Introduction
TS-1, an oral fluoropyrimidine anti-cancer agent,
contains tegafur (FT), a pro-drug of 5-fluorouracil (5-FU), as a
base component, as well as two modulators of 5-FU, gimeracil and
potassium oxonate (Oxo) (1).
Gimeracil inhibits dihydro-pyrimidine dehydrogenase (DPD), an
enzyme that degrades 5-FU, resulting in the maintenance of high
blood levels of 5-FU. Oxo, a selective inhibitor of orotate
phosphoribosyltransferase (OPRT), reduces gastrointestinal toxicity
through the suppression of 5-FU activation and
5-fluoro-2′-deoxyuridine-5′-monophosphate (FdUMP) synthesis in the
gastrointestinal mucosa. The overall response rate of TS-1 was
reported to be 21.8% in patients with recurrence of
taxane-resistant breast cancer in phase II clinical studies (Hino
M, et al, Proc ASCO 24: abs. 745, 2006). In late phase II
clinical studies, the overall response rate of TS-1 in patients
with advanced/recurrent breast cancer was 42.0% (2). In general, primary chemotherapy
recommended for recurrences of breast cancer includes
administration of anthracycline and taxane (3). Oral fluoropyrimidine anti-cancer
agents are less toxic (do not cause hair loss) and are easily
administered to elderly patients, making the agent beneficial.
Between August 2006 and May 2009, TS-1 was administered mainly as a
first-line treatment to 7 patients with locally recurrent breast
cancer. This study investigated TS-1 and reports the results
obtained.
Materials and methods
Between August 2006 and May 2009, 7 patients with
locally recurrent breast cancer were included in the study. The
mean age of the patients at the time of TS-1 administration was 64
years (range 56–71). Among the patients, 1 had stage I cancer, 4
had stage II and 2 had stage III cancers. The histological type of
the primary cancer was papillotubular carcinoma in 4 patients and
scirrhous carcinoma in 3. Lymph node metastases were classified as
n0 in 3 patients, n1α in 1 and n2 in 3. A total of 4 patients with
lymph node metastasis underwent postoperative chemotherapy (CAF
therapy). Hormone sensitivity was found to be positive in 3 cases
(2 cases of estrogen receptor [ER]+/progesterone
receptor [PgR]+ and 1 case of
ER+/PgR−) and negative in 4. Tamoxifen or
aromatase inhibitors were postoperatively administered to patients
with hormone-sensitive tumors. Hairy-related 2 (HER2) gene
expression was not observed in any patient. Although the patients
showed primary recurrence, distant metastases to the lung, liver
and bone were not observed at the time of TS-1 administration. TS-1
was administered as first-line treatment to 6 patients and as
second-line treatment, following administration of taxane, to 1
patient. The TS-1 monotherapy was performed for all 7 patients. The
sites of recurrence were the cervical lymph nodes in 4 cases, the
axillary lymph nodes in 2 and the thoracic wall in 1. The interval
between tumor resection and local recurrence varied widely from 1
year and 5 months to 28 years (See Table I).
 | Table ISeven cases of locally recurrent
breast cancer. |
Table I
Seven cases of locally recurrent
breast cancer.
| 1 Average age | 64 years (range
56–71) |
| 2 Clinical stage | Stage I (1 case),
Stage II (4 cases), Stage III (2 cases) |
| 3 Histology | Scirrhous (3 cases),
Papillotubular (4 cases) |
| 4 Lymph node
metastasis | n0 (3 cases), n1α (1
case), n2 (3 cases) |
| 5
Pre-chemotherapy | No therapy (6 cases),
docetaxel (1 case) |
| 6 Recurrent
sites | Cervical lymph node
(4 cases), axillary lymph node (2 cases), thoracic wall (1
case) |
| 7 Average
administration of TS-1 | 9.5 cycles (range
4–18) |
| 8 Efficacy of
TS-1 | CR (2 cases), PR (1
case), Long SD (1 case), SD (2 cases), PD (1 case)
Response rate 43% (including long SD; clinical benefit, 57%) |
The dose of TS-1 was set at 100 mg/body/day (body
surface area of ≥1.25 m2 and <1.5 m2).
Each treatment cycle lasted 6 weeks and comprised 4 weeks of
consecutive administration followed by 2 weeks of off-treatment.
This treatment cycle was repeated as long as the therapeutic
effects were observed. However, the dose of TS-1 was reduced to 80
mg/body/day, depending on the physical conditions of the patients,
and continued. The average duration of TS-1 administration was 9.5
cycles (range 4–18). The efficacy of TS-1 was assessed from the 2nd
or 3rd cycle after administration according to the Response
Evaluation Criteria in Solid Tumors (RECIST) guidelines (4).
Results
Among the 7 patients, 2 achieved complete response
(CR), 1 achieved partial response (PR), 2 had stable disease (SD),
1 had long SD and 1 had progressive disease (PD). The overall
response rate was 43%, and the clinical benefit rate, including
long SD, was 57% (Table I). A case
in which SD was confirmed to be maintained for at least 24 weeks
was judged to be long SD according to the RECIST guidelines.
Although the time to progression (TTP) was not calculated due to
the short duration of the observation periods in this study,
overall local control was favorable since only 1 patient showed PD
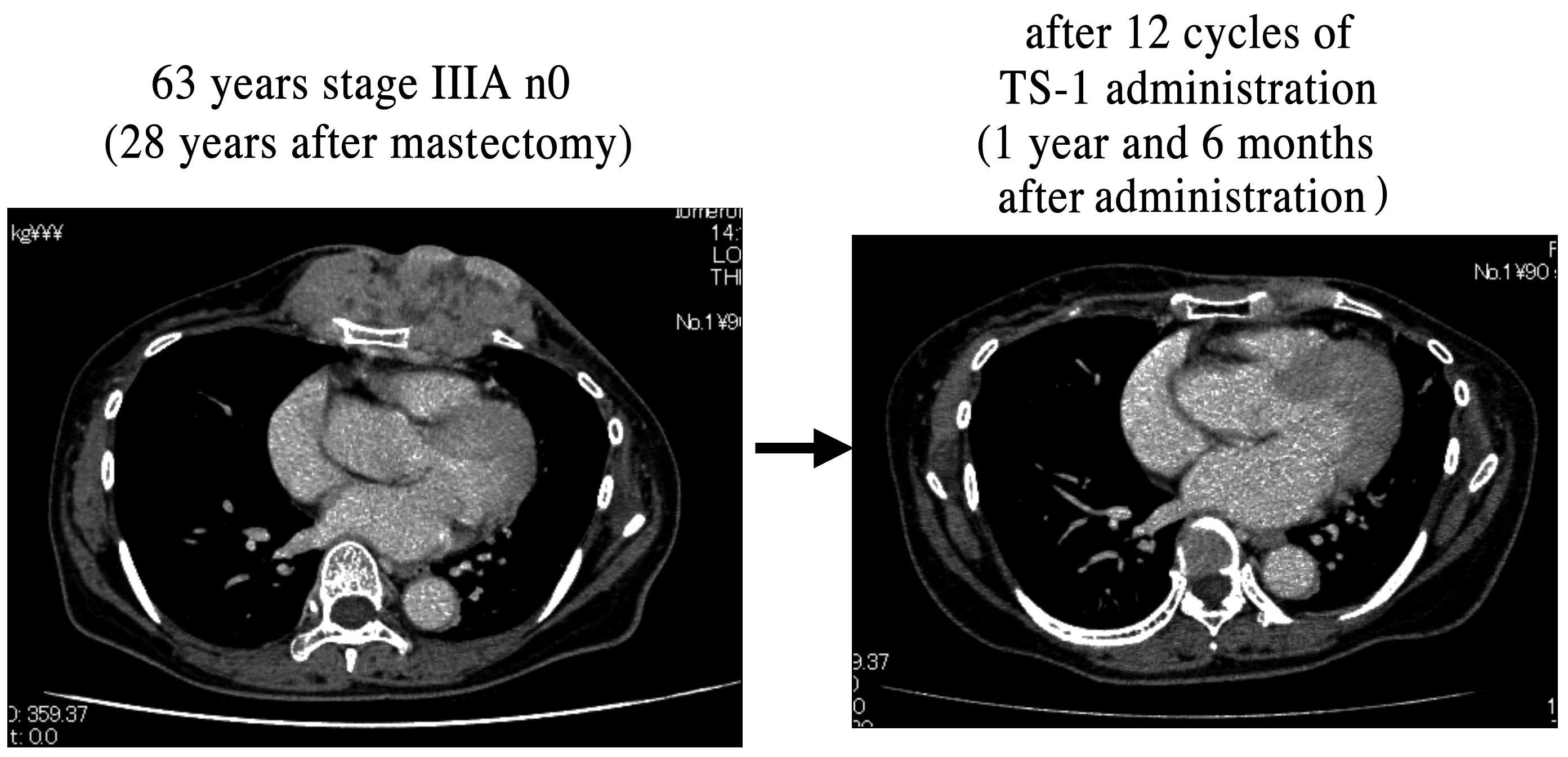
following the initial assessment of efficacy. In the CR group, 1
patient who had recurrence in the thoracic wall 28 years after
surgery achieved CR after 12 cycles of TS-1 administration
(Fig. 1). The mean duration of TS-1
administration was 9.5 cycles. The status of hormone sensitivity
did not affect the overall response rate. TS-1 administration
resulted in hand-foot syndrome, an adverse event, in 1 case (14%).
No patients withdrew from the treatment, and the study achieved
favorable compliance.
Discussion
Anthracycline and taxane are recommended for use in
the first- and second-line treatment of advanced recurrent breast
cancer (3). Although third-line
treatment has yet to be established, oral fluoropyrimidine
anti-cancer agents are often used for third-line treatment or in
later treatments in patients with anthracycline- and
taxane-resistant breast cancer. TS-1 contains FT, a pro-drug of
5-FU, as a base component and combines 2 modulators, i.e.,
gimeracil, to inhibit DPD which is a degradation enzyme of 5-FU,
and Oxo. TS-1 is different from the existing oral fluoropyrimidine
anti-cancer agents as it causes less gastrointestinal toxicity and
is administered in relatively higher doses (1). Capecitabine, an oral anti-cancer agent
with characteristics similar to that of TS-1, was approved for use
in Japan in April 2003. It has been reported that capecitabine has
been considered for use up-front or as a third-line treatment
(5). TS-1 was approved for use in
Japan in November 2005 and was largely used as third-line
treatment. The distinction between capecitabine and TS-1 for the
appropriate administration in the treatment of recurrent breast
cancer is a subject of further discussion. Although capecitabine
was already in use up-front in other countries (6), it was not commonly administered
up-front in Japan. However, a previous study suggested that
up-front administration of capecitabine holds great potential for
the treatment of patients with liver metastasis (5). Few studies have reported the up-front
administration of TS-1 either in Japan or abroad. The results of
the late phase II clinical studies conducted in Japan showed that
the overall response rates of TS-1 were high in local/regional
lymph nodes (62.1%), the skin (52.0%) and distant lymph nodes
(35.0%) (3) (Table II). This study examined the
efficacy of TS-1 as a first-line treatment in locally recurrent
breast cancer.
 | Table IIA phase II study of TS-1 for advanced
or recurrent breast cancer in Japan. |
Table II
A phase II study of TS-1 for advanced
or recurrent breast cancer in Japan.
| Recurrent site | No. of patients | CR | PR | MR | NC | PD | Response rate
(%) |
|---|
| Lung | 28 | 1 | 8 | 3 | 6 | 5 | 32.1 |
| Skin | 25 | 7 | 6 | 1 | 5 | 2 | 52.0 |
| Liver | 6 | 0 | 1 | 0 | 2 | 1 | 16.7 |
| Bone | 15 | 0 | 4 | 0 | 4 | 0 | 26.3 |
| Pleura | 5 | 0 | 1 | 0 | 1 | 2 | 20.0 |
| Local lymph node | 29 | 6 | 12 | 3 | 6 | 1 | 62.1 |
| Visceral lymph
node | 20 | 2 | 5 | 5 | 5 | 2 | 35.0 |
The overall response rate (43%) and clinical benefit
rate (57%) in cases of local recurrence obtained in our hospital
are consistent with those obtained in late phase II clinical
studies in Japan (42 and 57%, respectively). Although the TTP was
not calculated due to the short duration of the observation periods
in this study, the mean duration of administration period (9.5
cycles) was comparable to that of late phase II clinical studies in
Japan (average duration of overall response was 284.5 days), as
well as to a study by Yoneyama et al (7). Our study showed that the status of
hormone sensitivity did not affect the overall response rate, a
result that was similar to that of other late phase II clinical
studies conducted in Japan (2).
This study found that TS-1 is a promising drug for the effective
treatment of locally recurrent breast cancer. Since oral
anti-cancer agents, including capecitabine, do not induce response
in cases of cancer complicated by acute and progressive diseases
including lymphangitis carcinomatosa, treatment for
life-threatening cases should be tailored to the needs of the
patients.
TS-1, unlike other intravenously administered
anti-cancer agents, causes fewer adverse reactions (hair loss and
neutropenia) resulting in favorable compliance. Although 1 case of
hand-foot syndrome was observed, a reduction in the dose enabled
the continuation of TS-1 administration, and the patient achieved
long SD. When the dose of capecitabine was reduced in order to
continue administration, no differences in the TTP and overall
survival were observed between the reduced and full dose (8). Therefore, TS-1, another oral
anti-cancer drug, is anticipated to be as effective as
capecitabine, even at reduced doses. In this study, favorable
compliance from the patients resulted in increased therapeutic
efficacy. Vitamin B6 combined with capecitabine is reported to be
effective in alleviating hand-foot syndrome, an adverse reaction
(9). Therefore, the concomitant
administration of TS-1 with vitamin B6 is recommended. This study
suggested that up-front administration of TS-1 is effective in the
treatment of locally recurrent breast cancer. Taking the Hortobagyi
algorism (10) into consideration,
the administration of TS-1 may be one option for switching from
oral anti-hormone agents with fewer adverse reactions that can be
administered to elderly patients, as well as patients who have
difficulties in intravenous administration and those who experience
hair loss. Capecitabine has been shown to be effective in the
treatment of patients with liver metastasis (5). In future, it may be possible to select
either TS-1 or capecitabine for up-front administration depending
on the site of tumor recurrence.
Finally, in the treatment of recurrent breast
cancer, it is important not to aim to cure the cancer but to
prolong the survival period while managing the symptoms and
maintaining quality of life (11).
The majority of cases in this study achieved either long SD or SD,
resulting in a completely satisfactory outcome in terms of clinical
benefit and compliance for TS-1. The combined use of TS-1 with
other anti-cancer agents and antibody therapy suggests that further
extension of TTP can be achieved.
References
|
1
|
Shirasaka T, Shimamoto Y, Kato T, et al:
Invention of a tumor-selective 5-fluorouracil derivative named S-1
by biochemical modulation of 5-fluorouracil. JPN J Cancer
Chemother. 25:371–384. 1998.PubMed/NCBI
|
|
2
|
Saeki T, Takashima S, Sano M, et al: A
late phase II clinical study of S-1 in patients with progressed,
refractory breast cancer. JPN J Cancer Chemother. 31:539–547.
2004.PubMed/NCBI
|
|
3
|
The Japanese Breast Cancer Society. A
Manual For Evidence-Based Clinical Practice Guideline 1 Medication.
Kanehara Shuppan; Tokyo: pp. 91–96. 2007
|
|
4
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nohara T, Iwamoto M, Sumiyoshi K, et al:
Clinical effect and positioning of capecitabine for metastatic
breast carcinoma. JPN J Cancer Chemother. 35:1315–1318.
2008.PubMed/NCBI
|
|
6
|
William BD: Capecitabine monotherapy; safe
and effective treatment for metastatic breast cancer. Oncologist.
11:325–335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoneyama K, Yamada A, Koshida Y, et al: A
patient with pulmonary metastasis from breast cancer after surgery
who responded to S-1. JPN J Cancer Chemother. 34:1143–1162.
2007.PubMed/NCBI
|
|
8
|
Shiiki S, Sonoo H, Seki M, et al:
Therapeutic efficacy of capecitabine on advanced and recurrent
breast cancer with special reference to time to progression. JPN J
Cancer Chemother. 33:1431–1435. 2006.PubMed/NCBI
|
|
9
|
Shigemori C, Mizutani M, Iwata H, et al:
The experience of capecitabine in advanced or reccurent breast
cancer. Nyugan No Rinsyo. 21:148–152. 2006.
|
|
10
|
Hortobagyi GN: Treatment of breast cancer.
N Engl J Med. 339:974–984. 1998. View Article : Google Scholar
|
|
11
|
Greenberg PA, Hortobagyi GN, Smith TL, et
al: Long-term follow up patients with complete remission following
combination chemotherapy for metastatic breast cancer. J Clin
Oncol. 14:2197–2205. 1996.PubMed/NCBI
|