Introduction
Human papillomavirus (HPV) infection is associated
with a broad spectrum of benign and malignant neoplastic epithelial
changes. The association of HPV with neoplastic transformation has
been extensively investigated in lesions of the uterine cervix.
Moreover, the role of HPV in malignant transformation of the
cervical epithelium is well established. On the other hand, HPV-DNA
has been found to be less common in penile cancer cases (1). The incidence of penile cancer is lower
compared to cervical cancer. Although several epidemiological,
clinical and pathological studies have indicated that this virus is
sexually transmitted, detailed information on the association of
HPV with penile cancer has yet to be sufficiently elucidated as
compared to cervical cancer. HPV infection is the most common
sexually transmitted disease, thus the risk factor increases with
certain sexual behaviors. The highest prevalence of HPV infection
is observed in sexually active adolescents and young adults. The
virus affects the squamous epithelium of the male genitalia in a
similar way to the female genital tract (1–3).
The HPV viral oncogenes, E6 and E7, are the main
contributors to the development of HPV-induced cancers, probably
due to the integration of the viral genes in the host cell genome.
E6 and E7 react with the tumor suppressor gene products p53 and pRb
in host cell proteins, resulting in induced cellular
immortalization, transformation and carcinogenesis, due to their
interference with cell cycle and apoptosis control (4). p16INK4a belongs to the
inhibitors of cyclin-dependent kinase (CDK)-4 families (INK4a
family) that decelerate the cell cycle by inactivating the
cyclin-dependent kinase inhibitors. By interacting with CDK4 and
CDK6, p16INK4a inhibits the formation of the cyclin
D/CDK4 and CDK6 complex, which is a proliferation-stimulating
protein. The p16INK4a protein prevents the
phosphorylation of pRb family members. Normally, the overexpression
of p16INK4a results in the inhibition of E2F-dependent
transcription and of cell cycle progression at the G1 to S phase of
the cell cycle (5). The nuclear and
cytoplasmic overexpression of p16INK4a protein was
previously reported for certain cancers (5–10).
NF-κB was first discovered as a protein bound to the
κ immunoglobulin light chain gene enhancer in the nuclei of B cells
(11). NF-κB transcription factors
consist of five homologous subunits, such as RelA/p65, c-Rel, RelB,
p50/NF-κB1 and p52/NF-κB2. IκB-bound NF-κB dimers are IκB kinase
(IKK) complexes, comprising two catalytic (IKKα and IKKβ) and one
regulatory (IKKγ/NEMO) subunit (12). The causal relationship between
chronic inflammation and cancer is widely accepted. Numerous
investigations have identified nuclear factor-κB (NF-κB) as an
important modulator in driving chronic inflammation to cancer. This
transcriptional factor is indispensable for the malignant
progression of transformed cells associated with various
inflammatory cells and a network of signaling molecules.
Significant factors for cancer development include self-sufficiency
in growth signals, insensitivity to growth inhibitors, evasion of
apoptosis, limitless proliferation potential, tissue invasion and
sustained angiogenesis. Experimental evidence revealing specific
mechanisms by which NF-κB influences cancer initiation, promotion
and progression has been reported (13,14).
The expression and function of numerous cytokines, chemokines,
growth factors and survival factors are NF-κB-dependent. NF-κB
activation has been implicated in a variety of processes related to
transformation and oncogenesis (15).
To the best of our knowledge, the
immunohistochemical localization of NF-κB associated with HPV
infection in penile cancer was previously reported using materials
obtained from previous studies of Kenya, East Africa and Nagasaki,
Japan (16,17). The purpose of this study was to
determine the relationship among HPV infection,
p16INK4a, p53 and NF-κB in penile cancer in northern
Thailand.
Materials and methods
Tissue specimens
Penile tissue used in this study, was obtained from
51 surgical cases. Surgery was performed at Chiang Mai University
Hospital in northern Thailand. The specimens were fixed in 10%
formalin and embedded in paraffin for histochemical, in situ
hybridization (ISH) and HPV-DNA sequence studies. Histological
analysis was performed using 3.5-μm tissue sections stained with
hematoxylin and eosin. Penile cancer was classfied as i)
keratinizing and ii) non-keratinizing squamous cell carcinoma.
In situ hybridization
Paraffin-embedded tissue specimens were cut at
3.5-μm sections and collected on silane-coated glass slides. To
detect HPV-DNA, the ISH detection kit (Dako, Carpinteria, CA, USA)
was used. Wide spectrum HPV-DNA (Dako), ISH-positive control and
ISH-negative control probes, as well as HPV-positive control slides
were examined.
The ISH procedure using the HPV detection kit was
performed as follows: following hybridization with the probes,
alkaline phosphatase conjugated antibody against digoxigenin was
applied to the sections. The localization of HPV-DNA was detected
by using NBT/BCIP substrate and observed under a light
microscope.
Immunohistochemistry for NF-κB
Sections of 3.5 μm were placed on silane-coated
glass slides. The slides were deparaffinized to remove embedded
medium and dehydrated. They were then boiled in 0.01 mol/l citrate
buffer (pH 7.0) at 98˚C for 40 min for antigen retrieval and cooled
at room temperature for 30 min. After rinsing the slides in 0.01 M
phosphate-buffered saline (PBS) (pH 7.4), the endogenous peroxidase
activity was blocked with 3% H2O2 and
absolute methanol for 10 min. The tissue sections were covered with
1:50 dilution of mouse monoclonal anti-human NF-κB antibody (Cell
Signaling Technology Inc., Beverly, MA, USA) or control serum at
37˚C for 3 h. After being washed with PBS, the sections were
covered with EnVision (Dako) at 37˚C for 40 min and rinsed in PBS.
Antigenic sites on sections were demonstrated by reacting these
sections with a mixture of 0.05% 3,3′-diaminobenzidine
tetrahydrochloride in 0.05 M Tris-HCl buffer and 0.01% hydrogen
peroxide for 10 min. The sections were then counterstained with
methyl green for 10 min, dehydrated in ethanol, cleared in xylene
and mounted.
Results
Clinicopathological findings
The age of penile cancer patients ranged from 29 to
76 years (mean 56.9). Of the 51 cases of penile cancer included in
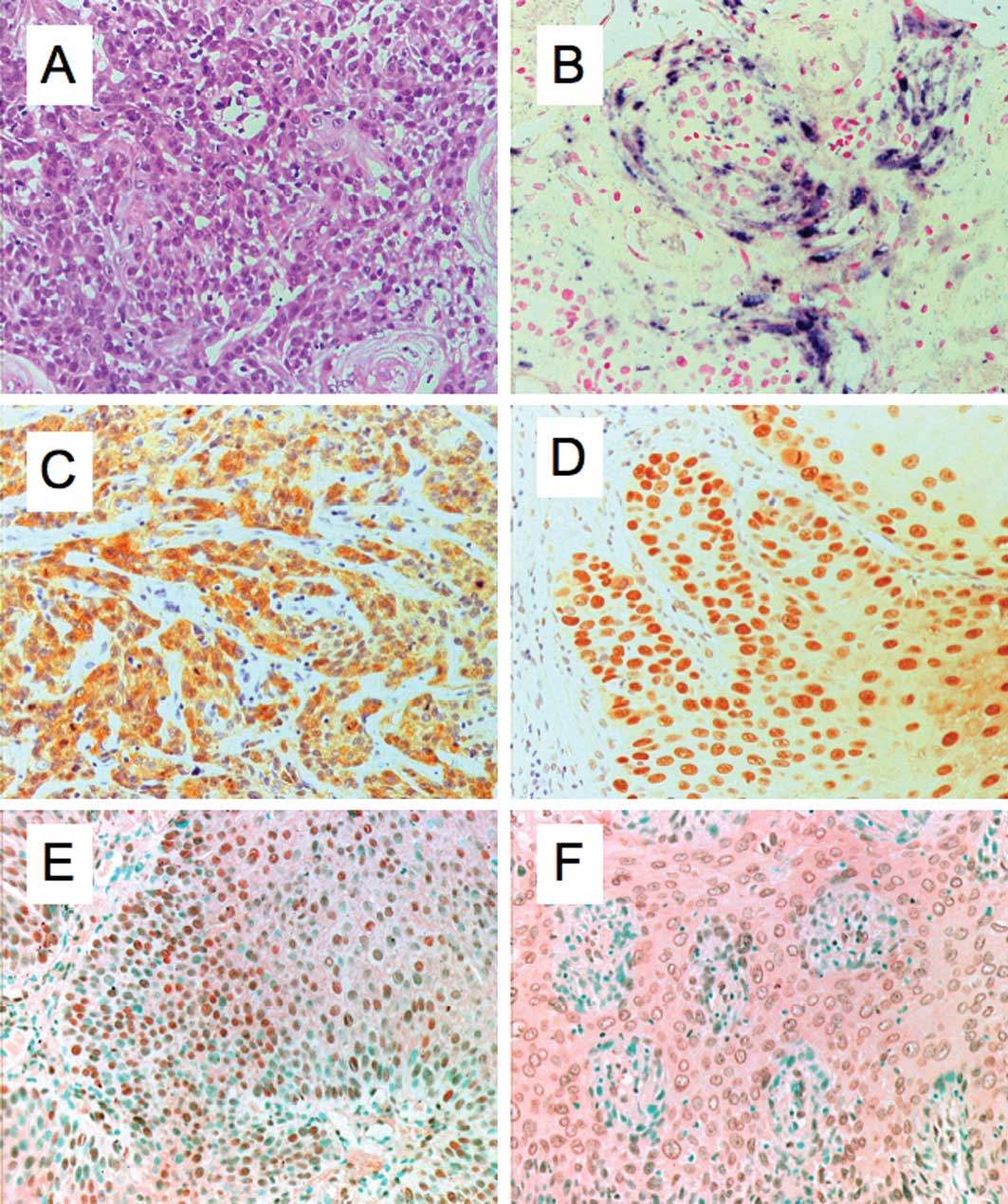
the study, 42 cases were classified as keratinizing (Fig. 1A) and 9 cases as non-keratinizing
squamous cell carcinoma.
Detection of HPV-DNA,
p16INK4a, p53 and NF-κB
Of the 51 cases, 39 (76.5%) were HPV-DNA-positive in
penile cancer, as determined by the ISH procedure (Fig. 1B). In the keratinizing squamous cell
carcinoma type, 32 (76.2%) out of 42 cases were HPV-DNA-positive.
On the other hand, in the non-keratinizing squamous cell carcinoma
type, 7 (77.8%) out of 9 cases were HPV-DNA-positive.
Immunohistochemical analysis for
p16INK4a, p53 and NF-κB was performed for all of the
specimens. The results in relation to HPV are summarized in
Table I. Of the 51 cases, 39
(76.5%) were HPV-positive and 12 (23.5%) were HPV-negative. Of the
39 HPV-positive cases, 24 (61.5) were p16INK4a-positive
(Fig. 1C) and 28 (71.8%) were
p53-positive (Fig. 1D). Of the 12
HPV-negative cases, 3 (25%) were p16INK4a-positive and 9
(75%) were p53-positive. Of the 39 HPV-positive cases, 29 (74.4%)
were NF-κB-positive in the cytoplasm (Fig. 1E) and 35 (73.3%) in the nucleus
(Fig. 1F). NF-κB was detected in
the nucleus and/or cytoplasm in 37 (94.9%) of the 39 HPV-positive
cases. Of the 12 HPV-negative cases, 4 (33.3%) were NF-κB-positive
in both the nucleus and cytoplasm.
 | Table IDetection of HPV-DNA,
p16INK4a, p53 and NF-κB in penile carcinoma. |
Table I
Detection of HPV-DNA,
p16INK4a, p53 and NF-κB in penile carcinoma.
| Cases | HPV DNA |
p16INK4a | p53 | NF-κB in nucleus | NF-κB in
cytoplasm | NF-κB in nucleus
and/or cytoplasm |
|---|
|
|
|
|
|
|
|
|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
|---|
| HPV-positive
cases | 39 | 76.5 | 24 | 61.5 | 28 | 71.8 | 35 | 89.7 | 29 | 74.4 | 37 | 94.9 |
| Keratinizing
SCC | 32 | 62.7 | 18 | 56.3 | 24 | 75.0 | 28 | 87.5 | 23 | 71.2 | 30 | 93.8 |
| Non-keratinizing
SCC | 7 | 13.7 | 6 | 85.7 | 4 | 57.1 | 7 | 100.0 | 6 | 85.7 | 7 | 100.0 |
| HPV-negative
cases | 12 | 23.5 | 3 | 25.0 | 9 | 75.0 | 4 | 33.3 | 4 | 33.3 | 4 | 33.3 |
| Keratinizing
SCC | 10 | 19.6 | 2 | 20.0 | 8 | 80.0 | 4 | 40.0 | 4 | 40.0 | 4 | 40.0 |
| Non-keratinizing
SCC | 2 | 3.9 | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total | 51 | 100.0 | 27 | 52.9 | 37 | 72.5 | 39 | 76.5 | 33 | 64.7 | 41 | 80.4 |
Discussion
Penile cancer is a relatively rare disease in Europe
and the United States, with incidence rates varying from 0.5–1.5
per 100,000 men. On the other hand, in developing countries, it is
a more common type of cancer, with an incidence of 2–5 per 100,000
men. The highest frequency of penile cancer occurs in Asia, Africa
and Latin America (1).
HPV is a large family of DNA viruses with more than
100 different HPVs, and with approximately 40 HPVs infecting the
genital mucosa following sexual transmission. HPVs are classified
into two groups depending on whether it causes benign or malignant
tumors. The high-risk group includes HPV-16, -18, -31, -33, -35,
-39, -45, -51, -54, -56, -58, -59, -66, -68 and -69; and the
low-risk group includes HPV-6, -11, -26, -30, -34, -40, -42, -43,
-44, -53, -55, -57, -61, -62, -64, -67, -70, -71, -73, -74, -79,
-81, -82, -83 and -84 (2). The most
common oncogenic HPV genotypes causing cervical cancer are HPV-16
and-18. HPV-16 is the most prevalent high-risk type and is found in
cervical cancer, with HPV-18 being the second most prevalent.
However, in our investigation, HPV-18 was found to be the most
prevalent type in penile cancer in northern Thailand (18). HPVs are believed to be the primary
causal agents for the development of benign and malignant tumors in
mucosal and skin lesions, and high-risk types such as HPV-16 and
-18 are associated with more than 90% of all cervical cancers.
HPV-6 and-11 are usually associated with common genital condyloma.
However, we cannot exclude the possibility of cancer formation from
low-risk HPV types. The prevalence of HPV-DNA is significantly
greater in cancer of the genital organs than in other organs.
HPV-related disease is well documented in lesions of the female
genital organs, and results have been obtained using a wide range
of epidemiological, clinical and molecular techniques. The
prevalence of HPV in the tumor tissue has been reported to vary
considerably. The frequency of HPV-positive (Fig. 1B) penile cancer (76.5%) reported in
this study (Table I) is similar to
that reported by Picconi et al (71%) (19) and Cupp et al (66%) (20). Higher expression rates were reported
by Senba et al (79%) (18),
Sarkar et al (82%) (21) and
Tornesello et al (83%) (22). Lower expression rates were reported
by Tornesello et al (46%) (1), Rubin et al (42%) (23) and Cubilla et al (31%)
(24).
HPV is a DNA tumor virus whose genome is organized
in three regions, including the early gene (E1 to E7), the late
gene (L1 and L2) regions and the upper regulatory region. The
nuclear protein in E6 and E7 of HPV is considered to be one of the
two major proteins involved in malignant transformation that is
consistently transcribed in HPV-positive cervical cancers. HPV E6
and E7 oncoproteins are essential factors for HPV oncogenesis.
Inactivation of tumor suppressor p53 and pRb is a common event in
the carcinogenesis of human cells. E7- and E6-induced genetic
instability leads to the activation of oncogenes and inactivation
of tumor suppressor genes. In HPV infection, significant
interactions are noted between pRb and p53 proteins, which are
important molecules in the cell cycle and apoptosis control.
Notably, pRb and p53 proteins are mutated in many human types of
cancer. Both E6 and E7 oncogenes interact with pRb and p53, which
inhibit the activities of these tumor suppressors. The cell cycle
in the S phase normally leads to apoptosis via pRb activity.
However, in HPV-infected cells, this process is counteracted by the
viral E6 protein, which targets p53 for proteolytic degradation
(4). The E7 protein interacts with
pRb, an important negative point of entry into the S phase of the
cell division cycle. In the hypophosphorylated state, a combination
of E7 and pRb activates the E2F transcription factors that trigger
the expression of proteins necessary for DNA replication and cell
cycle progression. Phosphorylation of pRb by G1 cyclin-dependent
kinases releases E2F, leading to cell cycle progression in the S
phase. Since E7 is able to bind to unphosphorylated pRb, it may
prematurely induce cells to enter the S phase by disrupting pRb-E2F
complexes (25). The loss of p53
function is implicated in the pathogenesis of tumors as well as in
the prognosis of many neoplasms. The mutant protein accumulates in
the nucleus of tumor cells and is identified by immunohistochemical
reaction. The frequency of the overexpression of the p53 protein
product was reported to be 41–89% in penile squamous cell
carcinomas (8,26,27).
Our data showed that overexpression of the p53 protein product was
detected in 37 (72.5%) of 51 penile cancer cases with and without
HPV infection.
The p16INK4a is a cyclin-dependent kinase
inhibitor that prevents the phosphorylation of pRb family members.
Normally, the overexpression of p16INK4a results in the
inhibition of E2F-dependent transcription and of cell cycle
progression at the G1 to S checkpoint (5). The repression of the
p16INK4a gene expression by hypermethylation or mutation
is a common occurrence in cancer. There is a close association
between p16INK4a overexpression and high-risk HPV
infection. Therefore, overexpression of p16INK4a is
suggested to be a useful marker for evaluating HPV activity in
cancer lesions and its precursors (6,9). The
overexpression frequency of p16INK4a was reported to be
55–97% in cervical squamous cell carcinoma (6–8) and
48–80% in cervical adenocarcinoma (28,29).
The overexpression frequency of p16INK4a was previously
reported to be 29–50% in penile squamous cell carcinoma (9,10).
Klaes and co-workers reported that p16INK4a is a
specific biomarker used to identify dysplastic cervical epithelia
in sections of cervical biopsy samples or cervical smears (7). As shown in Table I, overexpression of the
p16INK4a protein product was observed in 24 (61.5%) of
39 HPV-positive cases, and in 3 (25.0%) of 12 HPV-negative cases.
Therefore, overexpression of p16INK4a was higher in
HPV-positive vs. HPV-negative cases. Moreover, overexpression of
p16INK4a was found to have a higher prevalence in
cervical vs. penile cancer (30,31).
In our data, no significant association of a p16INK4a
(Fig. 1C) and p53 (Fig. 1D) abnormality with HPV-DNA was noted
in the penile cancer.
Two pathways are identified in NF-κB signaling. One
depends on NEMO, IKKβ activation, nuclear localization of RelA/p50
dimers, and is associated with inflammation. The other depends on
IKKα activation probably via the upstream kinase NIK and nuclear
localization of p52/RelB dimers. The two pathways involved in the
activation of NF-κB are implicated in oncogenesis (32). Activation of NF-κB was observed in
many types of cancer, including melanoma, lung cancer, colon
cancer, multiple myeloma, pancreatic cancer, esophageal
adenocarcinoma, leukemia and lymphoma. Increased NF-κB activity is
associated with viral infections. NF-κB activity is modulated for
many different viruses, such as HIV-1, HTLV-1, EBV, HBV, adenovirus
and HPV (33–38). NF-κB-dependent proliferation and
protection from apoptosis are likely to have significant effects on
the oncogenesis of HPV associated with cancers. As shown in
Table I and Fig. 1E and F, NF-κB was detected in the
HPV-positive (94.9%) and HPV-negative cases (33.3%) in the nucleus
and/or cytoplasm, respectively. HPV E6- and E7-positive cells have
shown IL-1b-induced NF-κB activation and elevated levels of NF-κB
components (35). E6 as opposed to
E7 expression was found to be associated with the nuclear location
of these components. It is frequently reported that HPV-encoded E6
and E7 oncoproteins are important regulatory proteins in host
cells, which are associated with the transcriptional activity of
NF-κB (33). A fraction of the E7
protein is found in association with the IκB kinase complex and
attenuates the induced kinase activity of IκB kinase α (IKKα) and
IKKβ, resulting in impaired IκBα phosphorylation and degradation.
While E7 obviates IKK activation in the cytoplasm, the E6 protein
reduces NF-κB p65-dependent transcriptional activity within the
nucleus. It is suggested that the HPV oncogene-mediated suppression
of NF-κB activity contributes to HPV escaping from the immune
system (34).
References
|
1
|
Tornesello ML, Duraturo ML, Losito S, et
al: Human papillomavirus genotypes and HPV 16 variants in penile
carcinoma. Int J Cancer. 122:132–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gross G and Pfister H: Role of human
papillomavirus in penile cancer, penile intraepithelial squamous
cell neoplasias and in genital warts. Med Microbiol Immunol.
193:35–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Senba M, Mori N and Wada A: Oncogenesis
and the link between inflammation and cancer due to human
papillomavirus (HPV) infection, and the development of vaccine
control strategies. Cancer Res J. 2:307–338. 2009.
|
|
4
|
Munger K, Baldwin A, Edwards KM, et al:
Mechanisms of human papillomavirus-induced oncogenesis. J Virol.
78:11451–11460. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang HS, Postigo AA and Dean DC: Active
transcriptional repression by the Rb-E2F complex mediates G1 arrest
triggered by p16INK4a, TGFb, and contact inhibition.
Cell. 97:53–61. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sano T, Oyama T, Kashiwabara K, Fukuda T
and Nakajima T: Expression status of p16 protein is associated with
human papillomavirus oncogenic potential in cervical and genital
lesions. Am J Pathol. 153:1741–1748. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klaes R, Friedrich T, Spitkovsky D, et al:
Overexpression of p16INK4a as a specific marker for
dysplastic and neoplastic epithelial cells of the cervix uteri. Int
J Cancer. 92:276–284. 2001.
|
|
8
|
Humbey O, Cairey-Remonnay S, Guerrini JS,
et al: Detection of the human papillomavirus and analysis of the
TP53 polymorphism of exon 4 at codon 72 in penile squamous cell
carcinoma. Eur J Cancer. 39:684–690. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferreux E, Lont AP, Horenblas S, et al:
Evidence for at least three alternative mechanisms targeting the
p16INK4a/cyclin D/Rb pathway in penile carcinoma, one of
which is mediated by high-risk human papillomavirus. J Pathol.
201:109–118. 2003.PubMed/NCBI
|
|
10
|
Prowse DM, Ktori EN, Chandrasekaran D,
Prapa A and Baithun S: Human papillomavirus-associated increase in
p16INK4a expression in penile lichen sclerosus and
squamous cell carcinoma. Br J Dermatol. 158:261–265.
2008.PubMed/NCBI
|
|
11
|
Sen R and Baltimore D: Inducibility of
kappa immunoglobulin enhancer-binding protein NF-kappa B by a
posttranslational mechanism. Cell. 47:921–928. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rothwarf DM, Zandi E, Natoli G and Karin
M: IKK-gamma is an essential regulatory subunit of the IkappaB
kinase complex. Nature. 395:297–300. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karin M and Greten FR: NF-κB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005.
|
|
14
|
Karin M: Nuclear factor-κB in cancer
development and progression. Nature. 441:431–436. 2006.
|
|
15
|
Kiriakidis S, Andreakos E, Monaco C,
Foxwell B, Feldmann M and Paleolog E: VEGF expression in human
macrophages is NF-κB-dependent: studies using adenoviruses
expressing the endogenous NF-κB inhibitor IkkappaBalpha and a
kinase-defective form of the IkkappaB kinase 2. J Cell Sci.
116:665–674. 2003.
|
|
16
|
Senba M, Buziba N, Mori N, Wada A, Irie S
and Toriyama K: Detection of human papillomavirus and cellular
regulators p16INK4A, p53, and NF-κB in penile cancer
cases in Kenya. Acta Virol. 53:43–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Senba M, Mori N, Wada A, et al: Human
papillomavirus genotypes in penile cancers from Japanese patients
and HPV-induced NF-κB activation. Oncol Lett. 1:267–272.
2010.PubMed/NCBI
|
|
18
|
Senba M, Kumatori A, Fujita S, et al: The
prevalence of human papillomavirus genotypes in penile cancers from
northern Thailand. J Med Virol. 78:1341–1346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Picconi MA, Eijan AM, Distefano AL, et al:
Human papillomavirus (HPV) DNA in penile carcinomas in Argentina:
analysis of primary tumors and lymph nodes. J Med Virol. 61:65–69.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cupp MR, Malek RS, Goellner JR, Smith TF
and Espy MJ: The detection of human papillomavirus deoxyribonucleic
acid in intraepithelial, in situ, verrucous and invasive carcinoma
of the penis. J Urol. 154:1024–1029. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sarkar FH, Miles BJ, Plieth DH and
Crissman JD: Detection of human papillomavirus in squamous neoplasm
of the penis. J Urol. 147:389–392. 1992.PubMed/NCBI
|
|
22
|
Tornesello ML, Buonaguro FM, Beth-Giraldo
E, Kyalwazi SK and Giraldo G: Human papillomavirus (HPV) DNA in
penile carcinomas and in two cell lines from high-incidence areas
for genital cancers in Africa. Int J Cancer. 51:587–592. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rubin MA, Kleter B, Zhou M, et al:
Detection and typing of human papillomavirus DNA in penile
carcinoma: evidence for multiple independent pathways of penile
carcinogenesis. Am J Pathol. 159:1211–1218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cubilla AL, Reuter VE, Gregoire L, et al:
Basaloid squamous cell carcinoma: a distinctive human papilloma
virus-related penile neoplasm. A report of 20 cases. Am J Surg
Pathol. 22:755–761. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nguyen DX, Nguyen MM, Lee D, Griep AE and
Lambert PF: Human papillomavirus type 16 E7 maintains elevated
levels of the cdk25A tyrosine phosphatase during deregulation of
cell cycle arrest. J Virol. 77:619–632. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lam KY and Chan KW: Molecular pathology
and clinicopathologic features of penile tumors: with special
reference to analyses of p21 and p53 expression and unusual
histologic features. Arch Pathol Lab Med. 123:895–904.
1999.PubMed/NCBI
|
|
27
|
Lopes A, Bezerra AL, Pino CA, Serrano SV,
Mello CA and Villa LL: p53 as a new prognostic factor for lymph
node metastasis in penile carcinoma: analysis of 82 patients
treated with amputation and bilateral lymphadenectomy. J Urol.
168:81–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Milde-Langosch K, Riethdorf S,
Kraus-Poppinghaus A, Riethdorf L and Loning T: Expression of
cyclin-dependent kinase inhibitors p16MTS1,
p21WAF1, and p27KIP1 in HPV-positive and
HPV-negative cervical adenocarcinomas. Virch Arch. 439:55–61. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zielinski GD, Snijders PJ, Rozendaal L, et
al: The presence of high-risk HPV combined with specific p53 and
p16INK4a expression patterns points to high-risk HPV as
the main causative agent for adenocarcinoma in situ and
adenocarcinoma of the cervix. J Pathol. 201:535–543.
2003.PubMed/NCBI
|
|
30
|
Kalof AN, Evans MF, Simmons-Arnold L,
Beatty BG and Cooper K: p16INK4a immunoexpression and
HPV in situ hybridization signal patterns: potential markers of
high-grade cervical intraepithelial neoplasia. Am J Surg Pathol.
29:674–679. 2005.PubMed/NCBI
|
|
31
|
Dehn D, Torkko KC and Shroyer KR: Human
papillomavirus testing and molecular markers of cervical dysplasia
and carcinoma. Cancer. 111:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Naugler WE and Karin M: NF-κB and cancer –
identifying targets and mechanisms. Curr Opin Genet Dev. 18:19–26.
2008.
|
|
33
|
Nees M, Geoghegan JM, Hyman T, Frank S,
Miller L and Woodworth CD: Papillomavirus type 16 oncogenes
downregulate expression of interferon-responsive genes and
upregulate proliferation-associated and NF-κB-responsive genes in
cervical keratinocytes. J Virol. 75:4283–4296. 2001.PubMed/NCBI
|
|
34
|
Spitkovsky D, Hehner SP, Hofmann TG,
Moller A and Schmitz ML: The human papillomavirus oncoprotein E7
attenuates NF-κB activation by targeting IκB kinase complex. J Bio
Chem. 277:25576–25582. 2002.PubMed/NCBI
|
|
35
|
Havard L, Delvenne P, Frare P, Boniver J
and Giannini SL: Differential production of cytokines and
activation of NF-κB in HPV transformed keratinocytes. Virology.
298:271–285. 2002.
|
|
36
|
Havard L, Rahmouni S, Boniver J and
Delvenne P: High levels of p105 (NF-κB1) and p100 (NF-κB2) proteins
in HPV 16-transformed keratinocytes: role of E6 and E7
oncoproteins. Virology. 331:357–366. 2005.
|
|
37
|
Mishra A, Bharti AC, Varghese P, Saluja D
and Das BC: Differential expression and activation of NF-κB family
proteins during oral carcinogenesis: role of high risk human
papillomavirus infection. Int J Cancer. 119:2840–2850. 2006.
|
|
38
|
James MA, Lee JH and Klingelhutz AJ: Human
papillomavirus type 16 E6 activates NF-κB, induces cIAP-2
expression, and protects against apoptosis in a PDZ binding
motif-dependent manner. J Virol. 80:5301–5307. 2006.
|