Introduction
Advanced gastric cancer often results in the
inability to ingest food or drink orally, a condition called
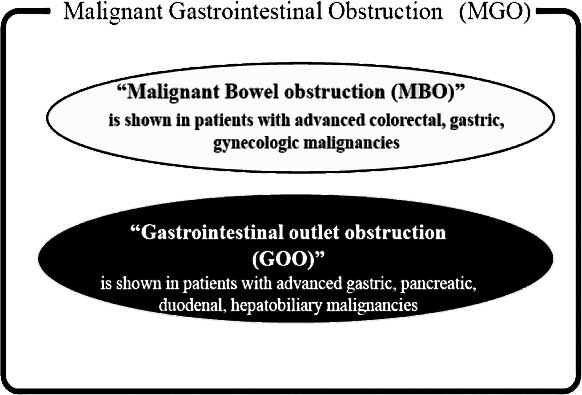
malignant gastrointestinal obstruction (MGO). MGO is clinically
defined as a gastrointestinal outlet obstruction (GOO) caused by a
large tumor, or malignant bowel obstruction (MBO) with peritoneal
dissemination (Fig. 1). MGO impacts
the quality of life (QOL) of patients by precluding oral intake and
by causing gastrointestinal symptoms, such as nausea, vomiting and
abdominal pain.
MGO is a common clinical complication in patients
with advanced abdominal malignancy, due to either a large primary
tumor or intestinal involvement with peritoneal carcinomatosis. As
previously mentioned, bowel obstruction negatively impacts QOL to a
great extent by impeding oral intake and inducing gastrointestinal
symptoms. Thus, the management of patients with MGO is a
significant issue for oncologists. Due to the poor general
condition of patients with MGO, surgical treatment, including
bypass procedures, is generally not recommended. Furthermore, the
placement of a nasogastric tube (NGT) to drain digestive secretions
and other fluids does not always result in symptom resolution or
QOL improvement in these patients due to the stress associated with
NGT placement.
Previous reports demonstrated the efficacy of
pharmacologic therapy consisting of analgetics, antiemetics and
antisecretory drugs for patients with MGO (1). Octreotide acetate (OA) is an analogue
of somatostatin that is increasingly used to relieve
gastrointestinal symptoms in patients with MGO (1–6). By
means of complex endocrine mechanisms, OA affects gastrointestinal
function by reducing gastric and intestinal secretion and bile flow
(7).
Orally administered S-1, currently the most
effective chemotherapeutic agent for gastric cancer in Japan,
cannot be used in patients with bowel obstruction (8–11).
Previously the authors of this study reported on a patient with
highly advanced gastric cancer with MBO, who was successfully
treated with combination chemoradiotherapy, including S-1 and OA
(12). The inclusion of OA in this
regimen enabled the patient to tolerate oral S-1 chemotherapy.
This is a pilot study of advanced gastric cancer
cases with MGO successfully treated with combination
chemoradiotherapy that included S-1. Given the seriousness of MGO,
the success of the OA-containing treatment regimen is
noteworthy.
Materials and methods
Chemoradiotherapy using S-1 and
cisplatin
The chemoradiotherapy regimen consisted of S-1
(Taiho Pharmaceutical Co., Ltd, Tokyo, Japan) and cisplatin (CDDP;
Randa®, Nihon Kayaku Co., Ltd., Tokyo, Japan). S-1 was
administered orally at a dose of 80 mg/m2/day on Days
1–21, while CDDP was infused at a dose of 6 mg/m2/day
for 1 h on Days 1–5, 8–12 and 15–19. Radiation therapy (5
days/week) at 2 Gy/day was started concurrently with chemotherapy
and was repeated hourly on Days 1–5, 8–12, 15–19 and 22–26.
Irradiation was planned using a computed tomography (CT) simulator
for two rectangular portals (anterior and left lateral) with a pair
of 45°-wedge filters, and was targeted at the primary tumor and
surrounding lesions, including the lymph nodes. The study was
approved by the ethics committee of our hospital, and all patients
gave written informed consent.
Patient characteristics
Table I shows the
clinicopathological findings of the patients. Gastric cancer
staging and the effects of chemoradiotherapy were evaluated
according to the criteria of the Japanese Research Society for
Gastric Cancer (13). The mean age
of the patients was 57 years (range 19–71) and there were 7 males
and 2 females. All 24 patients complained of appetite loss and
nausea caused by MGO. Due to the progression of peritoneal
dissemination, 2 of 9 patients received chemoradiotherapy after
non-curative resection due to positive lavage cytology (CY1). Only
1 patient had recurrence with peritoneal dissemination following
curative surgical resection, and that patient received
chemoradiotherapy 9 months after surgery. The analysis of the
clinical response showed that partial response (PR) or stable
disease (SD) was observed in 3 and 4 patients, respectively. Of a
total of 9 patients, 8 were able to resume oral liquid or solid
diet intake and were discharged from the hospital. The median
duration of OA (Sandostatin®; Novartis Pharma K.K.,
Tokyo, Japan) treatment and hospital stay was 28 days (range 7–70)
and 55 days (range 44–100), respectively. The patients continued to
receive outpatient combination chemotherapy consisting of S-1 and
CDDP, or other drugs. Grade 4 leukocytopenia was observed in only 1
patient and grade 3 toxicities of leukocytopenia, thrombocytopenia
and anemia were observed in 3, 1 and 2 patients, respectively. No
grade 3 or higher non-hematological toxicity was observed. The
median survival time was 305 days.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Case | Age/gender | Location | Stage | Hospital stay
(days) | Clinical response
(months) | Survival time |
|---|
| 1 | 68/F | LD | T4N1H0P1M0, stage
IV | 47 | SD | 10, deceased |
| 2 | 55/M | LD | T3N3H0P1M1, stage
IV | 40 | PR | 21, alive |
| 3 | 58/M | UM | T4N3H0P1M0, stage
IV | 55 | PR | 10, deceased |
| 4 | 70/M | MUL | T3N2H0P1M1, stage
IV | 42 | SD | 3, deceased |
| 5 | 70/M | UE, LD | T4N0H0P1M0, stage
IV | 86 | PR | 11, deceased |
| 6 | 64/F | LM | T4N2H0P0M0CY1, stage
IV | 55 | PD | 2, deceased |
| 7 | 41/M | UML | T3N3H1P1M1, stage
IV | 63 | PD | 3, deceased |
| 8 | 71/M | UML | T3N1H0P0M0CY1, stage
IV | 39 | SD | 17, alive |
| 9 | 19/M | L | rec (T2N0H0P0M0CY0,
stage IB) | 100 | SD | 8, deceased |
Case reports
Case 1
A 55-year-old male presented at our hospital with
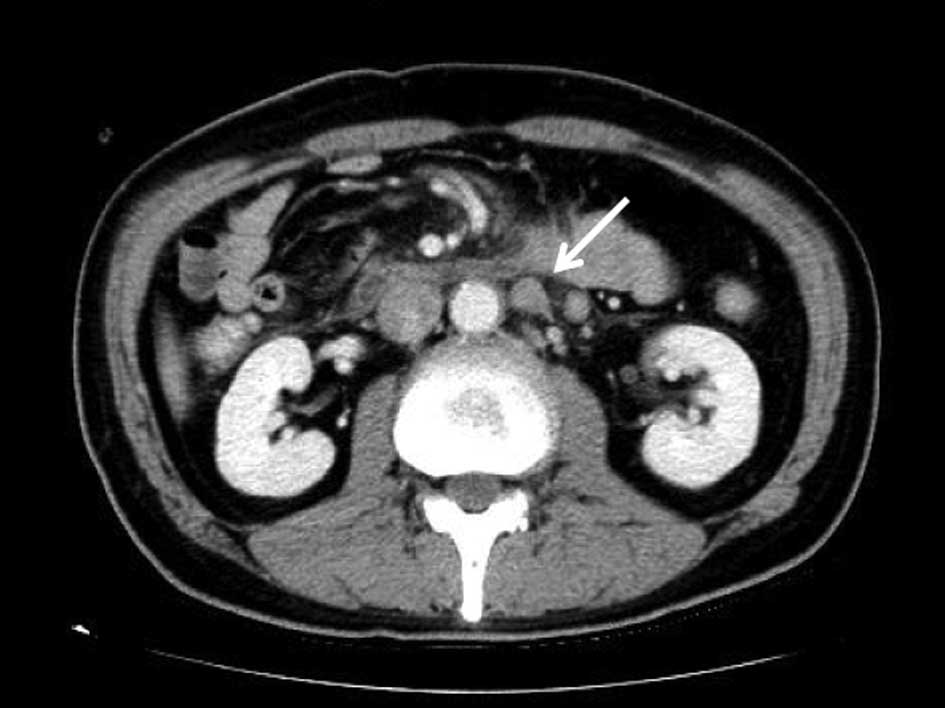
vomiting and abdominal distension. A gastrointestinal endoscopy
showed advanced gastric cancer with pyloric stenosis, while an
abdominal CT revealed lymph node metastases, including nodes in the
para-aortic region and multiple nodules plus ascites in the pelvic
cavity (Fig. 2A and B). The
clinical diagnosis, according to the Japanese classification of
gastric carcinoma (13), was stage
IV (cT3, cN3, cH0, cP1, cM1) gastric cancer. Due to severe
gastrointestinal symptoms caused by pyloric stenosis and peritoneal
dissemination, the patient was treated with OA intravenously at 300
μg/day to decrease bowel secretions.
After the improvement of the bowel obstruction
symptoms, the patient was able to resume the intake of water 1 day
after starting treatment with OA. After OA treatment resulted in
sufficient oral intake, chemoradiotherapy with S-1 and CDDP
commenced. Although Grade 3 leukopenia developed, it improved
following the administration of granulocyte colony-stimulating
factor (G-CSF). An abdominal CT following the last
chemoradiotherapy cycle revealed that the ascites had almost
completely disappeared and that the metastatic lymph nodes showed a
reduction in size (Fig. 2C and D).
The clinical response of the patient was determined to be PR. He
was able to resume oral intake and was discharged 6 weeks after
admission.
In a follow-up, 17 months after the initiation of
treatment, the patient’s peritoneal dissemination progressed.
However, he remains on outpatient chemotherapy.
Case 2
A 58-year-old male with nausea and lower back pain
was admitted to our hospital for further evaluation.
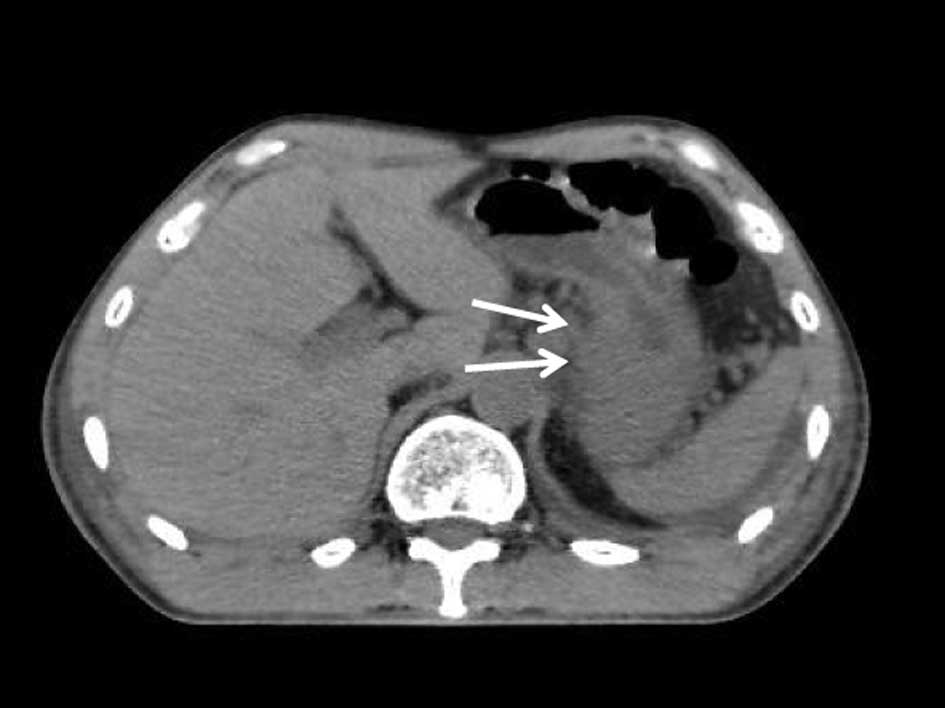
Gastro-intestinal endoscopy showed a type 3 tumor located on the
upper and middle parts of the stomach. Abdominal CT revealed
multiple lymph node metastases, peritoneal nodules plus ascites and
a large primary tumor (Fig. 3A and
B). Hydronephrosis and acute renal dysfunction caused by the
large tumor extending into the retroperitoneum resulted in a
urinary tract stent being inserted prior to chemotherapy. In
addition, to relieve gastrointestinal symptoms, the patient was
treated with OA intravenously at a dose of 300 μg/day to decrease
bowel secretions.
Following improvement of the bowel obstruction
symptoms, chemotherapy with half-dose S-1 (50 mg/body/day) was
initiated 2 days after starting treatment with OA. The patient’s
renal dysfunction gradually improved. Subsequently, the S-1 dose
was increased to a standard dose (100 mg/body/day), and low-dose
CDDP and radiation were included. Grade 3 leukopenia developed,
which resolved following G-CSF administration. An abdominal CT
following the last chemoradiotherapy cycle revealed that the
ascites had almost completely disappeared, and the tumor had
decreased in size (Fig. 3C and D).
The patient’s clinical response was determined to be PR. He was
able to resume oral intake and was discharged 8 weeks after
admission.
An outpatient chemotherapy regimen was administered
consisting of S-1 and biweekly CDDP. However, the patient succumbed
to the disease 10 months after the initiation of therapy due to
progression of the peritoneal dissemination.
Discussion
MBO is defined as a clinical syndrome in which a
patient has obstructive symptoms due to the presence of
carcinomatosis associated with abdominal or pelvic malignancy.
Gastrointestinal symptoms are caused by an increase in the
distension-secretion-motor activity of the obstructed bowel
(14). As the luminal contents
accumulate proximal to the obstruction, the bowel becomes
distended, and the increase in intraluminal pressure stimulates
intestinal fluid secretion, stretching the bowel wall even further.
In addition, upper abdominal cancer, such as advanced gastric,
pancreatic, duodenal and gallbladder cancer, may produce GOO. Both
MBO and GOO are identified as MGO (Fig.
1).
Patients with MGO are usually not eligible for
surgery since they present with multiple sites of obstruction and
are generally in poor health. These patients cannot be managed
adequately by conventional antiemetics, and NGT placement is often
the only treatment option for such inoperable patients. While NGT
placement may result in symptomatic relief in some patients with
MGO, common complications occur, including mucosal erosion and
hemorrhage, esophagitis and aspiration pneumonia.
The somatostatin analogue OA modulates
gastrointestinal function by reducing gastric acid secretion,
slowing intestinal motility, decreasing bile flow, increasing
mucous production and decreasing splanchnic blood flow (15). The inhibitory effects of OA cause
decreased water and sodium secretion by the intestinal epithelium,
thereby reducing bowel distension. Previous reports showed that the
relief of gastrointestinal symptoms by OA contributes to the
improvement of QOL in patients with MGO (3–6).
S-1 is an oral anticancer drug that combines
tegafur, a prodrug of 5-fluorouracil (5FU), with
5-chloro-2,4-dihydro-pyrimidine (CDHP) and potassium oxonate. S-1
has several advantages, including its high efficacy, excellent
tolerability, low side-effect profile and the convenience of
outpatient administration (8,9). The
Japan Clinical Oncology Group (JCOG) study (JCOG9912) reported that
S-1 was well tolerated, with a median survival as long as that of
5FU alone (control treatment) in advanced gastric cancer (11). Therefore, in Japan, S-1 is
identified as a first-line agent for treating unresectable, highly
advanced gastric cancer. CDDP is another key chemotherapeutic agent
used for the treatment of gastric cancer. Combination chemotherapy
with CDDP and S-1 results in high efficacy and tolerable toxicity
(16–18). In our clinical trial, radiation was
added to S-1 combined with low-dose CDDP chemotherapy to achieve
additional efficacy in patients with stage IV gastric cancer
(19–21). However, the oral formulation of S-1
prevents it from being administered to numerous patients with MGO
since they are unable to ingest even small capsules.
The clinical application of OA is somewhat limited
in MGO since symptom relief is only temporary. However, for 4
patients described in this study, who were initially unable to take
oral S-1 due to MGO, the administration of OA relieved their bowel
obstruction sufficiently to allow them to receive this valuable
type of adjunctive therapy. The use of OA enabled these patients to
complete an aggressive chemoradiotherapy regimen that included S-1.
After chemoradiotherapy plus OA, patient oral intake and QOL
substantially improved compared to their pre-treatment status.
Consequently, these patients were discharged without any
treatment-associated problems or NGT placement. Thus, in gastric
cancer patients with MGO, OA appears to be useful for managing
gastrointestinal symptoms and enabling patients to receive
effective treatment for this disease, resulting in a relatively
satisfactory QOL.
References
|
1
|
Mercadante S, Ferrera P, Villari P, et al:
Aggressive pharmacological treatment for reversing malignant bowel
obstruction. J Pain Symptom Manage. 28:412–416. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dean A: The palliative effects of
octreotide in cancer patients. Chemotherapy. 47(Suppl 2): 54–61.
2001. View Article : Google Scholar
|
|
3
|
Khoo D, Hall E, Motson R, et al:
Palliation of malignant intestinal obstruction using octreotide.
Eur J Cancer. 30A:28–30. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe H, Inoue Y, Uchida K, et al:
Octreotide improved the quality of life in a child with malignant
bowel obstruction caused by peritoneal dissemination of colon
cancer. J Pediatr Surg. 42:259–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shima Y, Ohtsu A, Shirao K, et al:
Clinical efficacy and safety of octreotide (SMS201-995) in
terminally ill Japanese cancer patients with malignant bowel
obstruction. Jpn J Clin Oncol. 38:354–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mercadante S, Spoldi E, Caraceni A,
Maddaloni S and Simonetti M: Octreotide in relieving
gastrointestinal symptoms due to bowel obstruction. Palliat Med.
7:295–299. 1993.PubMed/NCBI
|
|
7
|
Marie TF: The physiology of somatostatin
and its synthetic analogue, octreotide. Eur J Palliative Care.
1:20–22. 1994.
|
|
8
|
Sugimachi K, Maehara Y, Horikoshi N, et
al: An early phase II study of oral S-1, a newly developed
5-fluorouracil derivative for advanced and recurrent
gastrointestinal cancers. The S-1 Gastrointestinal Cancer Study
Group. Oncology. 57:202–210. 1999. View Article : Google Scholar
|
|
9
|
Koizumi W, Kurihara M, Nakano S and
Hasegawa K: Phase II study of S-1, a novel oral derivative of
5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative
Gastric Cancer Study Group. Oncology. 58:191–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakuramoto S, Sasako M, Yamaguchi T, et
al: Adjuvant chemotherapy for gastric cancer with S-1, an oral
fluoropyrimidine. N Eng J Med. 357:1810–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boku N, Yamamoto S, Fukuda H, et al:
Fluorouracil versus combination of irinotecan plus cisplatin versus
S-1 in metastatic gastric cancer: a randomized phase 3 study.
Lancet Oncol. 10:1063–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumagai K, Saikawa Y, Fukuda K, et al:
Octreotide acetate successfully treated a bowel obstruction caused
by peritoneally disseminated gastric cancer, thereby enabling the
subsequent use of oral S-1 chemotherapy. Int J Clin Oncol.
14:372–375. 2009. View Article : Google Scholar
|
|
13
|
Japanese Research Society for Gastric
Cancer. Japanese Classification of Gastric Carcinoma. 2nd English
edition. Kanehara; Tokyo: 1998
|
|
14
|
Ripamonti C, Twycross R, Baines M, et al:
Clinical-practice recommendations for the management of bowel
obstruction in patients with end-stage cancer. Support Care Cancer.
9:223–233. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mulvihill SJ, Pappas TN, Fonkalsrud EW and
Debas HT: The effect of somatostatin on experimental intestinal
obstruction. Ann Surg. 207:169–173. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saikawa Y, Kubota T, Kitajima M, et al:
Preoperative combination chemotherapy with S-1 and low-dose
cisplatin against highly advanced gastric carcinoma. Oncol Rep.
10:381–386. 2003.PubMed/NCBI
|
|
17
|
Koizumi W, Tanabe S, Saigenji K, et al:
Phase I/II study of S-1 combined with cisplatin in patients with
advanced gastric cancer. Br J Cancer. 89:2207–2212. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi T, Saikawa Y, Kitajima M, et al:
Histological complete response in a case of advanced gastric cancer
treated by chemotherapy with S-1 plus low-dose cisplatin and
radiation. Jpn J Clin Oncol. 33:584–588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saikawa Y, Kubota T, Kitajima M, et al: Is
chemoradiation effective or harmful for stage IV gastric cancer
patients? Oncol Rep. 13:865–870. 2005.PubMed/NCBI
|
|
21
|
Saikawa Y, Kubota T, Kumagai K, et al:
Phase II study of chemoradiotherapy with S-1 and low-dose cisplatin
for inoperable advanced gastric cancer. Int J Radiat Oncol Biol
Phys. 71:173–179. 2008. View Article : Google Scholar : PubMed/NCBI
|