Introduction
Nature is an important source of medicinal products.
Subsequently, numerous useful drugs have been developed from
natural sources. In particular, plants provide valuable anticancer
agents with novel structures and unique mechanisms of action
(1). Evidence of such successes in
natural product drug discovery include the isolation of
vinca-alkaloids, vinblastine and vincristine from the Madagascar
periwinkle Catharanthus roseus, as well as paclitaxel from
the bark of the Pacific Yew Taxus brevifolia (2). Various parts of the latter and other
Taxus species are used by Native Americans for a variety of
disease indications, including cancer (3). Similarly, Anemopsis californica
(A. californica), a perennial plant from the Saururaceae
family, native to Arizona, Southern California, Sonora and Mexico,
is commonly used among Native Americans and occasionally utilized
to treat illnesses with cancer-like symptoms. This knowledge was
obtained from their ancestors (personal communication with a Native
American medicine man whose descendants are Yaqui and
Cherokee).
Nevertheless, studies on the potential anticancer
activity of A. californica are rare, ambiguous (4) and largely performed with essential
oils, but provide some preliminary data supporting its anticancer
properties (5). Thus, this study
evaluated the effects of extracts obtained from different plant
parts (bracts, leaves, roots and stems) on the growth and migration
of human cancer cell lines, including HCT-8 colon, mammary
estrogen-independent Hs 578T and estrogen-dependent MCF-7/AZ cells.
For each plant part, three extract conditions were used, including
water, EtOH and EtOAc, in order to correlate to traditionally used
methods and compounds with different polarities as a starting point
for future bio-guided fractionation.
Materials and methods
Plant materials and preparation of
extracts
Whole plants of Anemopsis californica
(Saururaceae) were collected in Valencia County, located two miles
south of Los Lunas, New Mexico in August 2008. The plants were
identified by Dr Tim Lowrey, UNM Herbarium, Museum of Southwestern
Biology, University of New Mexico, Albuquerque, NM, USA. A voucher
specimen (No. 2185) was deposited at the UNM Herbarium collections
for further reference and is available via the NMBCC online
database (http://www.nmbiodiversity.org). The whole plants were
rinsed to remove dust and/or soil and dried in a plant drier at
38°C. The different parts were separated and cut into smaller
samples. Dried plant parts (50 g) were macerated in 500 ml solvent
(water, EtOH and EtOAc) for 24 h under constant shaking at 4°C. The
mixtures were filtered to remove particulate matter, lyophilized
and the resulting powders were stored in a desiccator at 4°C.
Table I shows the yields obtained
from the different parts and their particular solvents.
 | Table IPlant parts and extract conditions of
A. californica and their cytotoxicity against three human
cancer cell lines in vitro. |
Table I
Plant parts and extract conditions of
A. californica and their cytotoxicity against three human
cancer cell lines in vitro.
| Plant part | Solvent | Yield of extraction
(%) | IC50
(μg/ml) | IC20
(μg/ml) |
|---|
| | |
|
|
|---|
| | | HCT-8 | Hs 578T | MCF-7/AZ | HCT-8 | Hs 578T | MCF-7/AZ |
|---|
| Bracts | H2O | 18.08 | >200 | >200 | >200 | 40 | >200 | 100 |
| EtOH | 9.53 | >200 | >200 | >200 | 60 | >200 | >200 |
| EtOAc | 1.15 | 200 | 150 | 150 | 10 | 10 | 30 |
| Leaves
H2O | 14.58 | 180 | >200 | >200 | 30 | >200 | >200 | |
| EtOH | 4.07 | >200 | >200 | >200 | 40 | >200 | 180 |
| EtOAc | 1.53 | 180 | >200 | 200 | 50 | 120 | 40 |
| Roots | H2O | 8.83 | >200 | >200 | >200 | 120 | >200 | 40 |
| EtOH | 5.85 | >200 | 180 | 120 | 180 | 30 | 20 |
| EtOAc | 0.31 | 120 | 120 | 100 | 80 | 70 | 20 |
| Stems | H2O | 10.53 | >200 | >200 | >200 | >200 | >200 | >200 |
| EtOH | 1.59 | >200 | >200 | >200 | >200 | >200 | >200 |
| EtOAc | 0.38 | 100 | 100 | 100 | 10 | 10 | 10 |
Cell culture
The HCT-8 (ATCC no. CCL-244) and Hs 578T (ATCC no.
HTB-126) cell lines were obtained from the American Type Culture
Collection (ATCC). MCF-7/AZ is a variant of the human mammary
carcinoma cell family MCF-7 (6).
The cells were maintained at 37°C in the appropriate media
supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin and
10% fetal bovine serum (FBS) (Invitrogen, CA, USA), in a humidified
atmosphere containing 5 or 10% CO2.
In vitro cytotoxicity assay
The effect of the extracts on cell viability was
tested in accordance with Romijn et al (7). Briefly, mitochondrial dehydrogenase
activities were measured by an MTT reagent (Sigma, MO, USA). Cells
were seeded in 96-well plates at an initial density of
1.5×104 cells in 200 μl of the appropriate culture
medium. After a 24-h incubation, cells were treated with 10
concentrations (20, 40, 60, 80, 100, 120, 140, 160, 180 and 200
μg/ml) of the different crude extracts in culture medium. After a
24- and 72-h incubation, 100 μl medium was removed prior to the
addition of MTT. To determine the mean optical density (OD)
referring to cell viability in the three independent experiments,
eight wells were used for each condition and concentration.
IC50 and IC20 values were determined from the
graphs and are expressed as a percentage compared to
solvent-treated controls.
In vitro cell growth assay
Sulforhodamine B assay (SRB)
Cells were seeded in 96-well plates at an initial
density of 1.5×104 cells in 200 μl of the appropriate
culture medium. After a 24-h incubation, cells were treated with
increasing concentrations (20, 40, 60, 80, 100, 120, 140, 160, 180
and 200 μg/ml) of each crude extract. Concentrations were adjusted
to the results obtained after the MTT assays, and lower
concentrations and smaller increments (1, 5, 10, 15, 20, 25, 30,
40, 45, 50 or 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 μg/ml) were
used for most of the toxic extracts. Following a 72-h incubation,
the amount of cell protein in each well was estimated with the
Sulforhodamine B assay (Sigma) as described previously (8). In three independent experiments, eight
wells were used for each condition and concentration, to determine
the mean OD referring to cell growth. The percentage of growth
inhibition at the IC20 values was determined from the
graphs and compared to solvent-treated controls.
Cell counting assay
Cells were seeded in 25-cm2 culture
flasks at a density of 1.5×105 cells in 5 ml of the
appropriate culture medium. The cells were grown in the presence or
absence of the crude extracts in concentrations, determined in the
72-h MTT assays, harvested using trypsin/EDTA and counted with a
hemacytometer (Hausser Scientific, Horsham, PA, USA). At least
three independent experiments were performed to determine the mean
value, which is presented as a percentage as compared to the
solvent-treated controls.
In vitro wound-healing assay
Cells were grown in 6-well plates until confluency
in the appropriate medium and then washed twice with PBS. After
wounding the cells, 3 ml of medium in the presence or absence of
the crude extracts, at a concentration previously determined in the
24-h MTT assays, was added. After 24 h, the distances over which
the cells migrated were measured and expressed as migratory
velocity (μm/h). At least three independent experiments were
performed (9).
Statistics
Treatments were matched and performed at least three
times. Data were analyzed as means ± SD, using Student’s t-test
(95%).
Results
Extracts of Anemopsis californica and
cell viability
The MTT test was used to determine the cytotoxicity
of each extract on the cell lines studied. The IC50
values, as compared to solvent-treated control conditions, are
shown in Table I (left panel).
Additionally, concentrations at which 80% of the cells remain
viable (IC20) were determined, after 24 h (Table I, right panel) and 72 h (data not
shown), and used in subsequent experiments to eliminate confounding
effects due to cytotoxicity. The EtOAc extracts of the plant parts
(bracts, leaves, roots and stems) appear to be more toxic than the
aqueous and EtOH extracts in the cell lines tested. Additionally,
the aqueous and ethanol extracts of the stems at concentrations up
to 200 μg/ml did not affect the cell viability of these cell lines.
On the other hand, the EtOH and aqueous extracts of the bracts,
leaves and roots exerted variable effects.
EtOH and EtOAc extracts reduce cell
growth
Subsequently, the extracts were evaluated for growth
inhibitory activity against the cell lines studied. The EtOAc
extracts of the roots markedly inhibited the growth of the cell
lines by ≥50%, as determined by SRB and confirmed by cell count
after 72 h (Fig. 1, Table II, numbers in bold). EtOAc and EtOH
extracts of the leaves and the EtOH extract of the stems showed
growth inhibitory activity on MCF-7/AZ cells, while the growth of
HCT-8 colon cancer cells was significantly influenced by the EtOH
extract of the roots. The majority of extracts did not affect the
growth of the Hs 578T breast cancer cells and no activity was found
for each of the aqueous extracts (Table II).
 | Table IIGrowth inhibitory effect of crude
extracts of A. californica against three human cancer cell
lines in vitro. |
Table II
Growth inhibitory effect of crude
extracts of A. californica against three human cancer cell
lines in vitro.
| Plant part | Solvent | Growth (%) |
|---|
| |
|
|---|
| | HCT-8 | Hs 578T | MCF-7/AZ |
|---|
| Bracts | H2O | 95±3 | 100±4 | 107±5 |
| EtOH | 105±7 | 85±6 | 80±6 |
| EtOAc | 100±2 | 71±5 | 86±4 |
| Leaves | H2O | 102±3 | 94±4 | 103±3 |
| EtOH | 102±2 | 78±10 | 63±2 |
| EtOAc | 92±5 | 78±8 | 44±1 |
| Roots | H2O | 96±4 | 100±8 | 105±2 |
| EtOH | 14±5 | 85±2 | 91±6 |
| EtOAc | 46±4 | 27±2 | 47±2 |
| Stems | H2O | 110±1 | 89±10 | 102±4 |
| EtOH | 71±9 | 104±5 | 57±3 |
| EtOAc | 85±4 | 74±4 | 90±4 |
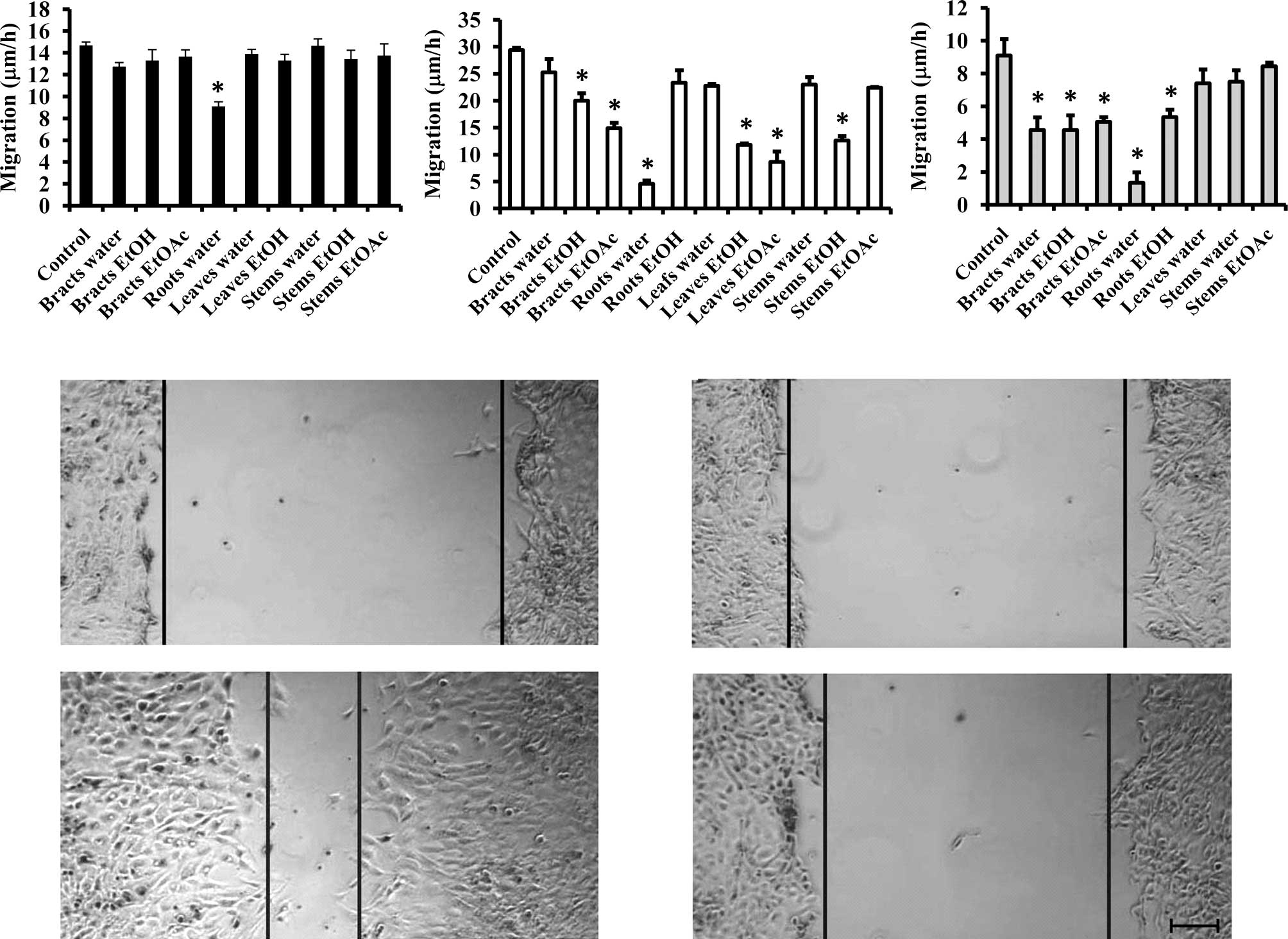
Aqueous extract of the roots inhibits
migration
Since the migration of cancer cells is affected by
their growth, we used the extracts that did not significantly
influence cell growth and tested them on the migratory capacity of
the cell lines. As shown in Fig. 2
(upper panel), the majority of the extracts did not influence the
migratory capacity of the colon cancer cell line, except for the
aqueous extract of the roots. An even more pronounced effect (by
>80%) was observed in the MCF-7/AZ and Hs 578T cell lines
(Fig. 2, lower panel). Furthermore,
several extracts were able to inhibit the migration of MCF-7/AZ and
Hs 578T breast cancer cells.
Discussion
Results of the present study showed that crude
extracts of the plant A. californica, obtained through
methods that correlate with its traditional use by Native
Americans, contain potent anticancer agents. While most of the
activity was found for extracts of the roots, results of other
plant parts and their particular extraction method could be
ignored. For example, the EtOAc and EtOH extracts of the leaves and
the EtOH extract of the stems inhibited the growth of MCF-7/AZ but
not Hs 578T breast cancer cells. This difference suggests that
these crude extracts contain compounds that have an impact on the
estrogen-dependency of MCF-7/AZ cells. No growth inhibitory
activity was found for the aqueous extracts, indicating that
potential compounds for this activity are of a less polar nature.
In a preliminary study conducted on A. californica roots,
cytotoxicity values were within the same range. However, a
discrepancy in the growth effect was noted (6). This can be explained by the fact that
the roots of A. californica were previously obtained from a
local herb store and little was known on how they were harvested
and processed; thus, there was no guarantee that the specimens were
unadulterated. This emphasizes the importance that WHO guidelines
should be followed at all times ensuring proper harvest and quality
assurance of medicinal plants (10).
Migratory and invasive capacities are important
characteristics that distinguish benign from malignant lesions. It
is now increasingly accepted that the migration and invasion
process offers a rich source of novel targets for therapy and that
inhibitors control tumor metastasis (11). Therefore, we determined the
influence of the extracts that did not affect cell growth on the
migratory capacity of the cell lines. We observed that a number of
extracts reduced the migratory capacity to a certain extent, mainly
in MCF-7/AZ and Hs 578T cells. Of particular interest is the
aqueous extract of the roots, which is able to inhibit migration of
all three cell lines and for MCF-7/AZ and Hs 578T by more than 80%.
This effect occurred at non-toxic concentrations, but did not
affect cell growth. The latter result suggests that the more polar
compounds are predominantly responsible for the reduced migratory
velocity. Only limited information on the composition of the
compound is available in the literature, mainly on essential oils
of leaves and roots. Herein, methyleugenol and elemicin were
identified as major constituents, next to thymol and piperitone
(12–15). A recent study relates these
compounds to the growth inhibition of AN3CA and HeLa cells
(5). However, no reports are
currently available to support the anti-migratory effect. This
suggests that other constituents are present in A.
californica, which explains the activity of the crude aqueous
root extract. A literature search of medicinal plant extracts
affecting the migration and invasion of cancer cells revealed that
flavonoids, alkaloids and phenylpropanoids are possible candidate
compound classes (16,17). In this regard, the flavonoid
evodiamine, one of the main constituents of Evodiae Fructus,
was found to inhibit tumor cell migration with low cytotoxicity and
an insignificant effect on cell growth (17). Further purification of the crude
extracts and isolation of the active constituents is necessary to
correlate our findings to these classes of molecules.
In conclusion, our investigation showed that aqueous
and EtOAc extracts of A. californica roots possess
pronounced anticancer activity against multiple human cancer cell
lines, an effect that could be observed independently of the
presence of the estrogen receptor. These extracts are currently
under consideration in our laboratory for bio-guided fractionation.
Additionally, the extracts of the leaves and stems, showing
specificity against the hormone-dependent MCF-7/AZ cells, warrant
further evaluation against known compounds affecting estrogen
receptor responsiveness.
Acknowledgements
This work was supported by the US National
Institutes of Health (1R15 AT002888-01A2), the NSF (0755469)
CHE-MPS/CHE-Undergraduate Programs in Chemistry, and the New Mexico
Tech startup funds. The authors are grateful to Margaret Garcia for
the plant identification.
Abbreviations:
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide
|
|
SRB
|
Sulforhodamine B
|
|
EDTA
|
ethylene diamine tetraacetic acid
|
References
|
1
|
Cragg GM, Grothaus PG and Newman DJ:
Impact of natural products on developing new anti-cancer agents.
Chem Rev. 109:3012–3043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–77. 2005.PubMed/NCBI
|
|
3
|
Moerman DE: Native American Ethnobotany.
5th edition. Timber Press; pp. 11–13. 1998
|
|
4
|
Childs RF and Cole JR: Phytochemical and
pharmacological investigation of Anemopsis californica. J
Pharm Sciences. 54:789–791. 1965. View Article : Google Scholar
|
|
5
|
Medina-Holguin AL, Holguin FO, Micheletto
S, Goehle S, Simon JA and O’Connel MA: Chemotypic variation of
essential oils in the medicinal plant, Anemopsis
californica. Phytochemistry. 69:919–927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daniels AL, van Slambrouck S, Lee RK,
Arguello TS, Browning J, Pullin MJ, Kornienko A and Steelant WF:
Effects of extracts from two Native American plants on
proliferation of human breast and colon cancer cell lines in
vitro. Oncol Rep. 15:1327–1331. 2006.PubMed/NCBI
|
|
7
|
Romijn JC, Verkoelen CF and Schroeder FH:
Application of the MTT-assay to human prostate cancer cell lines in
vitro: establishment of test conditions and assessment of
hormone-stimulated growth and drug-induced cytostatic and cytotoxic
effects. Prostate. 12:99–110. 1988. View Article : Google Scholar
|
|
8
|
Skehan P, Stroeng R, Scudiero D, Monks A,
McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S and Boyd MR:
New colorimetric cytotoxicity assay for anticancer drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Slambrouck S, Hilkens J, Bisoffi M and
Steelant WFA: AsialoGM1 and integrin α2β1 mediate prostate cancer
progression. Int J Oncol. 35:693–699. 2009.
|
|
10
|
WHO guidelines on good agricultural and
collection practices (GAPC) for medicinal plants. World Health
Organization. Geneva: 2003.
|
|
11
|
Mareel M and Leroy A: Clinical, cellular
and molecular aspects of cancer invasion. Physiol Rev. 83:337–376.
2003. View Article : Google Scholar
|
|
12
|
Horton WJ and Paul EG: 4-Allylveratrole
from Anemopsis californica. J Am Chem Soc. 79:2264–2266.
1957. View Article : Google Scholar
|
|
13
|
Acharya RN and Chaubal MG: Essential oil
of Anemopsis californica. J Pharm Sciences. 57:1020–1022.
1968. View Article : Google Scholar
|
|
14
|
Sanvordeker DR and Chaubal MG: Essential
oil of Anemopsis californica Part II: minor constituents. J
Pharm Sciences. 58:1213–1217. 1969.
|
|
15
|
Tutupalli LV and Chaubal MG: Composition
of essential oil from foliage of Houttuynia cordate and
chemosystematics of Saururaceae. Lloydia. 38:92–96. 1975.PubMed/NCBI
|
|
16
|
Ogasawara M, Matsubara T and Suzuki H:
Screening of natural compounds for inhibitory activity on colon
cancer cell migration. Biol Pharm Bull. 24:720–723. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SJ, Lee KW, Hur HJ, Chun JY, Kim SY
and Lee HJ: Phenolic phytochemicals derived from red pine (Pinus
densiflora) inhibit the invasion and migration of SK-Hep-1
human hepatocellular carcinoma cells. Ann NY Acad Sci.
1095:536–544. 2007.PubMed/NCBI
|