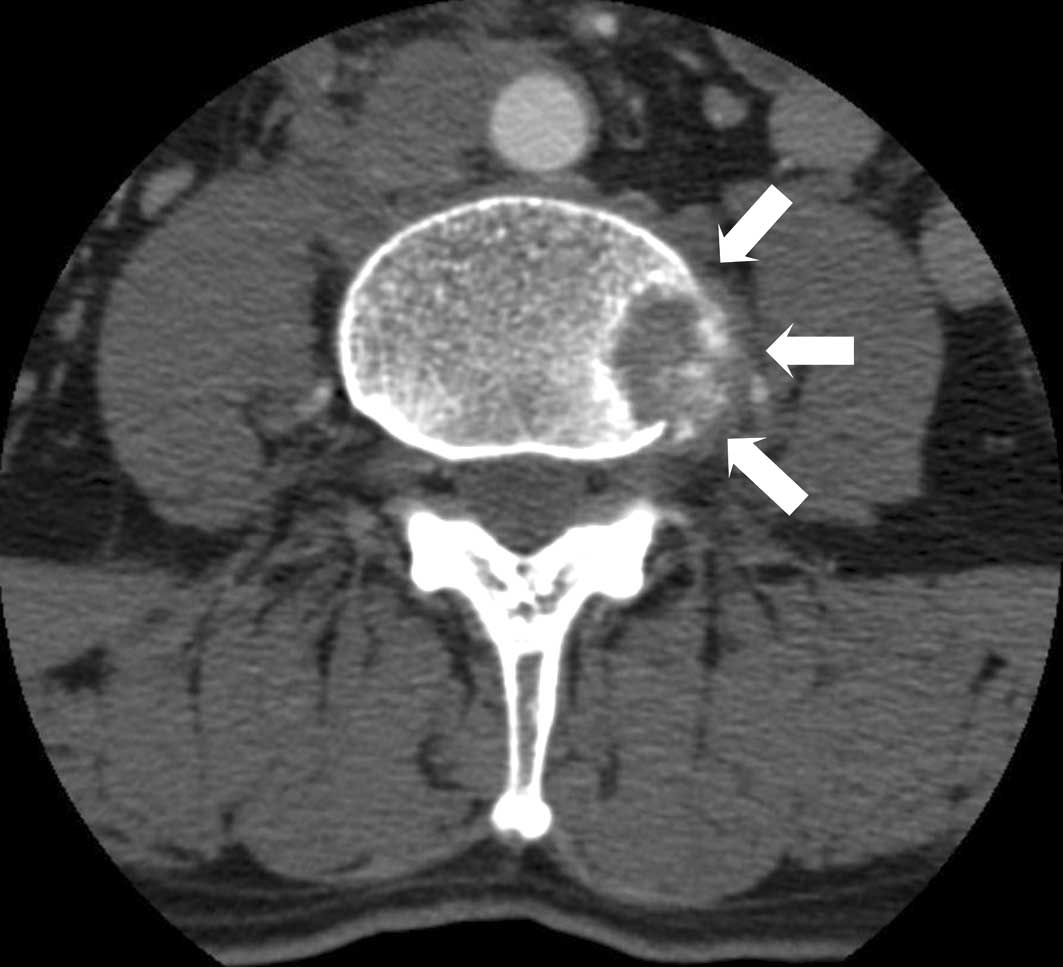
persistent back pain, and a thoracolumbar spinal CT showed an
osteolytic lesion extending into the paravertebral area at the 2nd
and 4th lumbar vertebral bodies (Fig.
5). Biopsy of the oeteolytic lesion at the 4th lumbar vertebral
body revealed metastatic adenocarcinoma upon pathology. A 5-FU- and
cisplatin-based combination of chemotherapy and radiotherapy was
administered. However, a follow-up bone scan and CT showed that the
metastatic lesions had progressed, and the patient succumbed to the
disease 15 months later.
Discussion
Teratomas are the most common testicular tumors
found in prepubertal children (2).
Prepubertal mature teratoma shows a benign clinical course;
however, teratoma in adults has a tendency to metastasize (3). In primary testicular tumors, teratoma
rarely undergoes transformation into a somatic malignant tumor. MTT
is used to describe a non-germ cell tumor arising in the teratoma.
MTT of ovarian cystic teratomas has been well documented, with an
incidence of approximately 2% (4).
However, MTT arising in mature teratomas in extraovarian sites is
rare (5). Subsequently, a review of
the literature found only three case reports of MTT in primary
testicular teratoma. One was a case of MTT of the testis with
colonic differentiation, while the other two cases did not describe
the specific pathology. Michal et al (6) first reported on a primary signet ring
stromal tumor of the testis; however, this case was not related to
MTT. Therefore, this is the first case report on MTT of the testis
with signet ring adenocarcinoma differentiation.
The mechanism of MTT in the testis remains poorly
understood. Two mechanisms for the development of MTT have been
postulated: malignant differentiation of the totipotential
embryonal carcinoma cell to a neoplasm of somatic phenotype, or
malignant transformation of mature teratoma elements (7). Koseoglu et al (5) also suggested the following clinical
mechanisms of MTT in the testis: i) chemotherapy- or
radiotherapy-induced MTT and ii) de novo MTT. Mediastinal,
retroperitoneal and metastatic MTT have typically been associated
with chemotherapy or radiotherapy. Mediastinal or retroperitoneal
mature teratomas are sensitive to the transforming effect of
chemotherapy or radiotherapy (8,9).
Teratomas in these regions are usually transformed into the
sarcomatous type as a result of chemotherapy or radiotherapy. In
addition, malignant transformation of metastatic mature teratomas
may frequently occur as a result of the same treatment. Some
authors have reported on the diagnosis of MTT of the testis with
malignant transformation of a pre-existing teratoma (5). However, these results involved
patients with MTT of the testis who underwent chemotherapy or
radiotherapy; few cases of MTT in a primary testicular tumor with
no previous treatment have been reported. In addition, MTT
associated with adenocarcinoma in retroperitoneum, mediastinum, or
metastatic teratomas is rare. Moreover, primary mature teratoma
with malignant transformation associated with an adenocarcinoma
phenotype is extremely rare. Therefore, the mechanism of primary
testicular MTT remains unknown.
Immunohistochemical study is a useful tool for the
identification of the exact type of adenocarcinoma. Park et
al (10) found an expression of
CDX-2 in 60.9% of stomach adenocarcinoma patients and also
suggested the value of determining tissue-specific
immunohistochemical stains in the diagnostic differentiation of
adenocarcinomas. These authors reported that the positive
predictive value of CDX-2(+), CK7(+), TTF-1(−) and CK20(−) in
signet ring type adenocarcinoma was 85.7%. In the present case, the
specimen found in the testis showed the same immunohistochemical
response as stomach adenocarcinoma. Kaseoglu et al (5) demonstrated that adenocarcinoma
originating from colonic glands in the testis showed CEA(+), CA
19-9(+), CK20(+) and CK7(−) upon immunohistochemical staining
results, which differed from this case.
Park et al (11) found that the incidence rates of MTT
were 0.8% in all mature teratomas in the ovary, and carcinoma
components were present in solid portions in cysts and thickened
cystic walls in ovarian MTT. Kido et al (12) reported that malignant tumors arising
in ovarian teratoma have a solid component region upon contrast
enhancement, with transmural extension and irregular invasion
through the septa to the adjacent organs. In our case, similar to
the ovarian teratoma, contrast enhancement during the pathologic
examination also identified adenocarcinoma in the solid portion of
the cyst.
Kuo et al (13) reported an excellent prognosis in
patients with signet ring stromal tumor of the testis. In addition,
Asano et al (8) reported no
recurrence after radical orchiectomy in patients with
non-metastatic MTT of the testis. However, when metastasis occurs,
prognosis of MTT of the testis is dependent on the histologic
phenotype. MTT of the testis with adenocarcinoma usually requires
an aggressive course of treatment. Kasai et al (9) reported on a patient who, despite
cisplatin-based chemotherapy, succumbed to MTT at 8 months after
orchiectomy. In the present case, the metastatic lesions also
progressed despite cisplatin-based combination chemotherapy.
Therefore, early detection of MTT is critical.
References
|
1
|
Comiter CV, Kibel AS, Richie JP, Nucci MR
and Renshaw AA: Prognostic features of teratomas with malignant
transformation: a clinicopathological study of 21 cases. J Urol.
159:859–863. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pohl HG, Shukla AR, Metcalf PD, et al:
Prepubertal testis tumors: actual prevalence rate of histological
types. J Urol. 172:2370–2372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shukla AR, Woodard C, Carr MC, et al:
Experience with testis sparing surgery for testicular teratoma. J
Urol. 171:161–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sumi T, Ishiko O, Maeda K, Haba T, Wakasa
K and Ogita S: Adenocarcinoma arising from respiratory ciliated
epithelium in mature ovarian cystic teratoma. Arch Gynecol Obstet.
267:107–109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koseoglu RD, Parlaktas BS, Filiz NO,
Erdemir F, Uluocak N and Tulunay O: Adenocarcinoma originating from
a mature teratoma of the testis. Kaohsiung J Med Sci. 23:265–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Michal M, Hes O and Kazakov DV: Primary
signet-ring stromal tumor of the testis. Virchows Arch.
447:107–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El Mesbahi O, Terrier-Lacombe MJ,
Rebischung C, Theodore C, Vanel D and Fizazi K: Chemotherapy in
patients with teratoma with malignant transformation. Eur Urol.
51:1306–1312. 2007.
|
|
8
|
Asano T, Kawakami S, Okuno T, et al:
Malignant transformation in a mature testicular teratoma left
untreated for more than 50 years since childhood. Scand J Urol
Nephrol. 37:177–178. 2003.PubMed/NCBI
|
|
9
|
Kasai T, Moriyama K, Tsuji M, Uema K,
Sakurai N and Fujii Y: Adenocarcinoma arising from a mature cystic
teratoma of the testis. Int J Urol. 10:505–509. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SY, Kim BH, Kim JH, Lee S and Kang
GH: Panels of immunohistochemical markers help determine primary
sites of metastatic adenocarcinoma. Arch Pathol Lab Med.
131:1561–1567. 2007.PubMed/NCBI
|
|
11
|
Park JY, Kim DY, Kim JH, Kim YM, Kim YT
and Nam JH: Malignant transformation of mature cystic teratoma of
the ovary: experience at a single institution. Eur J Obstet Gynecol
Reprod Biol. 141:173–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kido A, Togashi K, Konishi I, et al:
Dermoid cysts of the ovary with malignant transformation: Mr
appearance. AJR Am J Roentgenol. 172:445–449. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuo CY, Wen MC, Wang J and Jan YJ:
Signet-ring stromal tumor of the testis: a case report and
literature review. Hum Pathol. 40:584–587. 2009. View Article : Google Scholar : PubMed/NCBI
|