Introduction
Chemokines were originally discovered and studied
from the perspective of inflammation. However, their role in
modulating directional cell movement and the migration of cancer
cells has been demonstrated and is considered critical (1,2).
The human chemokine system includes almost 50
chemokines and 14 receptors (2).
The most commonly overexpressed chemokine in human cancer is CXCR4
(stromal cell-derived factor-1 receptor; fusin) and its ligrand
CXCL12 (stromal cell-derived factor-1 ligand; SDF1α). CXCR4
activation by CXCL12 stimulates several key migratory,
proliferative and survival signaling cellular pathways (3).
We hypothesized that CXCR4 and CXCL12, avidly
expressed by small-cell lung carcinoma (SCLC) cells and documented
to play important roles in the pathophysiology of metastasis, play
a prominent role in the dissemination of tumoral cells and their
subsequent invasion to a second malignancy.
Case report
A 69-year-old Caucasian male with a history of 60
packs/year tobacco consumption and coronary artery disease was
admitted to the hospital with a history of malaise, a 2-month
20-pound weight loss and a left kidney mass. The serum sodium level
was 133 meq/l and hemoglobin, 15.5 g/dl. Laparoscopic partial
nephrectomy was performed. A CT scan of the thorax was performed on
the 5th post-operative day. The scan showed a mass-like right lobe
opacification, bilateral pleural effusions and extensive
mediastinal and right hilar adenopathy. Three days after discharge
the patient was readmitted with acute dyspnea and atrial flutter.
Chest X-rays confirmed a large right pleural effusion. The serum
sodium level of 128 meq/l was due to a syndrome of inappropriate
anti-diuretic hormone secretion. A right thoracenthesis was
performed, and approximately 2 l of fluid was removed. A
bronchoscopy with transbronchial biopsy showed tumor invasion
within the right main stem bronchus and nests of small-cell
carcinoma with immunohistochemical stains positive for pankeratin,
synaptophysin, CD56 and TTF1.
The patient was discharged from the hospital on
demeclocycline for syndrome of inappropriate anti-diuretic hormone
secretion, and cytotoxic chemotherapy was commenced with a
combination of carboplatin and etoposide.
Materials and methods
Morphologic and immunohistochemical
analysis
A left partial nephrectomy specimen was obtained
measuring 4.5×3.7×3.2 cm. Sectioning of the tissue revealed a
single tan, focally hemorrhagic, well-circumscribed tumor, 2×2×2
cm, confined within the renal parenchyma. A histological
examination showed that this tumor mass was composed of a primary
renal oncocytoma containing multiple small islands of small-cell
carcinoma.
These foci of metastatic small-cell carcinoma were
confined to the oncocytoma. No metastatic small-cell carcinoma was
identified in the renal parenchyma surrounding the oncocytoma.
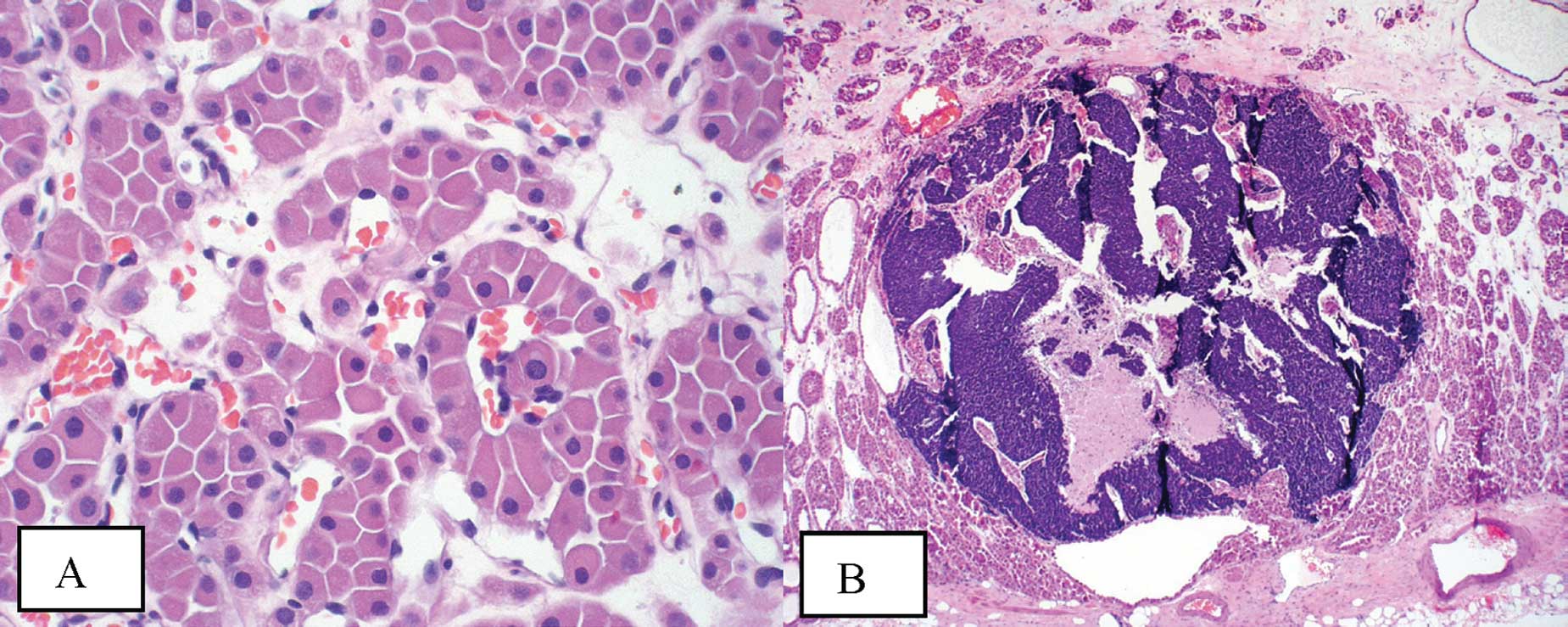
The oncocytoma itself exhibited classic histological
features. The tumor was composed of homogeneous cells with abundant
acidophilic granular cytoplasm and central to eccentrically located
round nuclei with even chromatin (Fig.
1A). These cells formed solid compact nests (alveolar pattern)
and areas of variably sized tubules set in the background of a
loose edematous or hyalinized stroma. Mitotic activity was not
appreciable. Immunohistochemical stains showed positive staining
for EMA and negative staining for vimentin and colloidal iron.
The foci of the small-cell carcinoma present within
the oncocytoma also exhibited classic features. The small foci were
composed of solid sheets of small cells with extremely scant
cytoplasm and nuclei with finely granular chromatin, absent or
inconspicuous nucleoli and prominent nuclear moulding. Mitotic
activity was rapid (Fig. 1B).
Immunohistochemical stains confirmed the diagnosis with the cells
showing positive staining for AE1/AE3, chromogranin, synaptophysin,
CD56 and TTF-1.
Diagnosis was consistent with tumor-to-tumor
metastasis with a primary SCLC as the site of origin and a renal
oncocytoma as the harboring malignancy.
Chemokine analysis
Processing of the surgical
specimens
Formalin-fixed and paraffin-embedded slides of
normal renal parenchyma and tumor tissues for each antibody were
hydrated. Antigens were retrieved in citrate buffer for 20 min and
cooled at room temperature.
The slides were blocked for peroxidase activity in
3% hydrogen peroxide (H202) for 5 min and
rinsed in Tris buffer. The slides were also blocked for possible
non-specific background staining with casein in PBS for 15 min.
Staining for CXCL12 (SDF1α, mAB clone 7801B, IG1
class; R&D Systems, Minneapolis, MN) and CXCR4 (fusin, goat
polyclonal AB, directed to the amino terminus of SDF1α sc-6729;
Santa Cruz Biotechnology, Santa Cruz, CA) was carried out at room
temperature for 30 min using a 1:50 dilution. Slides were rinsed in
Tris buffer.
For CXCL12, the secondary antibody, an anti-mouse
IgG-HRP labeled polymer was applied for 30 min. For CXCR4, an
anti-goat IgG-HRP labeled polymer was applied for 10 min. Slides
were rinsed in Tris buffer twice. The chromogen, DAB, was applied
for 5 min at room temperature and slides were rinsed in di
H20 and counterstained in hematoxylin.
Results
CXCL12 antibody staining of the renal specimen
showed an avid expression on the endothelial cell lining of
vascular channels, glomeruli and tubules within the histological
sections of the patient’s oncocytoma and kidney parenchyma
(Fig. 1C-F). CXCL12 also stained
the SCLC cells within the oncocytoma (Fig. 1C-F). We then explored the CXCL12 and
CXCR4 expression in a normal kidney control and confirmed their
expression as well (data not shown). CXCR4 staining was not noted
in the oncocytoma specimen nor were the small-cell carcinoma cells
nested within the tumor.
Discussion
Several hypotheses have been proposed to explain the
biology of tumor-to-tumor metastasis. In 1889, Paget in his ‘seed
and soil theory’ mentioned that gross tumor development is a
consequence of the provision of a fertile environment (the soil),
in which compatible tumor cells (the seeds) proliferate. A
mechanical theory was proposed by Ewing in 1928, describing site
specificity as a direct consequence of the anatomical location of a
primary tumor (4). High lipid and
glycogen content in the kidney were also proposed as a potential
mechanism to attract metastatic cells (4,5).
However, few malignancies metastasize to the kidneys.
More recent evidence links chemokines with the
pathogenesis of metastasis in more than 23 human cancer cells
including SCLC (6).
In vivo data showed that certain chemokines
serve as tissue-specific attractant molecules for tumor cells,
promoting tumor cell migration to a particular site through direct
action of the chemokine ligands on chemokine receptors via the
activation of heterotrimeric G proteins. The G protein subunits
then stimulate multiple signal transduction pathways, involving the
phosphatidylinositide 3 kinase (PIK-3)/Akt pathway and various Src
family kinases (6).
SCLC cells use the CXCR4 receptor to migrate to the
bone marrow tumor microenvironment, which is rich in CXCL12. This
migration is due to the activation of integrins after CXCR4/CXCL12
involvement, allowing the cells to interact with extracellular
matrix components (7).
The CXCR4/CXCL12 axis has been found to be
up-regulated in renal cell carcinoma and other malignancies such as
chronic lymphocytic leukemia, breast carcinoma, multiple myeloma,
melanoma and ovarian carcinoma and may constitute a novel
therapeutic target (1,7).
The development of tumor metastasis from a second
primary malignancy is uncommon and remains biologically puzzling.
Its low incidence has made its full biological characterization
evasive.
Initially described by Campbell, the event must meet
the following criteria to be considered tumor-to-tumor metastasis:
i) the presence of more than one primary tumor; ii) the recipient
tumor has to be a true neoplasm; iii) there must be evidence of
true metastasis from the second neoplasm; iv) the second malignancy
must grow or invade the tissues of the hosting tumor; v) the
metastatic growth must not be due to contiguous growth or embolism
of tumor cells, and vi) tumors that metastasize to the lymphatic
system where a lymphatic malignancy already exists are not
considered tumor-to-tumor metastasis (4,8–10).
Renal cell carcinoma and meningioma are the most
common malignant and benign recipients, respectively, whereas the
lung is the most common metastatic donor in both settings (10).
Though inconclusive, our results suggest that the
high tissue expression of CXCL12 observed in the tumor tissues is
also present in normal kidney parenchyma (control sample). Contrary
to our hypothesis, CXCR4 expression was not noted in the SCLC
metastasis in our case specimen. However, the lack of expression is
perhaps related to the interaction of the oncocytoma’s stromal
microenvironment and the metastatic nests of SCLC cells. In this
particular event the host for SCLC was indeed a renal neoplasm.
Unlike oncocytomas, renal cell carcinoma cells induce CXCR4
transcription via hypoxia inducible factor (HIF1). Moreover, the
up-regulation of prolyl and asparagynil hydroxylase have been
identified in oncocytomas (11).
These enzymes decrease the production of HIF1-α via proteasome
degradation by down-regulating the transcription of CXCR4 (11,12).
This partially explains the absence of CXCR4 receptors in our
specimen and likely plays a role in the low metastatic potential
that oncocytomas exhibit.
The molecular events between CXCR4 and its ligand
CXCL12 have yet to be elucidated in tumor-to-tumor metastasis. In
this regard, the question remains as to whether the mechanism of
the propagation of cells is related to the chemokine axis, the
oncocytoma’s microtumoral environment or, more likely, a
combination of both. This preliminary observation warrants further
investigation with functional molecular studies to characterize the
environment surrounding chemokines in tumor-to-tumor
metastasis.
Acknowledgements
The authors would like to thank Dr Jan Burger for
the guidance and invaluable suggestions in interpretation of the
chemokine staining.
References
|
1
|
Burger JA and Kipps TJ: CXCR4: a key
receptor in the crosstalk between tumor cells and their
microenvironment. Blood. 5:1761–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zlotnik A: Chemokines in neoplastic
progression. Semin Cancer Biol. 3:181–185. 2004. View Article : Google Scholar
|
|
3
|
Hartmann TN, Burger JA, Glodek A, Fujii N
and Burger M: CXCR4 chemokine receptor and integrin signaling
co-operate in mediating adhesion and chemoresistance in small cell
lung cancer (SCLC) cells. Oncogene. 27:4462–4471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campbell LV Jr, Gilbert E, Chamberlain CR
Jr and Watne AL: Metastases of cancer to cancer. Cancer. 3:635–643.
1968. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hart IR: ‘Seed and soil’ revisited:
mechanisms of site-specific metastasis. Cancer Metastasis Rev.
1:5–16. 1982.
|
|
6
|
Tanaka T, Bai Z, Srinoulprasert Y, Yang
BG, Hayasaka H and Miyasaka M: Chemokines in tumor progression and
metastasis. Cancer Sci. 6:317–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 7:540–550. 2004. View Article : Google Scholar
|
|
8
|
Singh EO, Benson RC Jr and Wold LE:
Cancer-to-cancer metastasis. J Urol. 2:340–342. 1984.
|
|
9
|
Ben-Izhak O and Lichtig C: Renal
oncocytoma harbouring metastatic lung carcinoma. Case report. Scand
J Urol Nephrol. 4:317–318. 1990.PubMed/NCBI
|
|
10
|
Altinoz MA, Santaguida C, Guiot MC and Del
Maestro RF: Spinal hemangioblastoma containing metastatic renal
cell carcinoma in von Hippel-Lindau disease. Case report and review
of the literature. J Neurosurg Spine. 6:495–500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koeman JM, Russell RC, Tan MH, Petillo D,
Westphal M and Koelzer K: Somatic pairing of chromosome 19 in renal
oncocytoma is associated with deregulated EGLN2-mediated
[corrected] oxygen-sensing response. PLoS Genet.
9:e10001762008.PubMed/NCBI
|
|
12
|
Ginouves A, Ilc K, Macias N, Pouyssegur J
and Berra E: PHDs overactivation during chronic hypoxia
‘desensitizes’ HIFalpha and protects cells from necrosis. Proc Natl
Acad Sci USA. 12:4745–4750. 2008.PubMed/NCBI
|