Introduction
Cases of adenocarcinoma in Barrett’s esophagus (BE)
are on the increase in Western countries. Clinical and histological
studies suggest a successive progression from gastroesophageal
reflux disease (GERD) to columnar-lined esophagus, also known as
BE, to dysplasia with a high risk of malignancy. The pathogenesis
of this sequence has yet to be completely elucidated, since more
cases of BE, dysplasia and adenocarcinoma were recently found among
patients without GERD symptoms than those that underwent endoscopy
because of GERD (1). This result
depends on the identifying criteria indicative for BE used by the
endoscopist who obtains the biopsy. The management of BE remains
controversial. Various guidelines exist and the international
consensus over issues, such as recognition of short-segment disease
and surveillance policies for uncomplicated and dysplastic disease,
is lacking (2).
The histological diagnosis of BE involves the
presence of columnar epithelium with goblet cells in the esophageal
mucosa. Dysplasia-intraepithelial neoplasia (IEN) serves as a
morphological marker for increased cancer risk. The most important
diagnostic markers of IEN are poor maturation, excessive crowding
of glands and cytonuclear features. It is believed that the
development of BE with intestinal goblet-type cells is related to
the process of proliferation and differentiation of pluripotential
epithelial stem cells in response to local injury, chronic
inflammation as well as the repair process being altered (3–5). The
practical utility of mucin stainings, endocrine cell count,
assessment of cell proliferation (Ki-67 and PCNA), expression of
EGF, TGFα and p53 is limited regarding the diagnosis and
differentiation of dysplastic and non-dysplastic BE (6,7).
One of the factors related to esophageal
adenocarcinoma pathogenesis is an aberrant arachidonic acid (AA)
metabolism through cyclooxygenase (COX) and 5- and 12-lipooxygenase
(5- and 12-LOX). Numerous published studies are related to the
expression of stem cell markers and pro-tumorigenic enzymes 5- and
12-LOX in BE. Findings of these studies showed a positive
expression in esophageal adenocarcinoma, with inconsistent results
in other lesions of esophago-gastric junction mucosa (4,8,9). This
study aimed to assess platelet 12-lipoxygenase (p12LOX) and stem
cell markers in BE mucosa and other gastro-intestinal mucosal
lesions.
Materials and methods
Patients
Between 2005 and 2007, endoscopical biopsies were
obtained from 110 patients, aged 33–71 years, with a clinical and
endoscopical diagnosis of GERD and the suggestion of BE, in the
Department of Gastroenterology, Medical University of Gdańsk,
Poland. The pathological examination of gastro-esophageal junction
biopsies performed by two independent pathologists confirmed the
initial diagnosis in 19 cases. Of the immunohistochemical
examinations performed in the BE cases, 5 of BE with low-grade
dysplasia, 10 of endoscopically suggested BE without pathological
confirmation (gastric carditis), 17 of gastric mucosal intestinal
metaplasia and 10 of sporadic colorectal low-grade adenomas were
included in the study.
Antibodies and proteins
The p12LOX antibodies were developed based on
whole-length recombinant human enzyme with 7212 being rabbit,
polyclonal and 7225 murine, monoclonal, anti-human antibodies
(American Diagnostica, Inc.; clones 12.05 and 25.20, dilution 1:200
and 1:300, respectively). The new antibodies were checked for
cross-reactivity against all human lipoxygenases. The recombinant
enzymes, except for p12LOX from our laboratory (5), were generous donations from Dr T.
Holman, University of California, Santa Cruz, CA, USA (5LOX,
15LOX-1 and 15LOX-2), and Dr A. Brash, Vanderbilt University
Medical Center, Nashville, TN, USA (12R-LOX and eLOX3). The
antibodies for nestin (196908, 1:50), CD44 (M7082, 1:50) and CD117
(K4011, 1:400) used in this study were from Dako, Dakopatts,
Denmark. The tissues of gastric adenocarcinoma were used for the
positive controls.
Immunohistochemistry
Paraffin-embedded tissue blocks were available for
immunohistochemical evaluation in all 110 cases. Standard
avidin-biotin-peroxidase complex technique was used for
immunohistochemistry performed on 4-μm paraffin sections of
formalin-fixed, paraffin-embedded tissue. Antigen retrieval in
heated citrate buffer at pH 6.0 with an incubation time of 30 min
was applied to the antibodies. The immunoreactivity was scored on a
3-point scale, with 1+ (low) reactivity in <10% of the
epithelial cell population, 2+ (moderate) in 10–40% of cells and 3+
(high) in >40% of cells.
Results
The immunoreactivity of the tested antibodies
varied. Stem cell marker CD117 was completely negative. The
positive immunoreactivity of the remaining antibodies is collated
in Table I. The majority of the
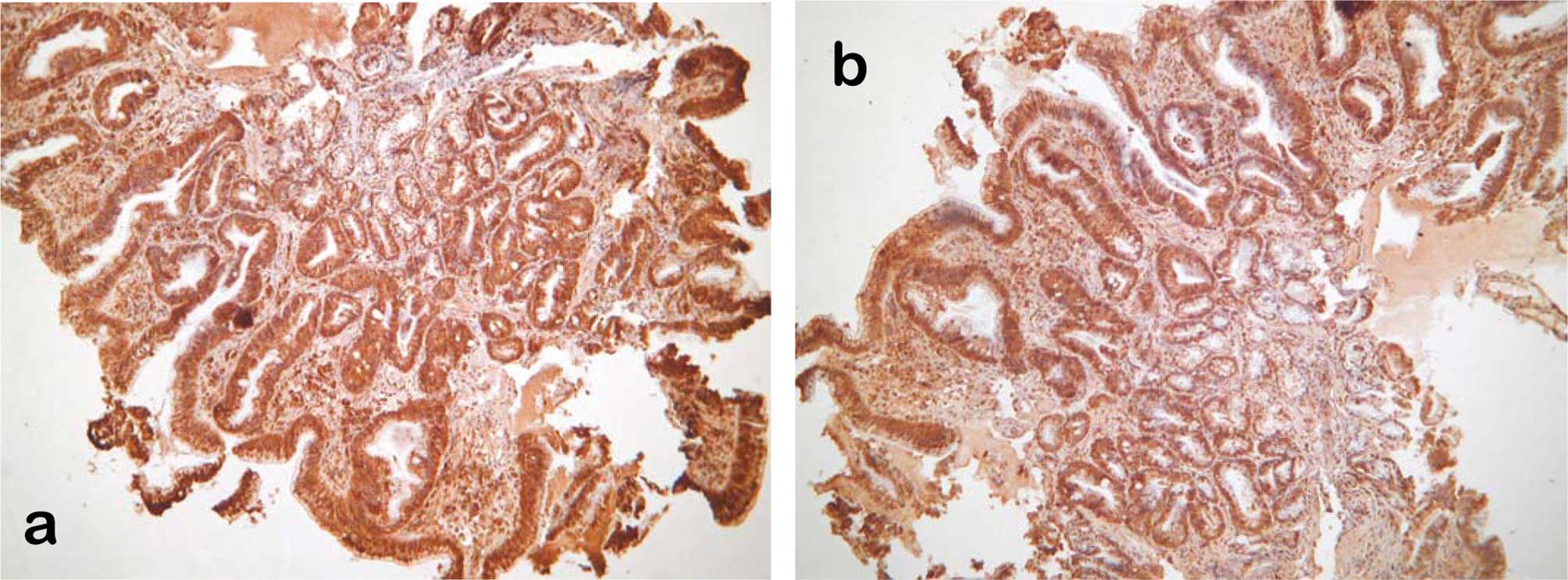
cases of Barrett’s mucosa showed moderate to high immunoreactivity
with p12LOX antibodies (Fig. 1a)
and more than half of the cases were immunopositive for stem-cell
antibodies CD44 (Fig. 1b) and
nestin. The highest immunoreactivity was observed in dysplastic BE
mucosa. The 5 cases of Barrett’s with IEN showed high positive
immunoreactivity with p12LOX, CD44 and nestin antibodies.
 | Table IThe positive immunoreactivity of
p12LOX, CD44 and nestin in Barrett’s metaplasia, GERD carditis and
colorectal adenomas. |
Table I
The positive immunoreactivity of
p12LOX, CD44 and nestin in Barrett’s metaplasia, GERD carditis and
colorectal adenomas.
| Antibodies | No. of patients | p12LOX ab7212 | p12LOX ab7225 | CD44 | Nestin |
|---|
| Barrett’s mucosa | 19 | 19 | 18 | 11 | 10 |
| Barrett’s
dysplasia | 5 | 5 | 5 | 5 | 5 |
| Gastric carditis | 10 | 3 | 1 | 1 | 1 |
| Gastric intestinal
metaplasia | 17 | 3 | 3 | 3 | 3 |
| Colorectal
adenoma | 10 | 7 | 1 | 0 | 0 |
In the comparative group, few clinically suspicious
cases not morphologically confirmed for BE showed mild focal
immunopositive reactions. A total of 3 of 17 cases in the gastric
mucosal intestinal metaplasia cases showed focal immunopositivity
for the tested markers restricted to the foci of low-grade mucosal
dysplasia. The majority of the colorectal adenomas with low-grade
dysplasia showed mild positive immunostaining for p12LOX ab7212
(rabbit, polyclonal), whereas p12LOX ab7225 (murine, monoclonal)
was positive in only 1 case with negative stem cell markers.
Discussion
The diagnostic incidence of BE in the endoscopical
biopsy material obtained from the OG junction mucosa of patients
with GERD, endoscopically suspected for BE, (13%) is comparable to
the incidence reported by other studies (1,10). Our
diagnostic criteria of BE and lesions suggestive for GERD gastric
carditis are the same as those of Montgomery (10).
Lipoxygenases (LOXs) are significant enzymes that
metabolize AA to hydroxyl-eicosatetraenoic acids (HETE) and
leukotrienes involved in inflammatory and carcinogenic processes.
Platelet 12-LOX metabolite 12S-HETE affects cell proliferation and
apoptosis on the signal transduction pathway mediated by ERK
(11). Limited information related
to the role and expression of LOX in BE is currently available.
5-LOX showed immunohistochemical overexpression during esophageal
adenocarcinogenesis (12). 5- and
12-LOX are regarded as pro-tumorigenic enzymes in colonic
carcinogenesis and their overexpression was also described in
various types of cancer [(13–16)
and references therein]. The new antibodies developed for this
study were the first on the market with specificity for the
whole-length enzyme and with proven lack of any cross-reactivity
with other human LOXs. Our studies have shown the usefulness of
these new antibodies for immunohistochemical studies of
parafin-embedded samples in melanoma, prostate, uteral and kidney
cancers (data not shown) in addition to the gastrointestinal
samples discussed in this study.
Our findings showed extremely high immunoreactivity
of the two p12LOX antibodies in non-dysplastic BE and Barrett’s
dysplasia, thereby confirming the pro-carcinogenic activity of
platelet 12-LOX and suggesting the diagnostic utility of the two
antibodies in GERD and BE.
CD44 is a cell surface molecule enrolled in
cell-cell and cell-extracellular matrix protein interactions. In
particular, its spliced variants 5 and 6 have been shown to play a
role in the progression of certain tumors, including gastric
carcinoma. According to Menges et al (9), the expression of CD44 noted in
Barrett’s carcinoma did not increase compared to non-dysplastic BE
and was completely negative in gastric mucosa. Other investigators
(8,17) showed that CD44 progressively
increases in Barrett’s dysplasia and adenocarcinoma.
Stem cell markers CD44 and nestin, as shown in our
study, are potential markers of malignant transformation in BE,
similar to intestinal metaplasia of the stomach (17,18).
Our study results also showed that p12LOX and stem-cell
immunoreactivity is much higher in BE when compared to other
gastrointestinal mucosal cancer precursor lesions and suggests a
more active pre-neoplastic transformation of Barrett’s mucosa.
Acknowledgements
The authors wish to thank American Diagnostica,
Inc., for providing p12LOX antibodies and Dr R. Hart for the
financial support (to J.J. and E.S.J.) of our research. This work
was financed in part by The Frank D. Stranaham Endowment Fund for
Oncological Research and The Frederick M. Douglass Foundation. In
memory of Professor K. Jaśkiewicz (pathologist), who was
instrumental in this research and passed away.
References
|
1
|
Fan X and Snyder N: Prevalence of
Barrett’s esophagus in patients with or without GERD symptoms: role
of race, age, and gender. Dig Dis Sci. 54:572–577. 2009.
|
|
2
|
Ramus JR, Caygill CP, Gatenby PA and
Watson A: Current United Kingdom practice in the diagnosis and
management of columnar-lined oesophagus: results of the United
Kingdom National Barrett’s Oesophagus Registry endoscopist
questionnaire. Eur J Cancer Prev. 17:422–425. 2008.PubMed/NCBI
|
|
3
|
Guillem PG: How to make a Barrett
esophagus: pathophysiology of columnar metaplasia of the esophagus.
Dig Dis Sci. 50:415–424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hattori T, Mukaisho K and Miwa K:
[Pathogenesis of Barrett’s esophagus – new findings in the
experimental studies of duodenal reflux models]. Nippon Rinsho (in
Japanese). 63:1341–1349. 2005.
|
|
5
|
Tang LH and Klimstra DS: Barrett’s
esophagus and adenocarcinoma of the gastroesophageal junction: a
pathologic perspective. Surg Oncol Clin N Am. 15:715–732. 2006.
|
|
6
|
Jaskiewicz K, Louw J and Anichkov N:
Barrett’s oesophagus: mucin composition, neuroendocrine cells, p53
protein, cellular proliferation and differentiation. Anticancer
Res. 14:1907–1912. 1994.
|
|
7
|
Rustgi AK: Models of esophageal
carcinogenesis. Semin Oncol. 33:S57–S58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lagorce-Pages C, Paraf F, Dubois S,
Belghiti J and Flejou JF: Expression of CD44 in premalignant and
malignant Barrett's oesophagus. Histopathology. 32:7–14. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Menges M, Goebel R, Pueschel W, Zeitz M
and Stallmach A: Expression of CD44v5 and -v6 in Barrett’s
carcinoma is not increased compared to that in nondysplastic
Barrett’s mucosa. Exp Mol Pathol. 72:207–212. 2002.
|
|
10
|
Montgomery EA: Biopsy Interpretation of
Gastrointestinal Tract Mucosa. Lippincott Williams & Wilkins;
pp. 37–70. 2005
|
|
11
|
Chen FL, Wang XZ, Li JY, Yu JP, Huang CY
and Chen ZX: 12-lipoxygenase induces apoptosis of human gastric
cancer AGS cells via the ERK1/2 signal pathway. Dig Dis Sci.
53:181–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Wang S, Wu N, et al:
Overexpression of 5-lipoxygenase in rat and human esophageal
adenocarcinoma and inhibitory effects of zileuton and celecoxib on
carcinogenesis. Clin Cancer Res. 10:6703–6709. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bednar W, Holzmann K and Marian B:
Assessing 12(S)-lipoxygenase inhibitory activity using colorectal
cancer cells overexpressing the enzyme. Food Chem Toxicol.
45:508–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong Z, Hebert JR, Bostick RM, et al:
Common polymorphisms in 5-lipoxygenase and 12-lipoxygenase genes
and the risk of incident, sporadic colorectal adenoma. Cancer.
109:849–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoque A, Lippman SM, Wu TT, et al:
Increased 5-lipoxygenase expression and induction of apoptosis by
its inhibitors in esophageal cancer: a potential target for
prevention. Carcinogenesis. 26:785–791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skrzypczak-Jankun E, Chorostowska-Wynimko
J, Selman SH and Jankun J: Lipoxygenases – a challenging problem in
enzyme inhibition. Current Enzyme Inhibition. 3:119–132. 2007.
|
|
17
|
Castella E, Ariza A, Fernandez-Vasalo A,
Roca X and Ojanguren I: Expression of CD44H and CD44v3 in normal
oesophagus, Barrett mucosa and oesophageal carcinoma. J Clin
Pathol. 49:489–492. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gulmann C, Grace A, Leader M, Butler D,
Patchett S and Kay E: CD44v6: a potential marker of malignant
transformation in intestinal metaplasia of the stomach? An
immunohistochemical study using tissue microarrays. Eur J
Gastroenterol Hepatol. 15:981–986. 2003. View Article : Google Scholar : PubMed/NCBI
|