Introduction
Tumor stage is the most potent and widely accepted
parameter predictive of survival for patients with stage I–IV
non-small cell lung cancer (NSCLC) (1). Although locoregional control of NSCLC
can be achieved by curative resection, more than 70% of patients
with stage I disease suffer either a locoregional relapse or
distant metastasis. A number of prognostic molecular markers have
been described for patients with NSCLC, but none are currently in
use in treatment decision making (2).
Ribonucleotide reductase (RR) is a highly regulated
rate-limiting enzyme in the conversion of ribonucleoside
diphosphate to 2′-deoxyribonucleoside diphosphate, which is
essential for DNA synthesis (3). In
humans, one large subunit (M1) and two small subunits (hRRM2 and
p53R2) of RR have been identified (4). Inhibition of RR activity has been
tested as a potential treatment modality in anticancer settings
(5). The two RR small subunits,
p53R2 and hRRM2, have an 80% similarity in the protein sequence
(4). An in vitro assay
showed that recombinant p53R2 protein, as well as hRRM2, interacts
with hRRM1 to form a holoenzyme with the ability to convert CDP to
dCDP (6,7). Piao et al demonstrated that
p53R2 negatively modulates serum-induced MEK-ERK activity and
inhibits the MEK-ERK-mediated malignancy potential of human cancer
cells (8). Overexpression of p53R2
is associated with clinical response in myelodysplatic
syndrome/acute myelogenous leukemia (9). It has been suggested that the opposite
regulation of hRRM2 and p53R2 in the invasion potential may play a
critical role in determining the invasion and metastasis phenotype
in cancer cells (10). The
implications of p53R2 expression, a regulatory subunit of RR, have
yet to be determined and deserve further investigation.
Subsequently, an immunohistochemical method was evaluated to
determine the association between p53R2 expression and the clinical
outcome of early stage NSCLC.
Materials and methods
Patients and samples
From January 2000 to December 2006, a total of 92
consecutive patients underwent surgical treatment for NSCLC at the
China Medical University Hospital in Taichung, Taiwan. Patients who
had pre-operative chemotherapy or radiotherapy were excluded from
this study. Prior to surgery, written informed consent for the use
of paraffin-embedded tissues and for information regarding
sociodemographic characteristics was obtained from each patient.
The study protocol was approved by the Institutional Review Board
of the China Medical University Hospital. All of the available
paraffin blocks were reviewed by a thoracic pathologist.
The study population consisted of 69 men and 23
women (mean age 65.3 years; range 36–83). The procedures included
sampling of hilar and mediastinal lymph nodes, and the pathology of
the specimens confirmed stage I (T1-2N0M0) in 66 patients and stage
II (T1N1, T2N1 and T3N0) in 26 patients. Histological
classification and grade were assessed by light microscopy
according to the World Health Organization criteria (1). Clinical data including gender, age
(≤65 vs. >65 years), smoking habits, histopathology (squamous
cell carcinoma vs. adenocarcinoma vs. others), tumor stage by TNM
(T1 vs. T2), lymphovascular invasion and tumor differentiation were
collected from the patient charts and are shown in Table I.
 | Table IClinicopathological characteristics of
the patients with early stage lung cancer. |
Table I
Clinicopathological characteristics of
the patients with early stage lung cancer.
| Parameters | Patient no. (%) |
|---|
| Age (years) |
| ≤65 | 40 (43.5) |
| >65 | 52 (56.5) |
| Gender |
| Female | 23 (25.0) |
| Male | 69 (75.0) |
| Smoking status |
| Negative | 46 (50.0) |
| Positive | 46 (50.0) |
| Tumor type |
| AD | 52 (56.5) |
| SCC | 40 (43.5) |
| Tumor stage |
| I | 66 (71.7) |
| II | 26 (28.3) |
| Lymphovascular
invasion |
| Negative | 76 (82.6) |
| Positive | 16 (17.4) |
|
Recurrence/metastasis |
| Negative | 59 (64.1) |
| Positive | 32 (35.9) |
| Differentiation |
| Well | 12 (13.0) |
| Moderate | 55 (59.8) |
| Poor | 25 (27.2) |
Postoperative follow-up visits were scheduled 1 and
2 months after surgery, every 3 months during the first 2 years and
every 6 months following that, or more frequently if required. The
median duration of follow-up after a curative resection was 4.8
years.
Tissue microarray and immunohistochemical
staining
Formalin-fixed and paraffin-embedded specimens were
sectioned at 3 μm. The sections were then deparaffinized in xylene,
rehydrated through serial dilutions of alcohol and washed in
phosphate-buffered saline (PBS) (pH 7.2), which was the buffer used
for all subsequent washes. For p53R2 detection, sections were
heated in a microwave oven twice for 5 min in citrate buffer (pH
6.0) and then incubated with a polyclonal anti-p53R2 (Santa Cruz,
CA, USA) for 90 min at 25˚C. The conventional streptavidin
peroxidase method (LSAB kit K675; Dako, Copenhagen, Denmark) was
performed to develop signals, and the cells were counterstained
with hematoxylin. Negative controls were obtained by omitting the
primary antibody. The signal intensities were evaluated
independently by three observers. Cases with 0–10% positive nuclei
were defined as having negative immunostaining, and cases with
>10% positive nuclei were exhibited positive immunostaining. The
antibody dilution buffer was used to replace antibodies to serve as
a negative control.
Statistical analysis
Data were collected using an MS-Excel spreadsheet.
The data were analyzed using the JMP Statistical Discovery Software
version 6.0 (SAS Institute, Cary, NC, USA). For categorical data,
the Fisher’s exact test or the binomial test of proportions was
employed. Survival rates were estimated using the Kaplan-Meier
method, and statistical analysis was carried out using the log-rank
test for equality of the survival curves. Multivariate survival
analysis was carried out on variables that were found to be
significant with univariate analysis using the Cox proportional
hazards model. Results from this model are reported as relative
risk with 95% CI. Statistical significance was set at
P<0.05.
Results
Relationship of p53R2 expression with
clinicopathological parameters
To elucidate the role of p53R2 in tumor progression,
92 patients with early stage lung cancer, including 66 with stage I
and 26 with stage II, were enrolled in this study. p53R2 protein
expression in lung tumors was analyzed by immunohistochemistry in a
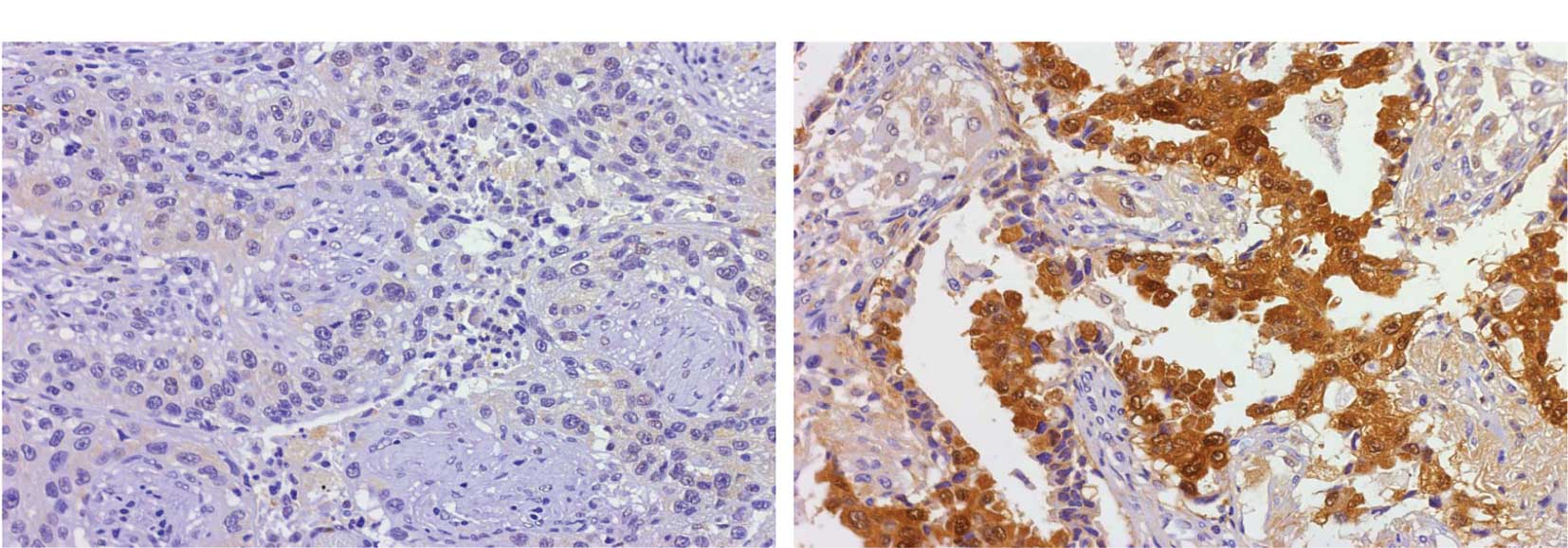
tissue array section. p53R2 was expressed in 32 (34.8%) patients.
The p53R2 proteins were expressed in the cytoplasm of the lung
tumor cells as shown in Fig. 1.
Table II shows that no correlation
occurred between p53R2 and the clinicopathological parameters.
However, p53R2 expression in the well-differentiated tumor cells
had a higher trend of expression than in the poorly differentiated
tumor cells (P=0.051).
 | Table IIAssociation between p53R2 protein
expression and clinical characteristics in patients with early
stage lung cancer. |
Table II
Association between p53R2 protein
expression and clinical characteristics in patients with early
stage lung cancer.
| Parameters | p53R2 protein
expression | P-value |
|---|
|
| |
|---|
| Negative | Positive | |
|---|
| Age (years) |
| ≤65 | 25 | 15 | |
| >65 | 25 | 17 | 0.207 |
| Gender |
| Female | 11 | 12 | |
| Male | 39 | 30 | 0.481 |
| Smoking status |
| Negative | 24 | 22 | |
| Positive | 26 | 20 | 0.834 |
| Tumor type |
| AD | 26 | 26 | |
| SCC | 24 | 16 | 0.401 |
| Tumor stage |
| I | 35 | 31 | |
| II | 15 | 11 | 0.817 |
| Lymphovascular
invasion |
| Negative | 41 | 35 | |
| Positive | 9 | 7 | 1.000 |
|
Recurrence/metastasis |
| Negative | 32 | 28 | |
| Positive | 18 | 14 | 0.829 |
| Differentiation |
| Well | 4 | 8a | |
| Moderate | 29 | 26 | |
| Poor | 17 | 8a | 0.121 |
p53R2 is an unfavorable prognostic factor
in early stage NSCLC
The effects of clinical characteristics on the
clinical outcome of patients with early stage NSCLC were calculated
by univariate analysis. Among the characteristics, p53R2 protein
expression, tumor stage and tumor recurrence/metastasis were
significant prognostic factors (Table
III; P=0.022 for p53R2 protein, P<0.0001 for tumor stage,
P<0.0001 for lymphovascular invasion, P=0.0003 for tumor
recurrence/metastasis and P<0.0001 for tumor differentiation).
Patients with stage I lung cancer (median survival 1,151 days) had
longer survival rates than those with stage II of the disease
(median survival 642 days). Patients with no lymphovascular
invasion (median survival 1,103 days) had longer survival rates
than those with lymphovascular invasion (median survival 555 days).
Patients with no tumor recurrence/metastasis (median survival 1,082
days) had longer survival rates than those with
recurrence/metastasis (median survival 868 days). Patients with
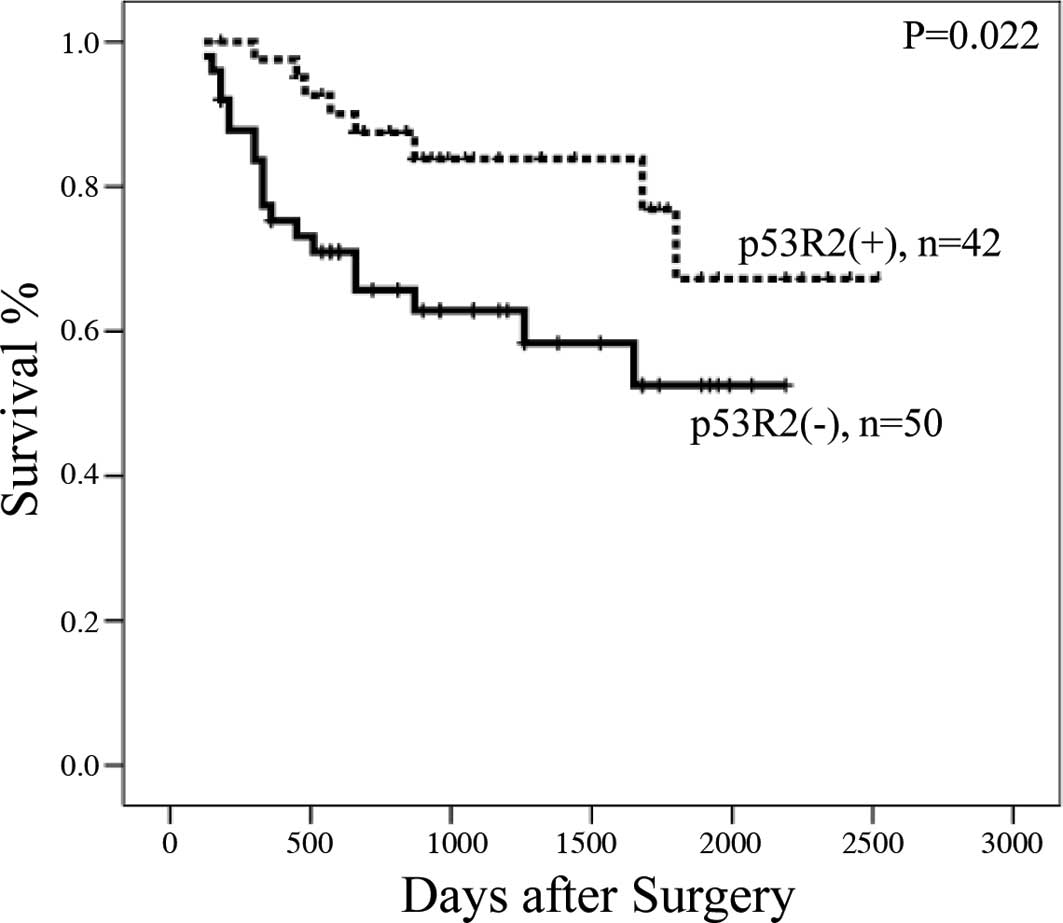
p53R2 protein expression (median survival 900 days) also had longer
survival rates than those without p53R2 expression (median survival
660 days) (Fig. 2). Moreover, Cox’s
regression analysis showed that p53R2, tumor stage and
recurrence/metastasis acted as significant independent prognostic
factors (Table IV; P=0.044, 95% CI
1.004–9.454 for p53R2 protein; P=0.025, 95% CI 0.036–0.801 for
tumor stage and P=0.020, 95% CI 0.094–0.816 for tumor
recurrence/metastasis). Patients without p53R2 protein had a
3.483-fold higher risk than patients with p53R2. The results
suggest that the presence of p53R2, tumor stage and
recurrence/metastasis are similarly significant as unfavorable
prognostic factors in early stage NSCLC.
 | Table IIIUnivariate analysis of the influences
of clinical characteristics on the overall survival of patients
with early stage NSCLC. |
Table III
Univariate analysis of the influences
of clinical characteristics on the overall survival of patients
with early stage NSCLC.
| Prognostic
factor | No. | Median survival
(days) | 3-year survival
(%) | Log-rank P-value |
|---|
| P53R2 |
| Negative | 50 | 660 | 36.0 | |
| Positive | 42 | 900 | 40.1 | 0.0220 |
| Age (years) |
| ≤65 | 40 | 1,074 | 36.0 | |
| >65 | 52 | 957 | 30.8 | 0.5750 |
| Gender |
| Female | 23 | 1,033 | 30.5 | |
| Male | 69 | 999 | 36.3 | 0.6020 |
| Tumor type |
| AD | 52 | 1,020 | 30.8 | |
| SCC | 40 | 991 | 40.0 | 0.8700 |
| Tumor stage |
| I | 66 | 1,151 | 43.9 | |
| II | 29 | 642 | 11.5 | <0.0001 |
| Lymphovascular
invasion |
| Negative | 76 | 1,103 | 59.2 | |
| Positive | 16 | 555 | 6.25 | <0.0001 |
|
Recurrence/metastasis |
| Negative | 59 | 1,082 | 37.3 | |
| Positive | 32 | 868 | 31.2 | 0.0030 |
|
Differentiation |
| Well | 12 | 1,105 | 33.0 | |
| Moderate | 55 | 1,140 | 40.0 | |
| Poorly | 25 | 670 | 24.0 | <0.0001 |
 | Table IVCox-regression analysis of various
potential prognostic factors in patients with early stage NSCLC
with different p53R2 protein expression. |
Table IV
Cox-regression analysis of various
potential prognostic factors in patients with early stage NSCLC
with different p53R2 protein expression.
| Variable | RR |
Unfavorable/favorable | 95% CI | P-value |
|---|
| P53R protein | 3.801 |
Negative/positive | 1.004–9.454 | 0.044 |
| Tumor stage | 5.920 | II/I | 0.036–0.801 | 0.025 |
| Lymphovascular
invasion | 1.360 |
Positive/negative | 0.124–4.345 | 0.734 |
|
Recurrence/metastasis | 3.610 |
Positive/negative | 0.094–0.816 | 0.020 |
Discussion
Numerous reports discussed p53R2 expression in
response to chemotherapy (9,11). The
present study analyzed the correlation of p53R2 and RRM2 protein
and their clinical significance in early stage lung cancer. A
recent study concluded that the overexpression of RRM2 and p53R2,
but not RRM1, in mice specifically induces lung neoplasms (12). p53R2 was also found to contain a
malignancy-suppressing activity (10). The role of p53R2 expression in tumor
formation has yet to be determined. Uramato et al reported
that p53R2 was detected in 46.2% of lung cancer patients and was
higher in patients with stage II–III, pathological T3-4 and N1-3
NSCLC (13). However, p53R2
expression could not be used as an independent prognostic marker in
NSCLC (13). Additionally, previous
reports showed that p53R2 expression is correlated with tumor
invasion, lymph node metastasis and tumor size in esophageal and
oral cancer (14,15). p53R2 expression in patients with
late stage cancer was higher than that in patients with early stage
esophageal cancer (14). The
present study analyzed p53R2 protein expression in early stage lung
cancer and found that only 45.6% (42 of 92) of the patients were
positive. p53R2 protein expression was similar to previous reports
(13). Additionally, we found that
p53R2 protein expression was negatively correlated with tumor cell
differentiation in early stage NSCLC (Table II). These findings appear to be
inconsistent with a previous report (13). Therefore, we suggest that p53R2
expression in early and advanced stages of lung cancer plays a
different role.
Previous reports showed that p53R2 protein
expression correlates with tumorigenesis (15). However, it was found that p53R2
expression was negatively related to the metastasis of colon
adenocarcinoma (16). The
invasion-suppressing ability of p53R2 was also found in
oropharyngeal, prostate, pancreatic and colon cancer cell lines
(15,16). In addition, these authors determined
that the invasion-suppressing ability of p53R2 was not related to
RR enzymatic activity (10). Plao
et al concluded that p53R2 negatively modulates
serum-induced MEK-ERK activity and inhibits the MEK-ERK-mediated
malignancy potential to suppress invasion of human lung cancer
cells (8). The balance of R1 and R2
expression significantly modifies the transformation,
tumorigenicity and metastatic potential (17,18).
Altering the RR level may change the tumorigenicity (19). In this study, patients with p53R2(+)
had a significantly higher median disease-free survival rate (900
days) compared to those with p53R2(−) (660 days) (P=0.022)
(Fig. 2). Our previous study found
that the disease-free survival rate was significantly higher for
patients with RRM1(−) than that for patients with RRM1(+).
Additionally, patients with p53R2(+)/hRRM2(−) had a significantly
higher median survival rate (960 days) than that of the other three
groups of patients: p53R2(−)/hRRM2(−), 810 days; p53R2(−)/hRRM2(+),
600 days and p53R2(+)/hRRM2(+), 840 days. Thus, the expression of
p53R2 and RRM2 may correlate with patient survival. Further studies
should be conducted to verify the RRM1 expression in the same
patient group in order to understand the role of RR in lung cancer
progression.
In conclusion, we showed that the p53R2 expression
is negatively correlated with tumor cell differentiation, and the
presence of p53R2 protein is a favorable prognostic factor in early
stage lung cancer. Therefore, we propose that the expression of
p53R2 is, not only a chemotherapy indicator in late stage lung
cancer, but also a tumor progression marker for early stage lung
cancer.
Acknowledgements
This research was supported by grants from the
National Science Council (NSC 97-2314-B-040-010-MY3) of Taiwan,
R.O.C.
References
|
1
|
Montain C: Revision in the International
System for Staging Lung Cancer. Chest. 111:1710–1717. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
WHO. The World Health Organization
histological typing of lung tumors. Am J Clin Pathol. 77:123–136.
1982.
|
|
3
|
Reichard P: Ribonucleotide reductases: the
evolution of allosteric regulation. Arch Biochem Biophys.
397:149–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanaka H, Arakawa H, Yamaguchi T, et al: A
ribonucleotide reductase gene involved in a p53-dependent
cell-cycle checkpoint for DNA damage. Nature. 404:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cerqueira N, Pereira S, Fernandes PA and
Ramos MJ: Overview of ribonucleotide reductase inhibitors: an
appealing target in anti-tumour therapy. Curr Med Chem.
12:1283–1294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shao J, Zhou B, Zhu L, et al: In vitro
characterization of enzymatic properties and inhibition of the
p53R2 subunit of human ribonucleotide reductase. Cancer Res.
64:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guittet O, Hakansson P, Voevodskaya N, et
al: Mammalian p53R2 protein forms an active ribonucleotide
reductase in vitro with the R1 protein, which is expressed both in
resting cells in response to DNA damage and in proliferating cells.
J Biol Chem. 276:40647–4051. 2001. View Article : Google Scholar
|
|
8
|
Piao C, Lin M, Kim HB, et al:
Ribonucleotide reductase small subunit p53R2 suppresses MEK-ERK
activity by binding to ERK kinase 2. Oncogene. 28:2173–2184. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Link PA, Baer MR, James SR, et al:
p53-inducible ribonucleotide reductase (p53R2/RRM2B) is a DNA
hypomethylation-independent decitabine gene target that correlates
with clinical response in myelodysplastic syndrome/acute
myelogenous leukemia. Cancer Res. 68:9358–9366. 2008. View Article : Google Scholar
|
|
10
|
Liu X, Zhou B, Xue L, et al:
Metastasis-suppressing potential of ribonucleotide reductase small
subunit p53R2 in human cancer cells. Clin Cancer Res. 12:6337–6344.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furuta E, Okuda H, Aya K and Watabe K:
Metabolic genes in cancer: the role in tumor progression and
clinical implications. Biochim Biophys Acta. 1805:141–152.
2010.
|
|
12
|
Xu X, Page JL, Surtees JA, et al: Broad
overexpression of ribonucleotide reductase genes in mice
specifically induces lung neoplasms. Cancer Res. 68:2652–2660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uramoto H, Sugio K, Oyama T, et al: P53R2,
p53 inducible ribonucleotide reductase gene, correlated with tumor
progression of non-small cell lung cancer. Anticancer Res.
25:983–988. 2006.PubMed/NCBI
|
|
14
|
Okumura H, Natsugoe S, Yokomalura N, et
al: Expression of p53R2 is related to prognosis in patients with
esophageal squamous cell carcinoma. Clin Cancer Res. 12:3740–3744.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yanamoto S, Kawasaki G, Yamada S, et al:
Ribonucleotide reductase small subunit p53R2 promotes oral cancer
invasion via the E-cadherin/beta-catenin pathway. Oral Oncol.
45:521–525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Zhou B, Xue L, et al:
Ribonucleotide reductase subunits M2 and p53R2 are potential
biomarkers for metastasis of colon cancer. Clin Colorectal Cancer.
6:374–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou BS, Tsai P, Ker R, et al:
Overexpression of transfected human ribonucleotide reductase M2
subunit in human cancer cells enhances their invasive potential.
Clin Exp Metastasis. 16:43–49. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan H, Villegas C and Wright JA:
Ribonucleotide reductase R2 component is a novel malignancy
determinant that cooperates with activated oncogenes to determine
transformation and malignant potential. Proc Natl Acad Sci USA.
93:14036–14040. 1996. View Article : Google Scholar
|
|
19
|
Fan H, Huang A, Villegas C and Wright JA:
The R1 component of mammalian ribonucleotide reductase has
malignancy-suppressing activity as demonstrated by gene transfer
experiments. Proc Natl Acad Sci USA. 94:13181–13186. 1997.
View Article : Google Scholar : PubMed/NCBI
|