Introduction
Small-cell lung cancer (SCLC) accounts for
approximately 15% of all types of lung cancer (1). Despite the high sensitivity to
chemotherapy, the majority of patients develop relapse. Second-line
chemotherapy is considered for cases of relapsed SCLC; however, the
prognosis of such patients is usually poor. Topotecan has shown a
survival benefit against best supportive care and also a comparable
response rate and survival with combination chemotherapy of
cyclophosphamide, doxorubicin and vincristine (CAV) in relapsed
SCLC (2,3). Currently, topotecan is the only drug
approved by the US Food and Drug Administration for relapsed
SCLC.
Amrubicin (AMR), a totally synthetic
9-aminoanthra-cycline, is converted to an active metabolite,
amrubicinol, by reduction of its C-13 ketone group to a hydroxyl
group (4). In a phase I/II study of
patients with non-small-cell lung cancer, the recommended dose was
determined to be 45 mg/m2/day for 3 consecutive days
every 3 weeks (5). In a phase II
study of SCLC, 35 patients with extensive disease (ED) were treated
at the recommended dose, and a response rate of 75.8% and median
survival time (MST) of 11.7 months were reported (6).
In a phase II study evaluating the activity of AMR
in relapsed SCLC, the response rate and MST were 52%, 11.6 months
and 50%, 10.3 months in sensitive and refractory relapse,
respectively (7,8). In addition, AMR was compared with
topotecan in a randomized phase II study. In sensitive relapse
(n=36), response rates were 53% for AMR and 21% for topotecan, and
in refractory relapse, 17% for AMR and 0% for topotecan. Overall
survival (OS) was not significantly different between the two arms;
however, multivariate analysis showed that AMR had more influence
than topotecan on OS. Although the dose of AMR was 40
mg/m2/day, lower than the recommended dose,
hematological toxicity was severe in the AMR arm, and one
treatment-related death resulting from infection was observed in
the AMR arm (9).
AMR is one of the most active chemotherapeutic
agents for SCLC. However, currently it is not approved outside
Japan, and no extensive evidence of its effects exist. This study
examined relapsed SCLC patients treated with AMR in our hospital
and analyzed its efficacy and hematological toxicities.
Patients and methods
Patients
Between January 2002 and January 2009, 164 SCLC
patients were admitted to Kyoto University Hospital. Among them,
112 patients received chemotherapy alone, 36 chemoradiotherapy, 6
surgical resection with adjuvant chemotherapy and 10 best
supportive care. As a consequence, 92 patients received second-line
chemotherapy, including 69 that received AMR. In this study,
patients who responded to initial chemotherapy and relapsed >3
months after chemotherapy were defined as sensitive relapse (S),
while patients who did not respond to initial chemotherapy or
relapsed within 3 months were defined as refractory relapse (R)
patients. Patient data were obtained from our database. Consent was
obtained form all patients. The study was approved by the
Institutional Review Board.
Tumor evaluation and statistical
analysis
Tumor response was assessed according to the
Response Evaluation Criteria in Solid Tumors (10). Hematological toxicity was evaluated
according to the Common Terminology Criteria for Adverse Events
(CTCAE v3.0). Survival time was measured from the start of
chemotherapy to either the time of the patient succumbing to the
disease due to any cause or the date when patients were last known
to be alive. The survival curve was estimated using the
Kaplan-Meier method and compared using the log-rank test.
Individual clinical factors were compared using the χ2
test. Multivariate analysis was conducted according to the Cox
proportional hazards model. P<0.05 was considered to be
significant. Statistical analyses were performed using StatView,
version 5.0 (Abacus Concepts, Berkeley, CA, USA).
Results
Patient characteristics
Patient characteristics are listed in Table I. There were 27 S and 42 R cases.
The median age was 70 years for sensitive patients and 62 for
refractory patients. Most patients (~80%) were male in the two
groups. The proportion of ED cases was 63% in sensitive patients
and 88% in refractory patients. The proportion of performance
status (PS) 2–4 was higher in the R group. Patients had previously
received platinum agents, and ~40 and 70% had received etoposide
and irinotecan, respectively. AMR as second-line treatment was
received by 74% of patients and as third-line treatment by the
remaining patients.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | S (n=27) | R (n=42) | Total (n=69) |
|---|
| Age (years) | | | |
| Median | 70 | 62 | 66 |
| Range | 50–80 | 33–82 | 33–82 |
| Gender (%) | | | |
| Male | 23 (85) | 32 (76) | 55 (80) |
| Female | 4 (15) | 10 (24) | 14 (20) |
| Disease extent
(%) | | | |
| LD | 10 (37) | 5 (12) | 15 (22) |
| ED | 17 (63) | 37 (88) | 54 (78) |
| Response to
first-line treatment (%) | | | |
| CR | 8 (30) | 5 (12) | 13 (19) |
| PR | 19 (70) | 28 (67) | 47 (68) |
| SD | 0 (0) | 5 (12) | 5 (7) |
| PD | 0 (0) | 4 (9) | 4 (6) |
| PS at the timing of
AMR (%) | | | |
| 0–1 | 21 (78) | 27 (64) | 48 (70) |
| 2–4 | 6 (22) | 15 (36) | 21 (30) |
| No. of prior
chemotherapy regimens (%) | | | |
| 1 | 19 (70) | 32 (76) | 51 (74) |
| 2 | 8 (30) | 10 (24) | 18 (26) |
| Previous chemotherapy
(%) | | | |
| Platinum | 27 (100) | 42 (100) | 69 (100) |
| Etoposide | 13 (48) | 17 (40) | 30 (43) |
| Irinotecan | 20 (74) | 29 (69) | 49 (71) |
| Topotecan | 1 (4) | 3 (7) | 4 (6) |
Response and treatment delivery
Table II shows
results of the response and treatment delivery. There were 1
complete response (CR), 18 partial response (PR), 5 stable disease
(SD) and 3 progressive disease (PD) cases in the S group (response
rate 70%) and 1 CR, 15 PR, 8 SD and 18 PD cases in the R group
(response rate 38%). The median number of treatment cycles was 4
(range 1–8) in the S group and 2 (range 1–14) in the R group. The
median dose of AMR was 40 mg/m2 in the S group and 35
mg/m2 in the R group, respectively.
 | Table IIResponse and treatment delivery. |
Table II
Response and treatment delivery.
| S | R | Total |
|---|
| CR | 1 | 1 | 2 |
| PR | 18 | 15 | 33 |
| SD | 5 | 8 | 13 |
| PD | 3 | 18 | 21 |
| Response rate
(%) | 70 | 38 | 51 |
| No. of treatment
cycles |
| Median | 4 | 2 | 3 |
| Range | 1–8 | 1–14 | 1–14 |
| Dose
(mg/m2/day) |
| 25 | 0 | 1 | 1 |
| 30 | 6 | 11 | 17 |
| 35 | 6 | 19 | 25 |
| 40 | 9 | 8 | 17 |
| 45 | 6 | 3 | 9 |
Survival
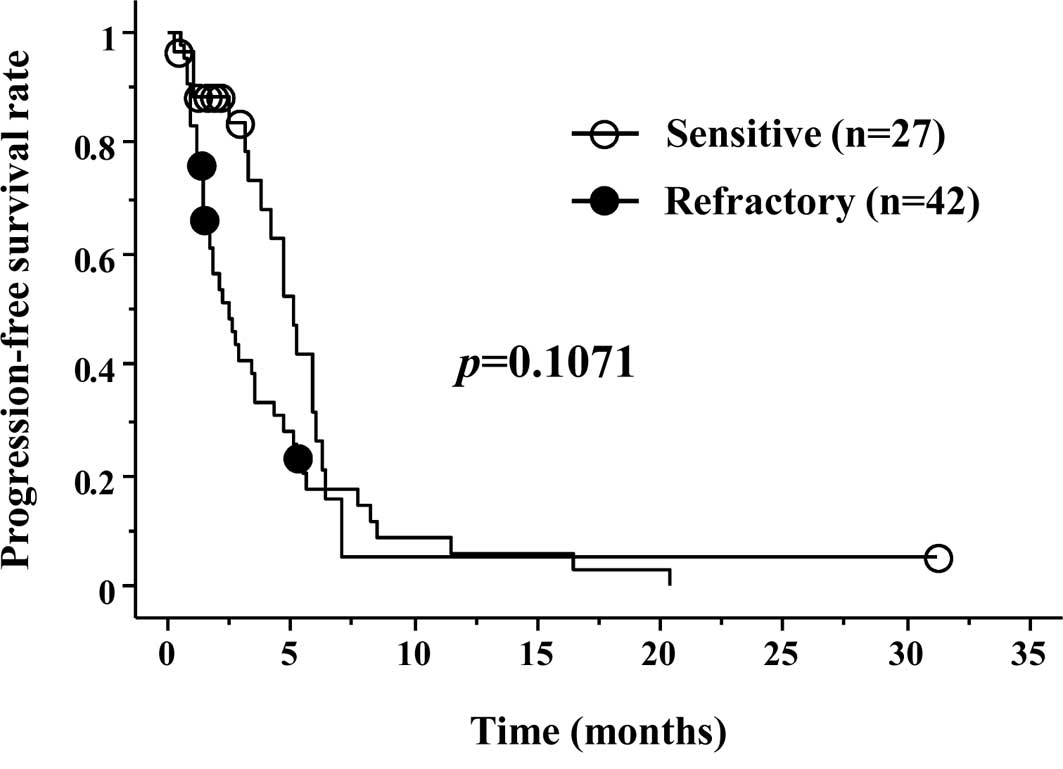
Progression-free survival (PFS) is shown in Fig. 1. Median PFS was 3.2 months in the S
group and 1.9 months in the R group, respectively (p=0.1071). OS
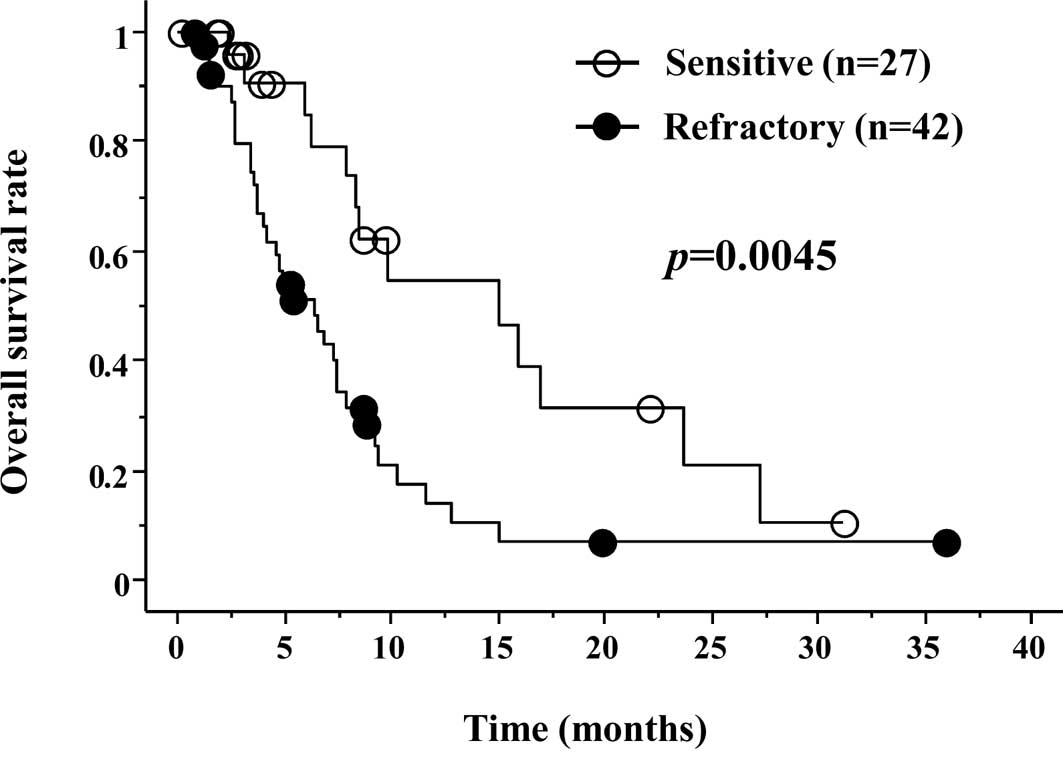
was significantly better in the S group (Fig. 2). Median OS was 6.2 months in the S
group and 4.8 months in the R group, respectively (p=0.0045).
Univariate and multivariate analyses showed that PS of 0–1 or 2–4
for AMR, and the relapse pattern (sensitive or refractory) were
both independent prognostic factors for OS (Table III).
 | Table IIIUnivariate and multivariate analyses
for overall survival. |
Table III
Univariate and multivariate analyses
for overall survival.
| Variables | MST (months) | Univariate | Multivariate |
|---|
| |
|
|
|---|
| | p-value | Risk ratio | 95% CI | p-value |
|---|
| Age |
| <70 | 5.3 | 0.9299 | 0.544 | 0.277–1.069 | 0.0775 |
| ≥70 | 4.9 | | | | |
| Gender |
| Male | 5.4 | 0.3841 | 2.148 | 0.942–4.901 | 0.0691 |
| Female | 5.0 | | | | |
| PS |
| 0–1 | 6.4 | 0.0039 | 0.388 | 0.197–0.763 | 0.0060 |
| 2–4 | 2.5 | | | | |
| Relapse pattern |
| Sensitive | 6.2 | 0.0045 | 3.533 | 1.656–7.540 | 0.0011 |
| Refractory | 4.8 | | | | |
Hematological toxicities
Results of the hematological toxicities are listed
in Table IV. The frequency of
grade ≥3 hematological toxicities was leukopenia (41%), neutropenia
(51%), anemia (14%), thrombocytopenia (17%) and febrile neutropenia
(12%). The number of patients requiring the administration of
granulocyte-stimulating factor (G-CSF), red blood cell transfusion
and platelet transfusion were 34 (50%), 7 (10%) and 2 (3%),
respectively. No treatment-related death was noted.
 | Table IVHematological toxicities. |
Table IV
Hematological toxicities.
| Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ≥Grade 3 (%) |
|---|
| Leukopenia | 12 | 16 | 18 | 10 | 28 (41) |
| Neutropenia | 10 | 18 | 17 | 18 | 35 (51) |
| Anemia | 19 | 32 | 6 | 4 | 10 (14) |
|
Thrombocytopenia | 18 | 8 | 8 | 4 | 12 (17) |
| Febrile
neutropenia | 0 | 0 | 8 | 0 | 8 (12) |
Discussion
SCLC is highly sensitive to chemotherapy. Therefore,
the main treatment strategy for SCLC is systemic chemotherapy.
Currently, both cisplatin + etoposide and cisplatin + irinotecan
are considered to be standard chemotherapeutic regimens for SCLC
(11). Despite the high initial
sensitivity to chemotherapy, the majority of patients develop
relapse, and the prognosis of patients with relapsed SCLC is
usually poor. The effects of topotecan, a DNA topoisomerase-I
inhibitor, are extensively evaluated in patients with relapsed
SCLC. Currently, topotecan is the only drug approved by the US Food
and Drug Administration for relapsed SCLC. AMR has shown promising
anti-tumor activity in previous phase II studies for relapsed SCLC,
as well as superiority in a randomized phase II study compared to
topotecan (7–9). AMR is at present being examined for
SCLC worldwide. However, it has yet to be approved outside Japan.
Therefore, clinical data regarding AMR is limited.
In a randomized phase II study comparing AMR with
topotecan, the response rate in the AMR arm was better in S with
53% in S and 17% in R cases, respectively (9). However, in previous single-arm phase
II studies of AMR, a comparable response rate was noted between S
and R cases [52 and 50% in Onoda et al (7); 40 and 50% in Kato et al
(8)]. Considering that R is
extremely chemo-resistant, the anti-tumor activity of AMR is
considerably promising. Although inferior to S, a response rate of
38% was observed in the R group in our study.
PFS was not significantly different between S and R
patients. However, OS was significantly more favorable in the S
patients (6.2 vs. 4.8 months, p=0.0045). Multivariate analysis
demonstrated that PS of 0–1 or 2–4 for AMR, and the relapse pattern
(sensitive or refractory) were both independent prognostic factors
for OS. The results were consistent with our previous study
(12).
Hematological toxicities, particularly neutropenia,
were found to be severe, as shown in previous studies. Although the
majority of patients received AMR under the recommended dose, 50%
required G-CSF support in our study. In previous single-arm phase
II studies of AMR, the frequency of grade ≥3 neutropenia and G-CSF
support were 83.3 and 70%, respectively, at a dose of 40
mg/m2, and 97 and 71%, respectively, at a dose of 45
mg/m2 (7,8). Since the recommended dose of 45
mg/m2 was determined in chemo-naïve patients, it may be
too high for previously treated patients. Igawa et al
evaluated the recommended dose of AMR in previously treated SCLC
patients and determined the dose to be 40 mg/m2 for
second-line and 35 mg/m2 for third-line treatment
(13).
In conclusion, in this retrospective study, we
confirmed that AMR has excellent anti-tumor activity, not only in
sensitive relapse, but also in refractory relapse, as shown in
previous phase II studies. These results warrant further evaluation
of AMR in both the second and first-line setting in limited- and
extensive-stage disease. We are currently conducting a phase II
study to assess the efficacy of consolidation chemotherapy
following AMR following standard chemoradiation in limited-stage
SCLC.
References
|
1
|
Jackman DM and Johnson BE: Small-cell lung
cancer. Lancet. 366:1385–1396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O’Brien ME, Ciuleanu TE, Tsekov H, et al:
Phase III trial comparing supportive care alone with supportive
care with oral topotecan in patients with relapsed small-cell lung
cancer. J Clin Oncol. 24:5441–5447. 2006.PubMed/NCBI
|
|
3
|
Von Pawel J, Schiller JH, Shepherd FA, et
al: Topotecan versus cyclophosphamide, doxorubicin and vincristine
for the treatment of recurrent small-cell lung cancer. J Clin
Oncol. 17:658–667. 1999.PubMed/NCBI
|
|
4
|
Inoue K, Ogawa M, Horikoshi N, et al:
Phase I and pharmacokinetic study of SM-5887, a new anthracycline
derivative. Invest New Drugs. 7:213–218. 1989.PubMed/NCBI
|
|
5
|
Negoro S, Fukuoka M, Nakamura S, et al:
Phase I-II study of amrubicin (SM-5887), a novel
9-aminoanthracycline, by iv administration for 3 consecutive days
in patients with advanced non-small cell lung cancer. Proc Am Soc
Clin Oncol. 14:3611995.
|
|
6
|
Yana T, Negoro S, Takada M, et al: Phase
II study of amrubicin in previously untreated patients with
extensive-disease small cell lung cancer: West Japan Thoracic
Oncology Group (WJTOG) study. Invest New Drugs. 25:253–258. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Onoda S, Masuda N, Seto T, et al: Phase II
trial of amrubicin for treatment of refractory or relapsed
small-cell lung cancer: Thoracic Oncology Research Group Study
0301. J Clin Oncol. 24:5448–5453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kato T, Nokihara H, Ohe Y, et al: Phase II
trial of amrubicin in patients with previously treated small cell
lung cancer (SCLC). Proc Am Soc Clin Oncol. 24:70612006.
|
|
9
|
Inoue A, Sugawara S, Yamazaki K, et al:
Randomized phase II trial comparing amrubicin with topotecan in
patients with previously treated small-cell lung cancer: North
Japan Lung Cancer Study Group Trial 0402. J Clin Oncol.
26:5401–5406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
11
|
Simon GR and Turrisi A: Management of
small cell lung cancer: ACCP evidence-based clinical practice
guidelines (2nd edition). Chest. 132:S324–S339. 2007. View Article : Google Scholar
|
|
12
|
Kim YH, Goto K, Yoh K, et al: Performance
status and sensitivity to first-line chemotherapy are significant
prognostic factors in patients with recurrent small cell lung
cancer receiving second-line chemotherapy. Cancer. 113:2518–2523.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Igawa S, Yamamoto N, Ueda S, et al:
Evaluation of the recommended dose and efficacy of amrubicin as
second- and third-line chemotherapy for small cell lung cancer. J
Thorac Oncol. 2:741–744. 2007. View Article : Google Scholar : PubMed/NCBI
|