Introduction
The management of patients with advanced-stage
epithelial ovarian cancer is best achieved through primary
cytoreductive surgery and subsequent combination chemotherapy. In
the cases of patients with non-optimal debulking, interval
cytoreduction is also considered when partial response is obtained.
The recommended doses and schedule for combination chemotherapy for
advanced-stage epithelial ovarian cancer are: paclitaxel (175
mg/m2) and carboplatin [area under the
concentration/time curve (AUC) 5–6 mg/min/ml] every 3 weeks for 6–8
cycles (1).
Many patients undergoing hemodialysis (HD) suffer
from various types of cancer. It has been reported that certain
types of cancers are more common among HD patients (2). However, few reports exist regarding
the pharmacokinetics of combination chemotherapy with paclitaxel
and carboplatin in HD patients with epithelial ovarian cancer
(3–5).
This study presented a case of a patient with
advanced-stage epithelial ovarian cancer with chronic renal failure
requiring HD. The patient received combination chemotherapy
consisting of paclitaxel and carboplatin, and interval debulking
surgery. The study analyzed the optimal carboplatin dose
administered and timing of HD.
Case report
The patient was a 52-year-old (gravida 4, para 2)
Japanese woman with chronic renal failure who required HD 3 times
per week for 14 years. The patient suffered from abdominal
distension. At the local clinic, left pleural effusion was
indicated by chest radiography, and a cytologic examination
revealed adenocarcinoma cells. Magnetic resonance imaging (MRI) of
the pelvis revealed tumors in both ovaries and an omental cake with
profuse ascites. Computerized tomography did not show any signs of
lymph node, lung and liver metastases. The serum levels of CA125
and CA72-4 were 3,008 U/ml (normal, <35 U/ml) and 114 U/ml
(normal, <5.4 U/ml), respectively; those of other tumor markers,
including CEA and CA19-9, were within the normal range.
The patient was admitted to our hospital and
underwent exploratory laparotomy. A histopathological examination
showed a serous papillary adenocarcinoma. The disease was
determined to be stage IVA epithelial ovarian cancer according to
the International Federation of Gynecology and Obstetrics (FIGO)
classification system. The patient was intravenously administered a
multidrug regimen consisting of paclitaxel and carboplatin. The
optimal dose of paclitaxel was determined to be 150
mg/m2, since it is metabolized mainly in the liver and
secreted in bile. The dose of carboplatin was calculated according
to Calvert’s formula (6):
carboplatin dose (mg/body) = AUC (mg/ml × min) × [glomerular
filtration rate (GFR) + 25]. The urinary volume per day of this
patient was 0 ml; thus, GFR was also determined to be 0. The
patient was initially administered 100 mg/body (AUC = 4 mg/ml ×
min) of carboplatin. Following premedication, paclitaxel was
administered for 3 h and carboplatin for 1 h. HD was initiated 24 h
after administration of carboplatin commenced, and was performed
for a period of 3 h.
For the pharmacokinetic analysis of free platinum
and paclitaxel, heparinized blood samples (5 ml) were collected at
specified times: 0 h (start of carboplatin infusion); 1 h (end of
carboplatin infusion); 1.5, 2, 4 and 24 h (before HD); 26 and 27 h
(after HD). The samples were centrifuged immediately at 3,000 rpm
at 4°C for 20 min, and plasma samples were obtained for the
measurement of paclitaxel. Aliquots of the plasma were
recentrifuged at 3,000 rpm for 10 min at 4°C in a Centrifree
Micropartition System (Amicon, Bevery, MA, USA) for the preparation
of ultrafilterable platinum. The free platinum and paclitaxel in
the plasma were analyzed according to the method of LeRoy et
al (7) and a high-performance
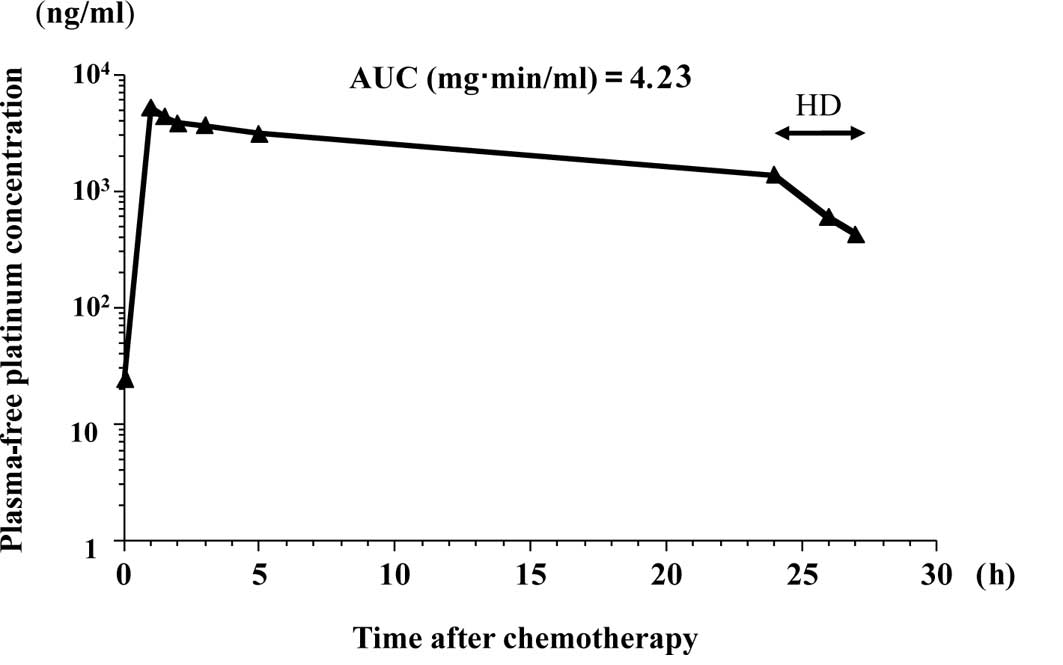
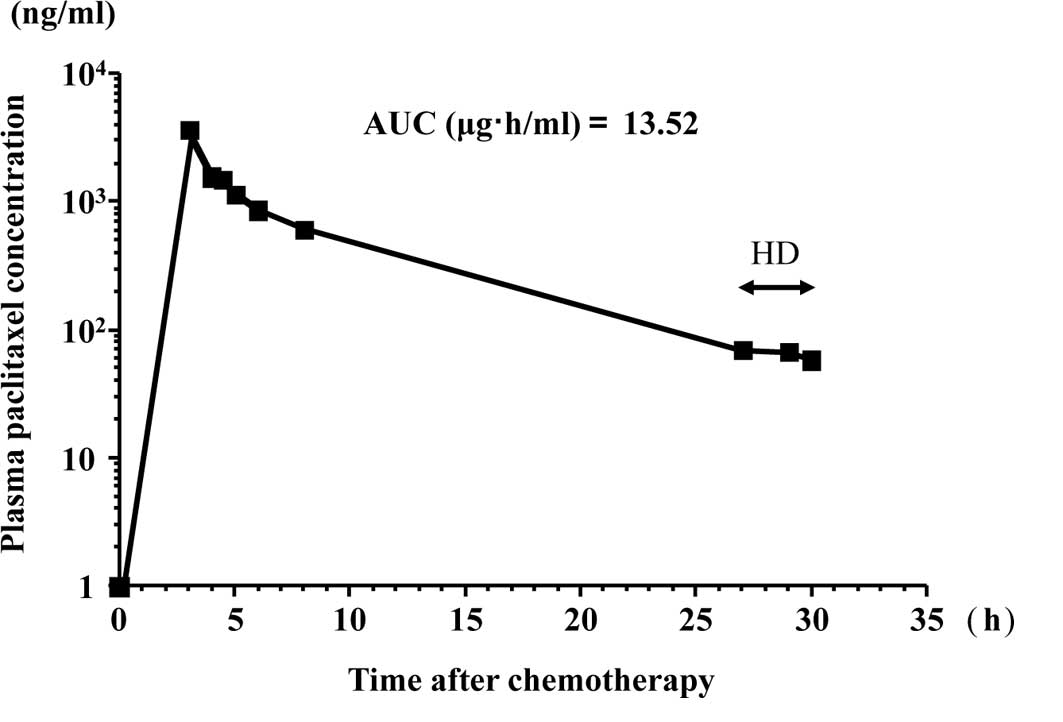
liquid chromatography UV method (8), respectively. Figs. 1 and 2 show the free platinum concentration in
the second course of the chemotherapy and the paclitaxel
concentration in the first course of the chemotherapy,
respectively. Table I summarizes
the maximum plasma concentrations (Cmax) and the AUCs of
free platinum and paclitaxel. Table
II shows the nadir blood cell counts.
 | Table IThe maximum plasma concentration and
the area under the concentration/time curve. |
Table I
The maximum plasma concentration and
the area under the concentration/time curve.
| Cycle | CBDCA (mg/body) | Cmax
(μg/ml) | AUC (mg·min/ml) | Paclitaxel
(mg/m2) | Cmax
(μg/ml) | AUC (μg·h/ml) |
|---|
| 1 | 100 | 3.86 | 3.48 | 150 | 3.60 | 13.5 |
| 2 | 150 | 5.12 | 4.23 | 150 | NA | NA |
| 3 | 175 | 7.30 | 5.55 | 150 | NA | NA |
| 4 | 150 | 5.71 | 4.59 | 150 | NA | NA |
 | Table IINadir blood cell count. |
Table II
Nadir blood cell count.
| Cycle | WBC nadir (/μl) | Neutrophil nadir
(/μl) | Platelets nadir
(103/μl) | Hemoglobin nadir
(mg/dl) |
|---|
| 1 | 3,800 | 2,774 | 24.3 | 6.0 |
| 2 | 1,600 | 720 | 14.6 | 6.8 |
| 3 | 900 | 297 | 3.3 | 6.1 |
| 4 | 2,000 | 1,000 | 6.3 | 6.6 |
Since mild side effects were observed and the AUC of
free platinum was smaller than anticipated in the first course, the
dose of carboplatin was increased to 150 mg/body in the second
course and to 175 mg/body in the third course. The optimal dose of
carboplatin was determined to be 150 mg/body for our patient. The
levels of serum CA125 were restored to normal after the patient
demonstrated a partial response to 4 courses of TC chemotherapy as
determined by MRI. Secondary cytoreductive surgery was performed
with macroscopically complete resection, followed by 4 courses of
TC therapy.
Discussion
The present study showed that combination
chemotherapy consisting of paclitaxel and carboplatin can be
administered to an advanced-stage epithelial ovarian cancer patient
with chronic renal failure requiring HD. Paclitaxel is metabolized
mainly in the liver and secreted in bile. Only 5–10% paclitaxel is
excreted unchanged by the kidney (9). Therefore, it was reported that the
pharmacokinetics of paclitaxel are not altered by HD. The values of
13.5 μg·h/ml for AUC and 3.6 μg/ml for Cmax when 150
mg/m2 of paclitaxel was administered in the present
study for 3 h are comparable to the values in patients with normal
renal function.
The optimal carboplatin dose and timing of HD have
yet to be determined. In the present study, the dose of the
administered carboplatin was calculated according to the Calvert
formula, considering that the GFR was equal to 0 ml/min and that
the extrarenal clearance was 25 ml/min. The extrarenal route of
elimination of free platinum corresponds to both the putative
hepatic elimination and the plasma and tissue protein binding,
which is irreversible for platinum compounds (6). In our case, the mean extrarenal
clearance of free platinum was 32 ml/min (range 29–36). In similar
studies involving HD patients, the extrarenal clearance of free
platinum was reported to be 20–30 ml/min (3,10). It
is likely that the extrarenal clearance of free platinum differs
among patients with chronic renal failure requiring HD. Therefore,
the measurement of free platinum may be useful in determining the
dose of carboplatin to obtain the correct AUC.
In the present study, HD was initiated 24 h after
the administration of carboplatin. Carboplatin is easily dialyzed
in HD patients, since it binds to plasma proteins at a low rate. If
no HD was performed, the AUC of the 4 consecutive courses of
chemotherapy would have reached 3.48 vs. 4.10, 4.23 vs. 4.98, 5.55
vs. 6.48 and 4.59 vs. 5.22, respectively. HD starting 24 h after
the infusion of carboplatin for a period of 3 h resulted in a 15%
reduction in the AUC of free platinum. This value was consistent
with that reported by Chatelut et al (3). It was reported that a relatively
higher dose of carboplatin (240–300 mg/m2) can be
administered in lung cancer patients with renal failure undergoing
HD (11–13). In these studies, HD was initiated
30–60 min after the end of carboplatin infusion. The HD setting
after the administration of carboplatin is a significant factor,
and delay in HD greatly influences the AUC of free platinum.
Therefore, to avoid the risk of hematological toxicity, the dose of
carboplatin should be calculated by the Calvert formula, and HD
should be initiated after 24 h. Jeyabalan et al demonstrated
that carboplatin of a target AUC of 5 (125 mg/body), when combined
with paclitaxel (175 mg/m2), was administered without
causing adverse myelosuppression in a patient with ovarian
carcinoma undergoing HD (14).
These authors did not carry out a pharmacokinetic analysis, and HD
was performed 24 h after the administration of carboplatin.
In conclusion, combination chemotherapy consisting
of paclitaxel and carboplatin is a feasible approach to improving
the treatment outcome of epithelial ovarian cancer patients with
chronic renal failure requiring HD. The measurement of free
platinum is useful in determining the ideal dose of carboplatin to
obtain an adequate AUC. Even in cases without the measurement of
free platinum, determining the dose of carboplatin according to the
Calvert formula and initiating HD after 24 h would ensure a
favorable therapeutic effect with limited side effects.
References
|
1
|
Berek JS and Natarajan S: Ovarian and
fallopian cancer. Berek & Novak’s Gynecology. 14th edition.
Berek JS: Lippincott Williams & Wilkins; pp. 1457–1647.
2007
|
|
2
|
Hollet JL: Screening, diagnosis and
treatment of cancer in long-term dialysis patients. Clin J Am Soc
Nephrol. 2:604–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chatelut E, Rostaing L, Gualano V, et al:
Pharmacokinetics of carboplatin in a patient suffering from
advanced ovarian carcinoma with hemodialysis-dependent renal
insufficiency. Nephron. 66:157–161. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe M, Aoki Y, Tomita M, et al:
Paclitaxel and carboplatin combination chemotherapy in a
hemodialysis patient with advanced ovarian cancer. Gynecol Oncol.
84:335–338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yokoyama Y, Futagami M, Higuchi T, et al:
Pharmacokinetic analysis of paclitaxel and carboplatin in a patient
with advanced ovarian cancer during hemodialysis – case report. Eur
J Gynaecol Oncol. 27:437–439. 2006.PubMed/NCBI
|
|
6
|
Calvert AH, Newell DR, Gumbrell LA, et al:
Carboplatin dosage: prospective evaluation of a simple formula
based on renal function. J Clin Oncol. 11:1748–1756.
1989.PubMed/NCBI
|
|
7
|
LeRoy AF, Wehling ML, Sponseller HL, et
al: Analysis of platinum in biological materials by flameless
atomic absorption spectrophotometry. Biochem Med. 18:184–191. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Longnecker SM, Donehower RC, Cates AE, et
al: High-performance liquid chromatographic assay for taxol in
human plasma and urine and pharmacokinetics in a phase I trial.
Cancer Treat Rep. 71:53–59. 1987.PubMed/NCBI
|
|
9
|
Sonnichsen DS and Relling MV: Clinical
pharmacokinetics of paclitaxel. Clin Pharmacokinet. 27:256–269.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motzer RJ, Niedzwiecki D, Isaacs M, et al:
Carboplatin-based chemotherapy with pharmacokinetic analysis for
patients with hemodialysis-dependent renal insufficiency. Cancer
Chemother Pharmacol. 27:234–238. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yanagawa H, Takishita Y, Bando H, et al:
Carboplatin-based chemotherapy in patients undergoing hemodialysis.
Anticancer Res. 16:533–536. 1996.PubMed/NCBI
|
|
12
|
Inoue A, Saijo Y, Kikuchi T, et al:
Pharmacokinetic analysis of combination chemotherapy with
carboplatin and etoposide in small-cell lung cancer patients
undergoing hemodialysis. Ann Oncol. 15:51–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takezawa K, Okamoto I, Fukuoka M, et al:
Pharmacokinetic analysis of carboplatin and etoposide in a small
cell lung cancer patient undergoing hemodialysis. J Thorac Oncol.
3:1073–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeyabalan N, Hirte HW and Moens F:
Treatment of advanced ovarian carcinoma with carboplatin and
paclitaxel in a patient with renal failure. Int J Gynecol Cancer.
10:463–468. 2000. View Article : Google Scholar : PubMed/NCBI
|