Introduction
Treatment of infection using antimicrobial drugs is
based on data generated with prophlogistic bacteria. Currently,
medical treatment strategy is determined according to the
guidelines for management (1). A
treatment guideline for nosocomial pneumonia and febrile
neutropenia (1) exists, but the
data implicating a causative agent at the time of pyrexia in a
tumor-bearing stage are not available. Therefore, a recommended
treatment guideline is absent in such conditions.
Lung cancer is a leading cause of cancer-related
mortality worldwide and is expected to remain a major health
concern for the foreseeable future. The majority of patients with
non-small cell lung cancer (NSCLC) are in advanced stages at
diagnosis and most are treated with cytotoxic agents such as those
used in patients with small cell lung cancer (SCLC) (2). The majority of cytotoxic agents for
anticancer therapy also adversely affect normal cells. It is
assumed that these unfavorable effects primarily impact rapidly
proliferating cells, such as bone marrow cells.
Increased susceptibility to infection and difficulty
in treatment due to neutropenia after cytotoxic anticancer drug
administration is a serious concern in terms of chemotherapeutic
continuation. Fever in neutropenic patients is a frequent
complication of chemotherapy. It occurs in 10–50% of patients with
solid tumors and in 80% of those with blood malignancies. It
usually requires treatment for 7–12 days, at an approximate daily
cost of more than US $1,500 and is associated with a mortality rate
of almost 10%. Thus, febrile neutropenia affects an increasing
number of individuals worldwide and poses a significant burden on
health care and its economy (3).
Even without neutropenia, respiratory tract infection can be
precipitated by lung cancer, thereby disordering pulmonary local
blood and air flow. In addition, it appears that degradation of the
immunologic status occurs from aggravation of the host nutritional
status. When general steroids are used for the therapeutic purpose
of edematous reduction of metastases to the brain, nausea and
vomiting can also cause a degradation of the immunologic state.
Cytotoxic anticancer drugs are usually administered
to advanced lung cancer patients, and fever increases the
difficulty in treatment continuation. The frequency of infectious
diseases in advanced lung cancer patients, regardless of the
administration of anticancer drugs, appears to be higher than in
healthy individuals. However, it is difficult to diagnose an
infectious disease in advanced lung cancer patients during
chemotherapy as no epidemiological investigative studies regarding
infection in lung cancer patients currently exist. Therefore, it is
difficult to determine whether pyrexia or the tumor itself is the
cause of infection.
Procalcitonin (PCT), a glycoprotein, is the
propeptide of calcitonin. It is devoid of hormonal activity. It
consists of 116 amino acids, has a molecular weight of 13 kDa
(4) and, under normal
circumstances, is produced in the C cells of the thyroid gland. PCT
is then cleaved via a specific protease to calcitonin, katacalcin
and an N-terminal residue (5). In
contrast to the short half-life of calcitonin (10 min), PCT has a
long half-life of 25–30 h in serum (6). In healthy humans, PCT levels are
undetectable (< 0.1 ng/ml). Severe generalized bacterial,
parasitic or fungal infections with systemic manifestation are
associated with increased PCT serum levels. Karzai et al
reported that PCT may be a superior marker in infections with
systemic manifestation (6).
Therefore, this study investigated the infectious
background of the febrile advanced lung cancer patients who
received chemotherapy. The value of PCT and c-reactive protein
(CRP) in the differential diagnosis of febrile conditions in
patients with advanced lung cancer was also evaluated.
Materials and methods
Study population
A total of 121 patients with advanced lung cancer
who were treated with a cytotoxic chemotherapy regimen between
September 2007 and September 2008 at Kyoto University Hospital were
recruited. Written informed consent pertaining to the utilization
of clinical materials was obtained from all patients. The study was
approved by the Ethics Committee of the Kyoto University Graduate
School and Faculty of Medicine. The inclusion criteria involved an
advanced lung cancer patient undergoing cytotoxic chemotherapy with
systemic infection based on any of the following characteristics:
fever (>38˚C) with cough, rapid breathing, difficulty in
breathing or convulsions and with a clinical diagnosis of pneumonia
or sepsis. Laboratory evaluation for blood, sputum and urine
culture were collected from the patients as part of the usual
routine investigations conducted. The patients were in the
nosocomial setting. Nosocomial pneumonia is defined as pneumonia
occurring ≥48 h after admission (7).
Laboratory methods
Blood samples obtained on the first day of fever
were stored at −80˚C until the serum PCT and CRP levels were
measured. The serum CRP level was determined using the
nephelometric method and the serum PCT level was measured by the
Lumi test-PCT (ILMA Kits BRAHMS Diagnostica GmbH, Berlin, Germany).
The analytical assay sensitivity was ~0.1 ng/ml. At least two blood
cultures were performed. Sputum was obtained for Gram staining and
culture and subsequently analysed by Taq Man real-time PCR for
Mycoplasma pneumoniae, Legionella pneumophila and
Chlamydophyla psittaci (8).
Urine was sampled for antigen testing on Streptococcus
pneumoniae and Legionella pneumophila (Binax NOW; Binax,
Scarborough, ME, USA) (9,10).
Results
Patient characteristics
Table I shows the
characteristics of the 61 advanced lung cancer patients who were
admitted to our institution between June 2007 and June 2008. The
patients were Japanese, and included 50 (83.3%) males and 10
(16.7%) females, with a median age of 69 years (range 35–80). Of
the 61 patients, 8 (11.5%) were non-smokers and 53 (88.3%) were
former or current smokers. The patients were treated with cytotoxic
agents in the febrile stage. The pathologic diagnoses and disease
stage are listed in Table I. There
were 71 episodes in 61 patients during the 12 months of the study,
i.e., 50.4% of our study population. Of the 71 episodes, causative
agents were found in 46 episodes, including 6 neutropenia cases
(Fig. 1, Group A). No causative
agents were found in 25 episodes, including 2 neutropenia cases
(Fig. 1, Group B). Table II shows the organisms isolated in
sputum, blood and urine. A total of 41 patients (57.7%) were
diagnosed with pneumonia using imaging modalities, 6 (8.5%) with
bacteremia using blood culture and 4 patients (5.6%) with urinary
tract infection using urine culture. Among the 41 pneumonia cases,
culture from sputum revealed pathologic bacteria in 21 (51.2%)
cases, fungal disease in 14 (34.1%) and cytomegalovirus in 1 (2.5%)
case. Antibiotic treatment was prescribed for all 41 cases.
 | Table ICharacteristics of the febrile
patients (n=61). |
Table I
Characteristics of the febrile
patients (n=61).
| Patient
characteristics | No. of cases (%) |
|---|
| Age (years) | 35–82 |
| Median | 69 |
| Gender |
| Female | 11 (18.0) |
| Male | 50 (82.0) |
| Smoking status |
| Non-smoker | 8 (13.1) |
| Smoker | 53 (86.9) |
| Former | 19 (28.4) |
| Current | 34 (58.5) |
| Tumor histology |
| Adenocarcinoma | 24 (39.3) |
| Squamous cell
carcinoma | 10 (16.4) |
| NSCLC | 4 (6.6) |
| Small cell
carcinoma | 23 (37.7) |
| Pathologic stage |
| IIIA | 11 (18.0) |
| IIIB | 18 (29.5) |
| IV | 32 (52.5) |
 | Table IIResults of identified causes of 71
episodes in 61 cases. |
Table II
Results of identified causes of 71
episodes in 61 cases.
| Causes | No. of episodes |
|---|
| Pneumonia |
| Streptococcus
pneumoniae | 6 |
| Clamidya
pneumoniae | 4 |
| MRSA | 3 |
| Moraxella
catarrhalis | 2 |
| Klebsiella
pneumoniae | 2 |
| Staphylococcus
aureus | 1 |
| Pseudomonas
aeruginosa | 1 |
| Streptococcus
G | 1 |
| Pseudomonas
fluorescens | 1 |
| Fungal diseases |
|
Aspergillosis | 4 |
| Pneumocystis
jiroveci | 4 |
|
Candidiasis | 1 |
| Causative fungus
unknown | 5 |
| Cytomegalo
virus | 1 |
| Cause unknown | 5 |
| Bacteremia |
| E. coli | 2 |
| Streptococcus
pyrogen | 1 |
| Streptococcus
epidermis | 1 |
| Streptococcus
G | 1 |
| Staphylococcus
aureus | 1 |
| Urinary tract
infection |
| Staphylococcus
agalactiae | 1 |
| Enterococcus
faecalis | 1 |
| Coaglase-negative
streptococcus | 1 |
| E.
coli | 1 |
| Cause unknown | 20 |
| Total | 71 |
Serum inflammation markers
Among the 71 febrile episodes, serum PCT and CRP
were measured in 50 cases. Serum CRP was elevated beyond the
cut-off level of our institute (0.2 mg/dl) in 69 (97.2%) cases.
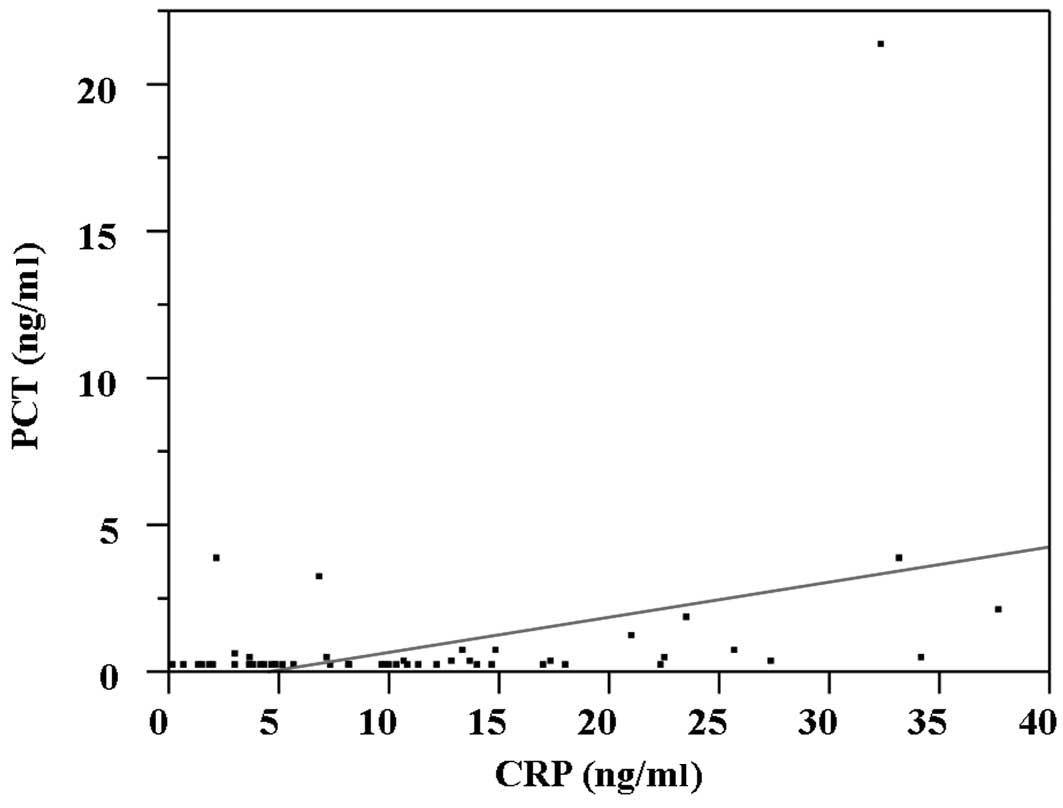
Serum CRP correlates with serum PCT (Fig. 2; r=0.38, p=0.0045). According to the
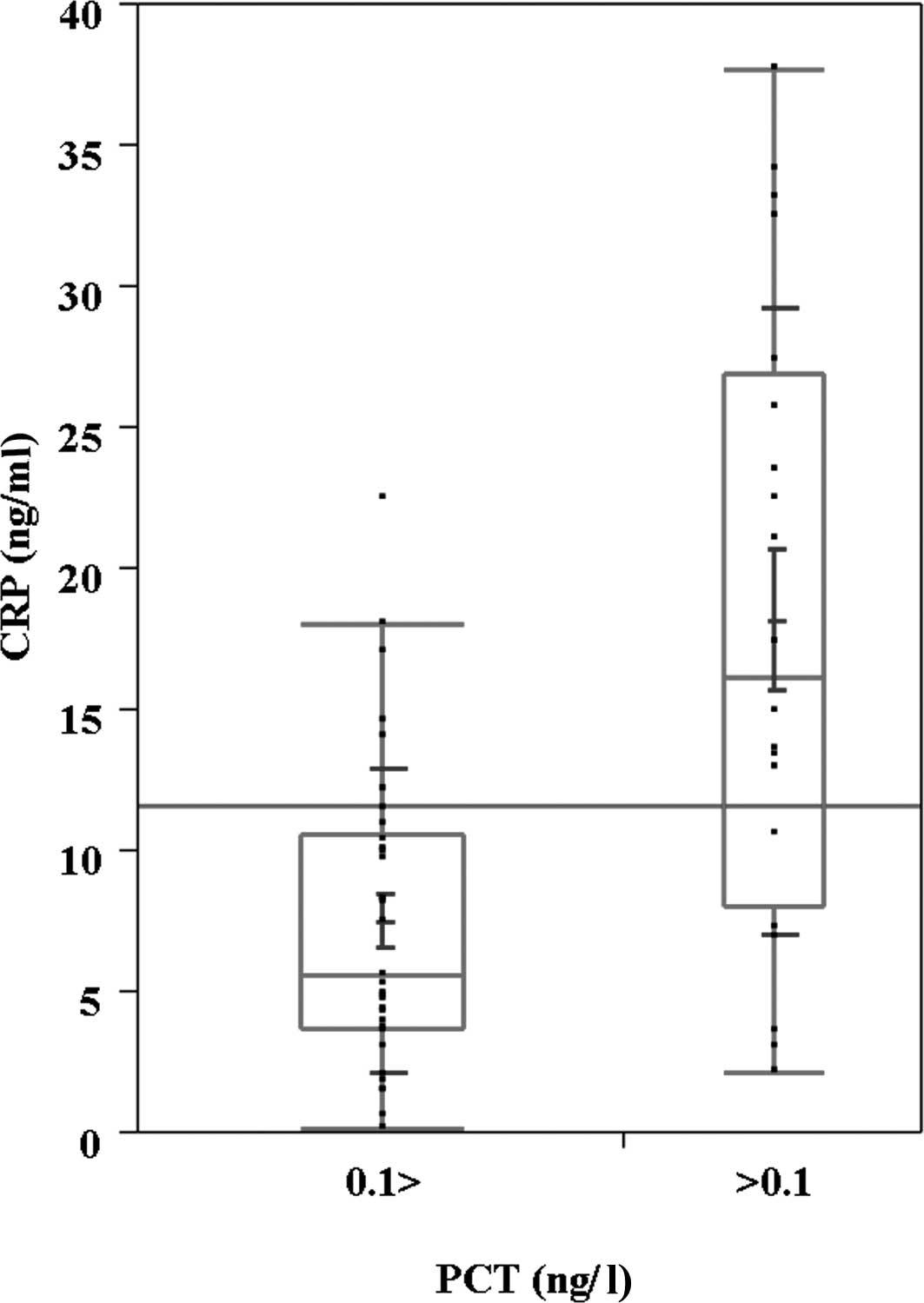
cut-off level of serum PCT (0.1 ng/ml), serum CRP was higher in the
PCT-positive patients (Fig. 3).
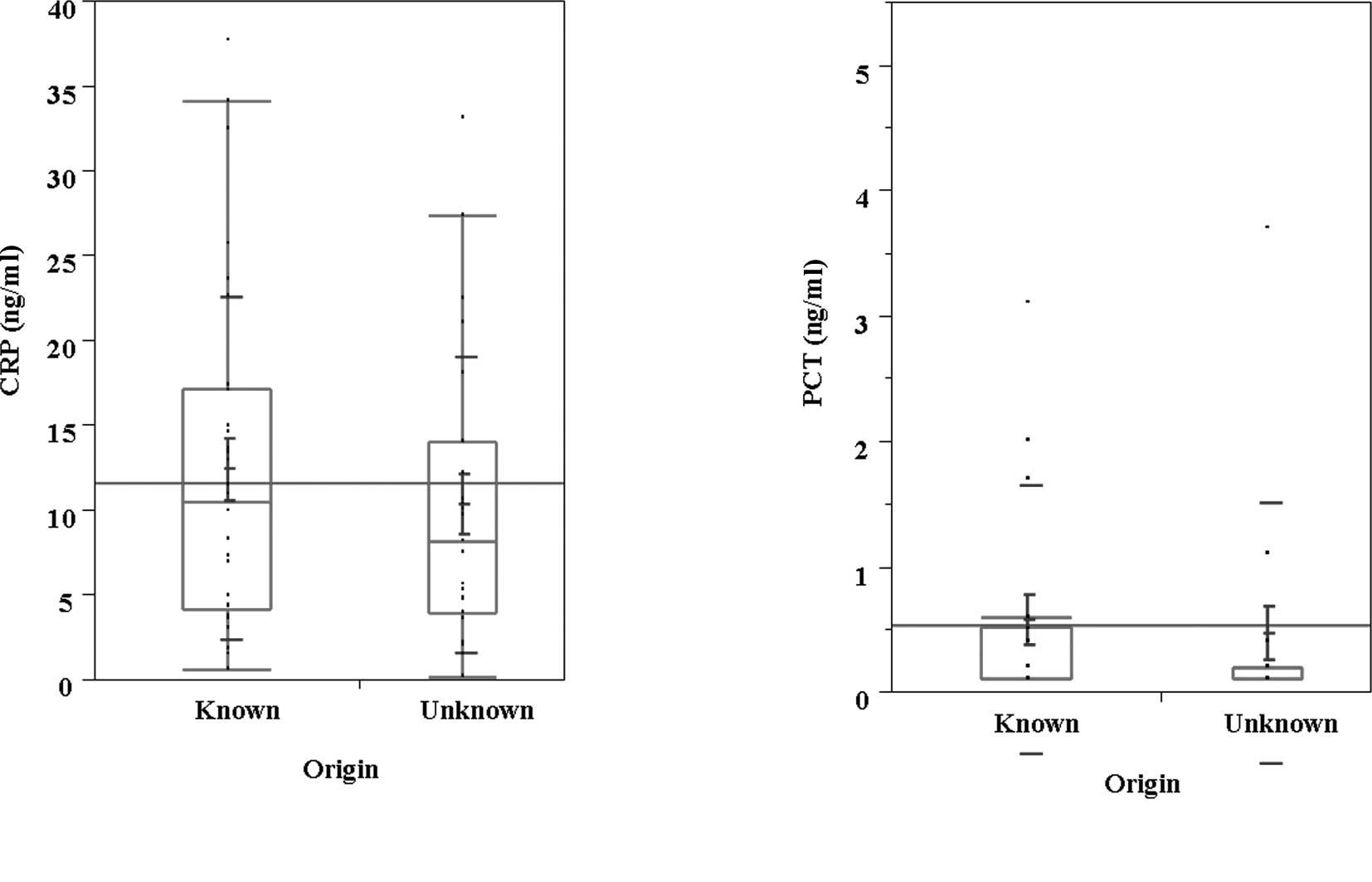
Serum PCT was also higher than serum CRP in the population whose
cause of infection was known (Fig.
4). Although serum PCT did not correlate with treatment outcome
among the 25 cases with known causes (Table III), serum PCT-positive patients
showed a poor outcome with antibiotics therapy in Table IV (Fisher's exact test, p=0.039).
Furthermore, serum PCT positivity was likely to discriminate
between infective fever and fever due to inflammation (Table V) (Chi-squared test, p=0.018).
According to the cut-off level of serum PCT, the duration of the
febrile state did not correlate with the serum PCT level (data not
shown).
 | Table IIITreatment outcomes of 25 cases with
known cause according to pro-carcitonin. |
Table III
Treatment outcomes of 25 cases with
known cause according to pro-carcitonin.
| No. of
episodes | Treatment response
of antibiotics |
|---|
| |
|
|---|
| | Improved | Deceased |
|---|
| Negative <0.1
ng/ml | 14 | 13 | 1 |
| Positive >0.1
ng/ml | 11 | 8 | 3 |
| Total | 25 | 21 | 4 |
 | Table IVTreatment outcomes of 50 episodes
according to pro-carcitonin. |
Table IV
Treatment outcomes of 50 episodes
according to pro-carcitonin.
| No. of cases | Treatment response
of antibiotics |
|---|
| |
|
|---|
| | Improved | Deceased |
|---|
| Negative
<0.1ng/ml | 32 | 30 | 2 |
| Positive
>0.1ng/ml | 18 | 12 | 6 |
| Total | 50 | 42 | 8 |
 | Table VInfectious origins of 50 episodes
according to pro-carcitonin. |
Table V
Infectious origins of 50 episodes
according to pro-carcitonin.
| No. of cases | Infectious
origin |
|---|
| |
|
|---|
| | Known | Unknown |
|---|
| Negative <0.1
ng/ml | 32 | 13 | 19 |
| Positive >0.1
ng/ml | 18 | 14 | 4 |
| Total | 50 | 27 | 23 |
Discussion
This study examined the infectious background of
patients with advanced lung cancer who received cytotoxic
chemotherapy. Among the serum PCT-positive population, causative
agents are more readily identified. Serum PCT levels correlate with
serum CRP levels. In our study population, the positive serum PCT
resulted in poor clinical outcome.
To the best of our knowledge, this is the first
study analyzing PCT plasma levels and infectious background in
advanced lung cancer patients receiving chemotherapy. Cultures from
sputum yielded pathogenic bacteria in 21 episodes (29.6%).
Gram-negative bacteria accounted for 66.7% and gram-positive
organisms for 33.3% of all microorganisms recovered.
Nosocomial pneumonia occurs following admission to
the hospital. This type of pneumonia was neither present nor in a
period of incubation at the time the patient was admitted (11). Nosocomial pneumonia is the leading
cause of death from hospital-acquired infections and thus a major
public health concern. The estimated prevalence of nosocomial
pneumonia within the Intensive Care Unit ranges from 10 to 65%,
with case fatality rates ranging from 20–55% in the majority of
reported findings (1,12,13).
When pneumonia arises in the hospitalized patient, aerobic
gram-negative bacilli, particularly Pseudomonas aeruginosa,
Enterobacter sp. and Staphylococcus aureus are the
major causative organisms (14).
Other common causes of nosocomial pneumonia include Hemophils
influenzae, Streptococcus pneumoniae, aspiration with
anaerobes, Legionella sp. and viruses. Respiratory syncytial
virus, influenza A and B, and parainfluenza are responsible for
more than 70% of nosocomial viral disease (14). Although there appears to be little
difference between the bacterial background in nosocomial pneumonia
with normal hosts and our study population (Table II), the frequency of fungal disease
or the rate of detection of pneumococcal pneumonia in our study was
relatively high. Consequently, a larger sample may result in more
accurate information concerning the causes of microbacterium in our
study population, thereby aiding in the treatment modality to be
used.
PCT was previously proposed as a new marker of
severe bacterial infection (6,15,16)
that may be particularly useful in the discrimination between
septic complication and non-infectious fever in transplant patients
(17). Although PCT appears to be
ubiquitously produced by the body, initial concerns were that, as
in the case of traditional cytokines, PCT is produced mainly by
white blood cells. PCT concentrations rise in neutropenia to
similar concentrations to those observed in immunocompetent
patients (18,19). However, the usefulness of PCT in the
detection of infection in such patients remains to be elucidated.
Higher concentrations, such as 2.5 mg/l (20) or 5.0 mg/l (18), are suggestive of severe sepsis over
bacteremia and localized infection but low concentrations do not
necessarily exclude infection. This phenomenon depends on the
causative organism with PCT responses noted in cases of
gram-positive cocci and gram-negative bacteremia, but not
coagulase-negative staphylococcus (18). It is likely that the value of PCT in
febrile neutropenics is in monitoring the response to therapy
rather than diagnosis. However, this finding is not universal,
since it has been noted that PCT may remain low despite evidence of
significant infection (19,21). Furthermore, PCT does not
discriminate between causes of fever early in the illness (22) and is unable to distinguish between
fungal and bacterial infection (19). However, in our study, serum PCT
positivity was able to discriminate between infective fever and
fever due to inflammation since the serum PCT was higher than that
of CRP in the population whose cause of infection was known.
The limitations of our study, however, include small
sample size, heterogeneity of treatment regimens and its
retrospective nature. Alternative methods, such as TRACE, appear to
be more sensitive than the lumi test (23). However, we showed the causative
organisms of febrile advanced lung cancer patients who received
cytotoxic chemotherapy. The ability of PCT to discriminate between
infective fever and fever due to inflammation was also
demonstrated. It can therefore be concluded that a prospective
clinical trial is required in order to evaluate whether antibiotics
should be employed on febrile advanced lung cancer patients during
cytotoxic chemotherapy treatment according to their serum PCT
levels.
References
|
1
|
Guidelines for the management of adults
with hospital-acquired, ventilator-associated, and
healthcare-associated pneumonia. American Thoracic Society. Am J
Respir Crit Care Med. 171:388–416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pfister DG, Johnson DH, Azzoli CG, et al:
American society of clinical oncology treatment of unresectable
non-small-cell lung cancer guideline: update 2003. J Clin Oncol.
22:330–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klastersky J: Management of fever in
neutropenic patients with different risks of complications. Clin
Infect Dis. 39(Suppl 1): S32–S37. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacobs JW, Lund PK, Potts JT Jr, Bell NH
and Habener JF: Procalcitonin is a glycoprotein. J Biol Chem.
256:2803–2807. 1981.PubMed/NCBI
|
|
5
|
Le Moullec JM, Jullienne A, Chenais J, et
al: The complete sequence of human preprocalcitonin. FEBS Lett.
167:93–97. 1984.
|
|
6
|
Karzai W, Oberhoffer M, Meier-Hellmann A
and Reinhart K: Procalcitonin – a new indicator of the systemic
response to severe infections. Infection. 25:329–334. 1997.
|
|
7
|
Hospital-acquired pneumonia in adults:
diagnosis, assessment of severity, initial antimicrobial therapy,
and preventive strategies. A consensus statement, American Thoracic
Society, November 1995. Am J Respir Crit Care Med. 153:1711–1725.
1996. View Article : Google Scholar
|
|
8
|
Herpers BL, De Jongh BM, van der Zwaluw K
and van Hannen EJ: Real-time PCR assay targets the 23s-5s spacer
for direct detection and differentiation of legionella spp.
and legionella pneumophila. J Clin Microbiol. 41:4815–4816.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Helbig JH, Uldum SA, Bernander S, et al:
Clinical utility of urinary antigen detection for diagnosis of
community-acquired, travel-associated, and nosocomial legionnaires'
disease. J Clin Microbiol. 41:838–840. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith MD, Derrington P, Evans R, et al:
Rapid diagnosis of bacteremic pneumococcal infections in adults by
using the binax now streptococcus pneumoniae urinary antigen test:
a prospective, controlled clinical evaluation. J Clin Microbiol.
41:2810–2813. 2003. View Article : Google Scholar
|
|
11
|
Finch RG and Woodhead MA: Practical
considerations and guidelines for the management of
community-acquired pneumonia. Drugs. 55:31–45. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ibrahim EH, Ward S, Sherman G and Kollef
MH: A comparative analysis of patients with early-onset vs.
late-onset nosocomial pneumonia in the ICU setting. Chest.
117:1434–1442. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kollef MH: The prevention of
ventilator-associated pneumonia. N Engl J Med. 340:627–634. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taylor GD, Buchanan-Chell M, Kirkland T,
McKenzie M and Wiens R: Bacteremic nosocomial pneumonia. A 7-year
experience in one institution. Chest. 108:786–788. 1995.PubMed/NCBI
|
|
15
|
Assicot M, Gendrel D, Carsin H, Raymond J,
Guilbaud J and Bohuon C: High serum procalcitonin concentrations in
patients with sepsis and infection. Lancet. 341:515–518. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gendrel D, Raymond J, Coste J, et al:
Comparison of procalcitonin with c-reactive protein, interleukin 6
and interferon-alpha for differentiation of bacterial vs. viral
infections. Pediatr Infect Dis J. 18:875–881. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuse ER, Langefeld I, Jaeger K and
Kulpmann WR: Procalcitonin in fever of unknown origin after liver
transplantation: a variable to differentiate acute rejection from
infection. Crit Care Med. 28:555–559. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giamarellou H, Giamarellos-Bourboulis EJ,
Repoussis P, et al: Potential use of procalcitonin as a diagnostic
criterion in febrile neutropenia: experience from a multicentre
study. Clin Microbiol Infect. 10:628–633. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hambach L, Eder M, Dammann E, et al:
Diagnostic value of procalcitonin serum levels in comparison with
c-reactive protein in allogeneic stem cell transplantation.
Haematologica. 87:643–651. 2002.PubMed/NCBI
|
|
20
|
Giamarellos-Bourboulis EJ, Grecka P,
Poulakou G, Anargyrou K, Katsilambros N and Giamarellou H:
Assessment of procalcitonin as a diagnostic marker of underlying
infection in patients with febrile neutropenia. Clin Infect Dis.
32:1718–1725. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blijlevens NM, Donnelly JP, Meis JF, De
Keizer MH and De Pauw BE: Procalcitonin does not discriminate
infection from inflammation after allogeneic bone marrow
transplantation. Clin Diagn Lab Immunol. 7:889–892. 2000.PubMed/NCBI
|
|
22
|
Ortega M, Rovira M, Filella X, et al:
Prospective evaluation of procalcitonin in adults with febrile
neutropenia after haematopoietic stem cell transplantation. Br J
Haematol. 126:372–376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prieto B, Llorente E, Gonzalez-Pinto I and
Alvarez FV: Plasma procalcitonin measured by time-resolved
amplified cryptate emission (trace) in liver transplant patients. A
prognosis marker of early infectious and non-infectious
postoperative complications. Clin Chem Lab Med. 46:660–666. 2008.
View Article : Google Scholar
|