1. Introduction
Cancer metastasis is a leading cause of death in
cancer patients and is a multistep process involving complex
interactions between tumor and host cells. To metastasize, tumor
cells must invade from the primary tumor by dissociating from the
tumor mass and be transported to close or distant secondary sites.
Single cells, homotypic clusters of cells or heterotypic emboli
then remain at the secondary site and use organ-specific and organ
non-specific mechanisms which invade the surrounding tissue and
respond to growth signals. A tumor cell should successfully
accomplish each step in the pathway or metastasis may not develop.
Positive and negative regulators exist for each step in the
metastatic cascade, implying the involvement of numerous different
genes.
KiSS-1 is a human metastasis suppressor gene that
inhibits the metastasis of human melanomas and breast carcinomas,
without affecting tumorigenicity (1). KiSS-1 encodes a carboxy-terminally
amidated peptide with 54 amino-acid residues. The peptide was
isolated from human placenta as the endogenous ligand of an orphan
G-protein-coupled receptor (metastin receptor, hOT7T175) and was
termed ‘metastin’ (2). Ohtaki et
al reported that metastin inhibits the chemotaxis and invasion
of metastin receptor-transfected CHO cells in vitro and
attenuates the pulmonary metastasis of metastin
receptor-transfected B16-BL6 melanomas in vivo (2). Results suggest the potential action
mechanisms of KiSS-1 and a novel therapeutic approach. Since the
report by Ohtaki et al, metastin has been considered an
agent with the potential to inhibit cancer metastasis, resulting in
the investigation of various cancer metastases. The literature
currently covers melanoma (2),
thyroid cancer (3), pancreatic
cancer (7,8), hepatocellular carcinoma (HCC)
(5,6), breast cancer (9), ovarian cancer (10), esophageal squamous cell carcinoma
(ESCC) (4), bladder cancer
(11,12) and kidney cancer (13). Additionally, it has been suggested
that metastin inhibits tumor invasion or migration through focal
adhesion kinase, paxillin, MAP kinase or Rho A (2,3,13,14).
2. Identification and basement study of
metastin related to cancer metastasis
Lee et al identified various candidate
metastasis-suppressor cDNAs by subtractive hybridization and
differential display by comparing human metastatic melanoma cell
lines (C8161) and non-metastatic hybrid clones (neo6/C8161.1
cells). One clone, termed KiSS-1, was expressed only in
neo6/C8161.1 cells. KiSS-1 cDNA has a single open reading frame
that encodes a protein of 164 amino acids with a predicted
molecular mass of 18 kDa (1).
Ohtaki et al found a rat orphan receptor (rOT7T175) that was
nearly identical to GPR54 during a search for novel
G-protein-coupled receptors (GPCRs) using a degenerate polymerase
chain reaction (PCR) strategy. To identify the endogenous ligand of
a human orphan receptor, these authors established a stable CHO
cell line expressing the human counterpart metastin receptor
(CHO/h175). Although hOT7T175 had 39.2% amino-acid identity to
human galanin receptor GALR2, the cells did not show any response
to a panel of known peptides, including galanin and galanin-like
peptides. On the other hand, the human placental extract induced a
robust increase in the intracellular calcium ion concentration
([Ca2+]i) in CHO/h175 cells. The amino-terminal sequence
obtained for the isolated peptide was identical to the partial
amino-acid sequence (Gly 68 to Ala 88) of the KiSS-1 gene product.
The sequence was isolated from human placenta as the endogenous
ligand of an orphan G-protein-coupled receptor and was termed
‘metastin’ (2).
Matrix metalloprotease (MMP) plays an important role
in development and morphogenesis by participating in extracellular
matrix re-modeling (15). Cancer
cells also utilize MMP for invasion and metastasis. Invading cells
are forced to proliferate within an embedded dense
three-dimensional matrix composed largely of type I collagen or
cross-linked fibrin (16). Takino
et al reported that metastin forms a complex with pro-MMP
and active MMP-2, while MMP-9, matrix type (MT) 1-MMP, MT3-MMP and
MT5-MMP cleaved the Gly118-Leu119 peptide bond of both full-length
metastin and metastin decapeptide (17). Metastin decapeptide induced the
formation of focal adhesion and actin stress fibers in cells
expressing the receptor. Moreover, digestion of the metastin
decapeptide by MMP abolished its ligand activity. Takino et
al proposed that i) metastin be used as an antimetastatic agent
in combination with the MMP inhibitor, or ii) MMP-resistant forms
of metastin be developed that may also be efficacious.
Metastin has attracted cancer investigations due to
its novelty and potential to inhibit cancer metastasis.
Subsequently, the pertinent literature related to metastin and
cancer metastasis is reviewed herein.
3. Metastin inhibits chemotaxis and invasion
of the metastin receptor-transfected CHO cells in vitro and
the attenuates pulmonary metastasis of metastin
receptor-transfected B16-BL6 melanoma in vivo
Ohtaki et al studied whether metastin
suppresses tumor metastasis (2).
These investigators examined the effect of peptide on cell
motility, a characteristic that is considered crucial for the
invasion of cancer cells. Metastin inhibited the chemotaxis of
metastin receptor-transfected CHO (CHO/h175) cells towards fetal
calf serum, but did not affect that of CHO mock transfectants
(CHO/mock). In an in vitro invasion assay that tested
migration through a Matrigel-coated porous filter, metastin
inhibited the invasion of CHO/h175 cells specifically with an
IC50 value of ~50 nM. Metastin inhibited the
fibronectin-induced chemotaxis of the metastin receptor-transfected
B16-BL6 mouse melanoma (B16-BL6/h175) cells, but not that of
mock-transfected melanoma (B16-BL6/mock) cells. Furthermore, Ohtaki
et al found that metastin induced the excessive formation of
focal adhesions (vinculin-positive patches) and stress fibers in
B16-BL6/h175 cells (2). The cells
began to express focal adhesions 5 min after the addition of
metastin and the majority of cells expressed focal adhesions
densely after 30 min. Excessive stress fibers were formed after 30
min, but not after 5 min. Metastin also induced the phosphorylation
of focal adhesion kinase (FAK) and paxillin, which is believed to
be essential for the formation of focal adhesions. The
phosphorylation of FAK occurred rapidly and almost ceased after 10
min, whereas that of paxillin lasted longer. The same authors
hypothesized that metastin attenuates cellular motility by
excessively inducing adhesive phenotypes (2). In spontaneous pulmonary metastasis
assays, metastin-treated mice transplanted with B16-BL16/h175 cells
showed a marked decrease in the number of metastasized tumor foci
compared to mice that had received a vehicle. On the other hand,
metastin did not affect the metastasis of B16-BL6/mock melanomas.
Therefore, the observed effect on tumor metastasis is likely due to
the direct action of metastin on tumor cells, although metastin may
have also exerted pharmacological effects on host mice.
4. Metastin receptors are overexpressed in
papillary thyroid cancer tissue and activate MAP kinase in thyroid
cancer cells
Ringel et al analyzed thyroid tissue samples
obtained at the time of surgery from 23 patients with thyroid
cancer (10 with follicular and 13 with papillary cancer) and from 2
patients with benign, non-functioning follicular adenomas (3). The expression of metastin receptor
mRNA levels, measured using real-time RT-PCR, was detected in 10 of
13 papillary thyroid cancers and in only 2 of 10 follicular thyroid
cancer types. Statistically, papillary carcinomas were more likely
to express metastin receptors than either follicular carcinoma or
normal thyroid tissue. In paired samples, metastin receptor
expression was overexpressed in the papillary cancers with adjacent
normal tissue specimens. A thyroid cancer cell line (ARO) that
expresses a metastin receptor was analyzed in order to evaluate the
signaling pathways activated by endogenous metastin receptors. It
was found that metastin increased ERK, but not Akt or p38
activation, in a dose-dependent manner under these conditions.
5. Metastin receptors, a promising target
for the suppression of metastasis and metastin, a potential
biomarker for predicting cancer presence and progression in
pancreatic cancer
Masui et al reported that KiSS-1 expression
is suppressed and metastin receptor expression is enhanced in
pancreatic cancer tissue when compared to that in normal tissue
(18). Exogenous metastin was
reported not to suppress cell proliferation, but significantly
reduce the in vitro migration of the pancreatic cancer cell
line (PANC-1) and induce the activation of ERK1 in two pancreatic
cancer cell lines (PANC-1 and AsPC-1) (18). In addition, three novel short
variant forms of metastin, i.e., FM053a2TFA, FM059a2TFA and
FM052a4TFA, were synthesized. These metastin variants significantly
suppressed the migration of PANC-1 cells and activated ERK1. The
results suggested that metastin receptors are a promising target
for the suppression of metastasis.
Katagiri et al reported plasma metastin in
pancreatic cancer patients (8).
Blood samples from 24 healthy volunteers, as well as 33 patients
with pathologically confirmed pancreatic cancer, were analyzed
prior to the latter patients being treated with chemotherapy,
radiation or surgery. Peptide levels in plasma were measured using
highly sensitive EIAs for metastin-LI. Plasma metastin-LI levels
also showed no significant correlation with tumor markers CEA,
CA19-9, CA125 or DUPAN-2. Moreover, levels in pancreatic cancer
patients were significantly higher than those in healthy volunteers
of a similar age. Notably, plasma metastin-LI levels in patients in
T3, T4, N0, M0 and M1 groups were significantly higher than those
in healthy volunteers, although plasma metastin-LI levels in
patients with T2 tumors were not significantly different from those
in healthy volunteers. Plasma metastin-LI levels in patients did
not significantly differ by UICC (International Union Against
Cancer) TNM classification (6th edition) (19). Therefore, Katagiri et al
hypothesized that a high level of metastin in pancreatic cancer
patients is secreted from normal tissue as a reaction to cancer
progression, as a self-defense mechanism. Although further
prospective studies are required, metastin has potential as a
biomarker for predicting cancer presence and progression (8).
6. Metastin/metastin receptor system in
hepatocellular carcinoma and breast cancer is influenced by
hormonal disorders
Ikeguchi et al analyzed the expression of
metastin and metastin receptor mRNA levels in HCC using real-time
RT-PCR (5). Tissue samples were
obtained from 60 patients with HCC who had received surgical
hepatectomy, but not pre-operative chemotherapy or radiation
therapy. Additionally, 8 normal liver tissues were obtained from 8
patients without HCC or liver cirrhosis. It was reported that
although the average level of metastin mRNA did not change among
normal and non-cancerous cirrhotic livers and carcinomas, a
significantly high level of metastin mRNA expression was detected
in 13 carcinomas (5). Moreover, the
percentage of the positive expression of metastin receptor mRNA
significantly increased from 12.5% in normal livers and 26.7% in
non-cancerous cirrhotic livers to 48.3% in the carcinomas. The
tumors with overexpression of metastin and metastin receptor mRNA
were in an advanced stage. Ikeguchi et al concluded that the
overexpressed metastin and metastin receptor mRNA was frequently
observed in HCC, and it was suggested that overexpressed metastin
and metastin receptor peptides mediate growth signals into cancer
cells in HCCs (5). Schmid et
al analyzed samples from 142 patients who underwent orthotopic
liver transplantation (OLT) for HCC by immunohistochemistry
(6). Metastin receptor
immunoreactivity was correlated with the pathological tumor grade
and stage. These authors showed, by multivariate analysis, that an
intense metastin expression in HCC was associated with the poor
prognosis of patients undergoing OLT.
Martin et al reported that frozen sections
from breast cancer primary tumors (matched tumor 124 and background
33) were immunostained with metastin antibody (9). The results of real-time RT-PCR showed
that the expression levels of metastin were higher in tumors
compared to background tissues and were significantly increased in
node-positive tumors compared to node-negative ones. The mRNA of
metastin expression was also elevated with increasing grade and TNM
status, but no such trends were noted with the metastin receptor.
The expression of metastin mRNA was higher in patients who had
succumbed to breast cancer than in those who had remained healthy,
whereas the expression of the receptor showed a decrease in the two
groups. Using immunohistochemistry, the expression of metastin was
higher in tumor sections than in normal tissues. The insertion of
metastin mRNA into the human breast cancer cell line MDA-MB-231
resulted in cells that were significantly more motile and invasive
in behavior, with reduced adhesion to the matrix, using respective
assays. Thus, metastin showed an increase in human breast cancer,
particularly in patients with aggressive tumors and fatal disease.
The overexpression of metastin was therefore correlated with poor
prognosis in patients with breast cancer, and acted as a possible
metastasis promoter in human breast cancer cells in vitro.
This study showed that metastin may have a potential role as a
tumor suppressor in breast cancer and that future studies should
include in vivo models.
By contrast, recently published data on metastin
and/or metastin receptor mRNA expression in other human
malignancies reported a strong correlation between metastin and/or
metastin receptor mRNA overexpression and an improved clinical
course (2–4,7,9). The
mechanisms regarding the reason for the expression of metastin mRNA
product showing an opposite impact on the clinical outcome of HCC
and breast cancer compared to other malignant tumors remain to be
elucidated. Ikeguchi et al postulated that elevated estrogen
levels modulate the expression patterns of metastin (5). Patients with HCC exhibit liver
cirrhosis and are therefore prone to a hyper-estrogenic state. The
fact that metastin/metastin receptor systems in HCC and breast
cancer may be influenced by hormonal disorders is supported by the
findings of Muir and Ohtaki, who reported that the plasma level of
metastin was 10,000-fold higher in pregnant than in non-pregnant
women (2,20). However, further studies are required
to clarify the possible link between estrogen signaling and
metastin and metastin receptor expression in tumors.
7. Metastin and metastin receptor signals
suppress the invasive phenotype of epithelial ovarian cancer
Hata et al analyzed the expression levels of
metastin, the metastin receptor gene, using real-time RT-PCR in 76
epithelial ovarian cancer surgical specimens (10). These authors reported that the
presence of residual tumor was negatively associated with metastin
and metastin receptor gene expression, and that patient age at
diagnosis was significantly associated with metastin gene
expression. It was also found that the prognosis of patients with a
low expression of the metastin receptor gene was significantly
worse than that of those with a high expression (10). However, metastin gene expression had
no impact on patient survival, but a combination of metastin and
metastin receptor gene expression significantly affected patient
prognosis. The results provided new insight into the biological
behavior of epithelial ovarian cancer. Furthermore, metastin and
metastin receptor signals may suppress the invasive phenotype of
epithelial ovarian cancer.
8. Loss of metastin or metastin receptor
mRNA expression may be an important biomarker for detecting lymph
node metastasis in esophageal squamous cell carcinoma
Ikeguchi et al reported on the mRNA levels of
metastin and metastin receptor in tumors and non-cancerous
epithelia of 71 patients with ESCC who underwent surgical
esophageal resection using real-time RT-PCR (5). The mRNA levels of metastin and
metastin receptor were analyzed quantitatively using real-time
RT-PCR and compared to the clinical findings. The mean metastin and
metastin receptor levels of the tumors were similar to those in
non-cancerous epithelia. Loss of metastin and metastin receptor
mRNA was detected in 38 and 61% of tumors, respectively. Ikeguchi
et al compared disease-specific survival among three groups
(group A: 17 patients with tumors with preserved expression of both
metastin and metastin receptor mRNA; group B: 34 patients with
tumors with loss of one of the two mRNA and group C: 14 patients
with tumors with loss of metastin and metastin receptor mRNA)
(4). The disease-specific 5-year
survival rates were 68, 31 and 32% in groups A, B and C,
respectively. To compare differences in the survival curves between
group A and groups B and C, the depth of tumor invasion and lymph
node metastasis were detected as strong prognostic factors in a
multivariate survival analysis. Loss of metastin or metastin
receptor mRNA expression may therefore be an important biomarker
for detecting lymph node metastasis in ESCC.
9. Expression levels of metastin mRNA
provide prognostic information in bladder carcinoma
Nicolle et al analyzed the expression of
metastin and metastin receptor mRNA levels in urothelial carcinoma
(UC) using real-time RT-PCR (12).
It was reported that in UC, from bladder specimens, the expression
levels of metastin mRNA showed a significant increase (carcinoma of
low grade < high grade) compared to normal bladder tissue
(12). The expression levels of
metastin receptor mRNA also showed high expression levels in
bladder carcinomas compared to normal bladder tissue and the
difference between low- and high-grade groups was significant (low
grade < high grade). When the expression levels were classified
(low, normal and high), a correlation was found between increased
aggressiveness of the tumor ranges and the expression levels of the
metastin receptor.
Using in situ hybridization, Sanchez-Carbayo
et al reported a low expression of metastin in 34% and a
high expression of metastin in 45% of high-grade bladder cancers.
No correlation was found between the expression levels of metastin
and the metastin receptor, respectively. These investigators also
suggested that the expression of metastin mRNA provided prognostic
information. Furthermore, complete loss of metastin mRNA was noted
in the invasive tumors of that study as compared to their
respective normal urothelium. The expression of metastin mRNA was
found to be significantly correlated with the histopathological
stage. Patients with a lower expression of metastin mRNA showed a
direct correlation with overall survival in a subset of bladder
tumors whose follow-up was available (n=69) (11). A potential tumor suppressor role in
bladder cancer was shown for metastin. Moreover, a predictive value
was observed by identifying the patients with a poor outcome.
10. Metastin inhibits the migration and
invasion of renal cell carcinoma via overexpression of the metastin
receptors
We reported the mRNA levels of metastin and metastin
receptors in tumor and normal tissues from fresh tissues of renal
cell carcinomas (RCCs) (matched tumor 25 and background 25)
(13). The results showed that the
metastin receptors are highly expressed in RCCs. Our results
present the possibility that metastin receptors are a target for
molecular therapy in the inhibition of RCC metastasis and local
invasion due to the high affinity between metastin and its
receptor. In the literature, the prognosis for RCC based on
histological subtypes and 5-year cancer-specific survival for clear
(conventional) cell carcinoma is shorter than that for papillary
and chromophobe carcinoma (21).
Although our 25 RCC cases did not include all three (clear cell
type, papillary type and unclassified) of these subtypes, no
differences in metastin and/or its receptor between clear-cell and
papillary carcinoma were found. Another notable finding presented
in this study is that metastin induced the excessive formation of
focal adhesions, which are located at the ends of stress fibers in
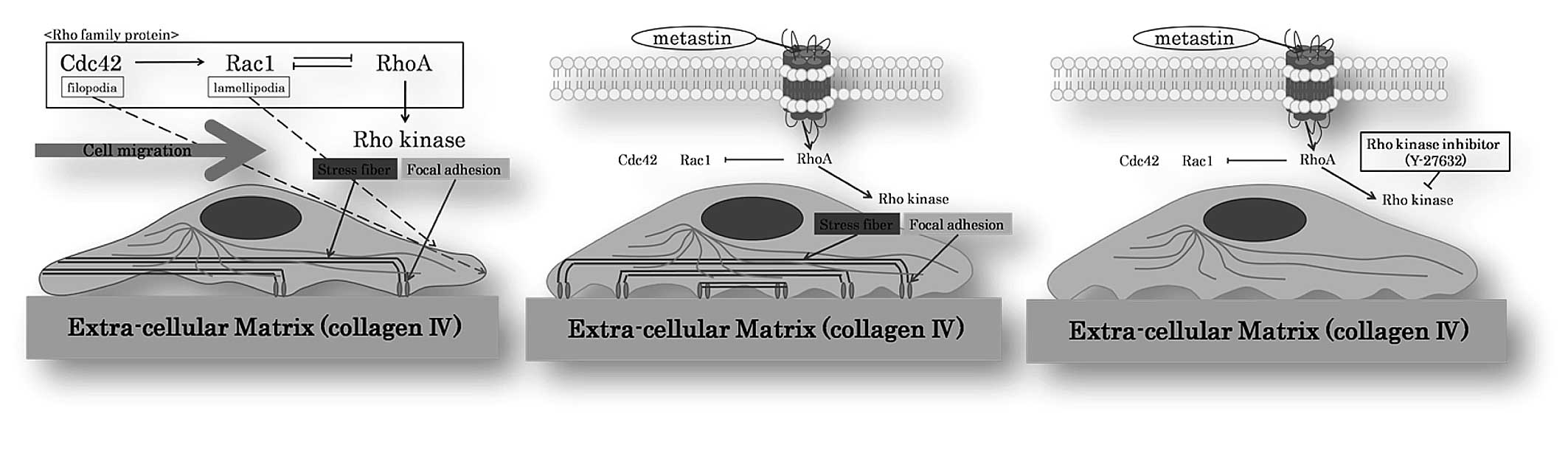
RCC cell lines (Caki-1 and ACHN cells) (Fig. 1B). This reaction was blocked when we
pre-treated Caki-1 and ACHN with the pharmacological Rho kinase
inhibitor Y-27632 (Fig. 1C).
However, the mechanism of how intracellular signals induce
excessive focal adhesion through the metastin receptor has yet to
be elucidated. In light of previous investigations, we hypothesized
that metastin changes the shape of RCC cells through this
Rho-GTPase pathway by activating one or a number of its members.
Using Caki-1, it was found that the expression of the von
Hippel-Lindau tumor suppressor protein (pVHL) was up-regulated by
hypoxia through RhoA-dependent activity and that Rho kinase
inhibitor Y-27632 inhibits this pVHL induction by hypoxia (22). Rho kinase is involved in the
formation of stress fibers and focal adhesion in fibroblasts.
Numerous observations suggest that RhoA regulates the formation of
focal adhesion associated with stress fibers by activating myosin,
whereas Cdc42/Rac1 regulates the formation of focal complexes
associated with filopodia and lamellipodia, and disassembles focal
adhesion and stress fibers through PAK. Rho regulates cell adhesion
and motility by sequence activation of its family members, i.e.,
Cdc42→Rac1⇔RhoA GTPase. This sequence is associated with
time-dependent changes in cell morphology from filopodia/actin
spikes to lamellipodia/membrane ruffles to stress fibers (Fig. 1A). Each member of the Rho-GTPase
family has its specific intracellular signal. Our current results
clearly show that metastin changes the shape of RCC cells through
this Rho-GTPase pathway by activating one or a number of its
members (Fig. 1B). This phenomenon
may provide fundamental knowledge for exploiting new treatments for
RCC cases. Cdc42/Rac1 and RhoA exert mutually antagonistic effects
at some point in the process of contact initiation, maturation and
turnover. The balance of activities between the Rho-GTPase family
and downstream signaling of Rho determines the final patterns of
adhesion and cytoskeleton organization. In addition, a phenomenon
exists whereby a high concentration of Cdc42 suppresses membrane
ruffling, which is dependent on Rac1. We did not find any
disruption of microtubules, as evidenced by tubulin staining in
metastin-supplied cells. This phenomenon is consistent with the
finding that metastin has no effect on Caki-1 and ACHN cell
proliferation. It is known that there is a pathway separate from
the that leading to cell adhesion, which mediates the
growth-promoting action of Rho (23). Although the detailed mechanism
should be investigated further, it is speculated that metastin may
have inhibited cell migration and invasion by regulating Rho kinase
in RCC. It is known that the metastasis and invasion of RCC involve
various factors. Thus, the pathways associated with metastin and
its receptors are crucial.
11. Conclusion
In conclusion, it is evident that further research
is necessary before the precise role and effect of metastin and the
metastin receptor in human cancer can be elucidated. However, since
cancer metastasis is difficult to treat, the results of basic and
clinical studies of metastin are noteworthy. Metastin should be
further investigated for the future treatment of cancer.
Abbreviations:
|
ESCC
|
esophageal squamous cell carcinoma
|
|
HCC
|
hepatocellular carcinoma
|
|
OLT
|
orthotopic liver transplantation
|
|
RCC
|
renal cell carcinoma
|
|
UC
|
urothelial carcinoma
|
References
|
1
|
Lee JH, Miele ME, Hicks DJ, et al: KiSS-1,
a novel human malignant melanoma metastasis-suppressor gene. J Natl
Cancer Inst. 88:1731–1737. 1997.PubMed/NCBI
|
|
2
|
Ohtaki T, Shintani Y, Honda S, et al:
Metastasis suppressor gene KiSS-1 encodes peptide ligand of a
G-protein-coupled receptor. Nature. 31:613–617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ringel MD, Hardy E, Bernet VJ, et al:
Metastin receptor is overexpressed in papillary thyroid cancer and
activates MAP kinase in thyroid cancer cells. J Clin Endocrinol
Metab. 87:23992002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ikeguchi M, Yamaguchi K and Kaibara N:
Clinical significance of the loss of KiSS-1 and orphan
G-protein-coupled receptor (hOT7T175) gene expression in esophageal
squamous cell carcinoma. Clin Cancer Res. 10:1379–1383. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ikeguchi M, Hirooka Y and Kaibara N:
Quantitative reverse transcriptase polymerase chain reaction
analysis for KiSS-1 and orphan G-protein-coupled receptor
(hOT7T175) gene expression in hepatocellular carcinoma. J Cancer
Res Clin Oncol. 129:531–535. 2003. View Article : Google Scholar
|
|
6
|
Schmid K, Wang X, Haitel A, et al: KiSS-1
overexpression as an independent prognostic marker in
hepatocellular carcinoma: an immunohistochemical study. Virchows
Arch. 450:143–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagai K, Doi R, Katagiri F, et al:
Prognostic value of metastin expression in human pancreatic cancer.
J Exp Clin Cancer Res. 28:92009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katagiri F, Nagai K, Kida A, et al:
Clinical significance of plasma metastin level in pancreatic cancer
patients. Oncol Rep. 21:815–819. 2009.PubMed/NCBI
|
|
9
|
Martin TA, Watkins G and Jiang WG: KiSS-1
expression in human breast cancer. Clin Exp Metastasis. 22:503–511.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hata K, Dhar DK, Watanabe Y, et al:
Expression of metastin and a G-protein-coupled receptor (AXOR12) in
epithelial ovarian cancer. Eur J Cancer. 43:1452–1459. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanchez-Carbayo M, Capodieci P and
Cordon-Cardo C: Tumor suppressor role of KiSS-1 in bladder cancer:
loss of KiSS-1 expression is associated with bladder cancer
progression and clinical outcome. Am J Pathol. 162:609–617. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nicolle G, Comperat E, Nicolaïew N, et al:
Metastin (KISS-1) and metastin-coupled receptor (GPR54) expression
in transitional cell carcinoma of the bladder. Ann Oncol.
18:605–607. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shoji S, Tang XY, Umemura S, et al:
Metastin inhibits migration and invasion of renal cell carcinoma
with overexpression of metastin receptor. Eur Urol. 55:441–449.
2009. View Article : Google Scholar
|
|
14
|
Harms JF, Welch DR and Miele ME: KISS1
metastasis suppression and emergent pathways. Clin Exp Metastasis.
20:11–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liotta LA, Thorgeirsson UP and Garbisa S:
Role of collagenases in tumor cell invasion. Cancer Metastasis Rev.
1:277–288. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fisher KE, Sacharidou A, Stratman AN, et
al: MT1-MMP- and Cdc42-dependent signaling co-regulate cell
invasion and tunnel formation in 3D collagen matrices. J Cell Sci.
122:4558–4569. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takino T, Koshikawa N, Miyamori H, et al:
Cleavage of metastasis suppressor gene product KiSS-1
protein/metastin by matrix metalloproteinases. Oncogene.
22:4617–4626. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masui T, Doi R, Mori T, et al: Metastin
and its variant forms suppress migration of pancreatic cancer
cells. Biochem Biophys Res Commun. 315:85–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sobin L and Witte KC: Anonymous: Pancreas.
TNM Classification of Malignant Tumours. Wiley-Lewis; New York: pp.
93–96. 2002
|
|
20
|
Muir AI, Chamberlain L, Elshourbagy NA, et
al: AXOR12, a novel human G protein-coupled receptor, activated by
the peptide KiSS-1. J Biol Chem. 276:28969–28975. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teloken PE, Thompson RH, Tickoo SK, et al:
Prognostic impact of histological subtype on surgically treated
localized renal cell carcinoma. J Urol. 182:2132–2136. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turcotte S, Desrosiers RR and Beliveau R:
Hypoxia upregulates von Hippel-Lindau tumor-suppressor protein
through RhoA-dependent activity in renal cell carcinoma. Am J
Physiol Renal Physiol. 286:F338–F348. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Narumiya S: The small GTPase Rho: cellular
functions and signal transduction. J Biochem. 120:215–218. 1996.
View Article : Google Scholar : PubMed/NCBI
|