Introduction
In Japan, hepatocellular carcinoma (HCC) is a major
health concern with an incidence of two million patients infected
with hepatitis C virus (HCV) and with 7–8% of patients with liver
cirrhosis developing de novo HCC every year. Moreover,
approximately 35,000 patients with HCC succumbed to the disease in
2009. A total of 70–80% of HCC patients are infected with HCV and
approximately 20% with hepatitis B virus (HBV) (1). It is estimated that the number of HCC
patients may increase in the next 10 years. Therefore, the
establishment of effective treatment modalities for HCC is
imperative.
Percutaneous ethanol injection (PEI) therapy is a
useful type of therapy for patients with small HCC, particularly
for those with poor hepatic functional reserve (2,3). PEI
therapy involves the injection of absolute ethanol into HCC using
ultrasound guidance, resulting in cellular dehydration, coagulation
necrosis and vascular thrombosis within the treated tumor (4). Patient outcome for PEI therapy is
comparable to the outcome of patients who undergo surgical
resection (5,6). However, the recurrence of primary HCCs
after PEI is common, and the rate of local residual recurrence
after PEI therapy is reported to range from 14 to 44% (7–10).
Therefore, to control local recurrence, combination therapy with
transcatheter arterial chemoembolization (TACE) and PEI has been
proposed.
TACE is widely used and is considered to be an
effective conservative treatment for HCCs. Embolization of the
hepatic artery results in selective ischemic necrosis of the tumor
tissue (11). However, complete
necrosis of the tumor by embolization of the hepatic artery alone
is almost impossible to achieve (12).
A number of clinical studies examined the
non-surgical treatment of small HCCs including TACE alone, PEI
alone and combined therapy with TACE and PEI (13–18).
Certain investigators reported the superior efficacy of combined
TACE-PEI therapy, compared to PEI alone. Koda et al
attempted to clarify the efficacy of combination TACE-PEI therapy
in patients with small HCCs (<3 cm) using randomized assignment
(19). The results, however,
revealed that patient survival was not different between combined
TACE-PEI therapy and PEI therapy alone. Stratified analysis showed
that for patients bearing HCC tumors <2 cm, combined TACE-PEI
therapy was superior to PEI alone. Consequently, the efficacy of
additional TACE to PEI as a recommended treatment for HCCs >2 cm
has yet to be determined.
Thus, using multicenter randomized assignment, this
study was conducted to examine the efficacy of TACE-PEI therapy
instead of PEI alone for patients with relatively small HCC tumors,
2–4 cm in diameter.
Patients and methods
Study design
This was a multicenter randomized control (RCT)
study. The study protocol was approved by the review board of each
hospital, and all patients provided informed written consent.
Patients
Between July 1997 and April 1999, patients diagnosed
with small HCCs for the first time were eligible to be enrolled as
study subjects. The criteria for enrollment to this study were: i)
age <70 years; ii) HCC nodules measuring 2–4 cm in maximum
diameter; iii) number of HCC nodules ≤3; iv) no portal thrombosis
or extrahepatic metastasis; v) hypervascular nodules, as determined
by dynamic computed tomography (CT) scan and/or arteriography; and
vi) no previous treatment for HCC prior to entry. Exclusion
criteria included any severe comorbidity (such as uncontrolled
diabetes mellitus, heart failure, renal failure or other cancer),
as well as any patient who was unable to understand the protocol or
manage self-care.
The diagnosis of HCC was made by dynamic CT and/or
abdominal sonography. To assist the diagnosis of HCC, a needle
biopsy was performed in all 27 patients. Tumor vascularity was also
evaluated by dynamic CT and/or angiography from the hepatic
artery.
Randomization was performed using a sealed-envelope
method. Patients were divided into two groups: the TACE-PEI group,
in which patients were treated with TACE followed by PEI and the
PEI-alone group, in which patients were treated with PEI therapy
alone.
Treatment procedure
Patients with HCC were treated by trained
specialists at each institution. The precise techniques of ethanol
injection are described elsewhere (7). Briefly, after local anesthesia, one
21-gauge needle was inserted into the lesion under ultrasound (US)
guidance, and absolute ethanol was injected. In one session, 2–8 ml
of ethanol was injected into several sites in and around the lesion
according to the lesion size. After the procedure, the patients
remained in bed for 3 h. This procedure was performed twice a week.
The treatment was repeated until dynamic CT demonstrated entire
tumor necrosis.
In addition, TACE [precise techniques are described
elsewhere (12,13)] was performed by super-selectively
introducing a catheter into the hepatic artery that fed the tumor.
A mixture of an ionized oil and doxorubicin hydrochloride (0.6–1.0
mg per kg of body weight) was injected, followed by a gelatin
sponge.
Diagnosis of the remaining tumors was based on image
findings, particularly dynamic CT. In addition, the positivity of
serum α-fetoprotein (AFP >10 ng/ml) or serum protein induced by
vitamin K absence II (PIVKA-II >40 mAU/ml) facilitated the
diagnosis.
Follow-up
The patients were under regular observation for the
detection of recurrence by measurement of tumor markers (AFP and/or
PIVKA-II), ultrasonography and/or dynamic CT scans every 3 months.
The primary endpoint was a recurrence indicated in any of the above
examinations. The secondary endpoint was patient death. The
recurrence of HCC was classified as local residual or new nodular
recurrence in lesions other than the tumor treated. Local
recurrence was defined as tumors within or adjacent to the tumor
being treated. The recurrent tumors were treated with PEI or
TACE-PEI. In the PEI-alone group, however, TACE was performed when
≥3 recurrent tumors developed.
Statistical analysis
The statistical significance of the patient
characteristics between the two groups was determined by the
Chi-square or Mann-Whitney U test. The mean cancer-free time and
survival time were calculated using the Kaplan-Meier method, and
significance was determined by the generalized Wilcoxon’s test.
P<0.05 was considered to be significant.
Results
A total of 30 patients fulfilled the criteria for
enrollment in this study. Patients were stratified and randomized
into two treatment arms: 16 patients were treated with a
combination of TACE and PEI (TACE-PEI group) and 14 received only
PEI therapy (PEI-alone group). However, three patients withdrew
from the study, and the final number of patients analyzed was 27
(TACE-PEI group, 13; PEI-alone group, 14). Of the 27 patients, 4
had cirrhosis and 23 had chronic hepatitis. Hepatitis B surface
antigen was positive in 5 of the 27 patients (18.5%) and the HCV
antibody was positive in 20 of the 27 patients (74.1%). No
significant differences were noted between the two groups in the
baseline characteristics (Table
I).
 | Table IClinical characteristics according to
the treatment group. |
Table I
Clinical characteristics according to
the treatment group.
| PEI (n=14) | TACE-PEI (n=13) | P-value |
|---|
| Age (years) (mean ±
SD) | 63.6±6.2 | 65.8±7.3 | NS |
| Gender (M/F) | 7/7 | 9/4 | NS |
| Etiology of liver
disease |
| HBV | 3 | 0 | |
| HCV | 9 | 9 | |
| HBV + HCV | 1 | 1 | |
| NBNC | 2 | 3 | NS |
| Chronic
hepatitis | 13 | 10 | |
| Cirrhosis | 1 | 3 | NS |
| Albumin (g/dl) | 3.5±0.3 | 3.8±0.4 | NS |
| Total bilirubin
(mg/dl) | 1.1±0.6 | 1.2±0.8 | NS |
| ALT (UI/l) | 82±50 | 145±99 | NS |
| AST (UI/l) | 65±53 | 129±65 | NS |
| Prothrombin time
(%) | 63±3.6 | 77±15 | NS |
| α-fetoprotein (ng/ml)
[median (range)] | 13 (4–97) | 16 (4–373) | NS |
| Tumor lesions |
| Single nodule | 11 | 8 | |
| 2–3 nodules | 3 | 5 | NS |
| Greatest tumor
dimension (mm) | 26.4±7.4 | 26.5±6.8 | NS |
The median (interquartile range) follow-up period
was 33.2 (24.6) months [TACE-PEI group, 39.7 (46.7) months and PEI
alone group, 33 (42.7) months].
Primary endpoint
Recurrence
Tumor recurrence was detected in 10 patients treated
with PEI alone and in 11 patients treated with TACE-PEI. The
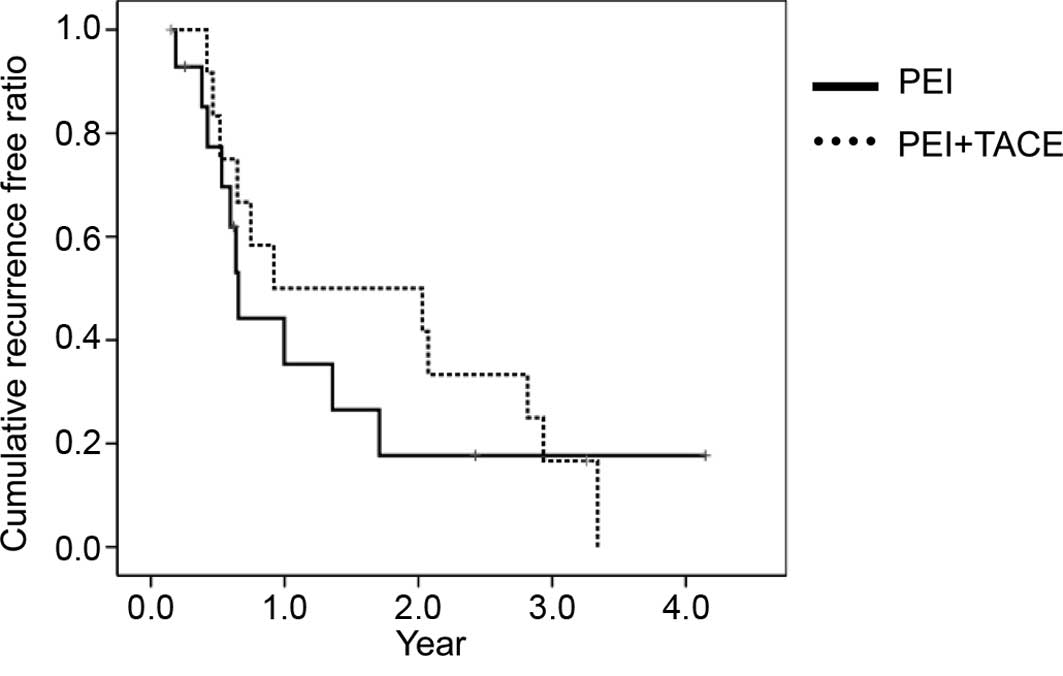
cumulative cancer-free time was calculated using the Kaplan-Meier
method (Fig. 1). The mean
cancer-free time was 16.7 months (95% CI 7.3–26.0) for the PEI
alone group and 22.9 months (95% CI 12.4–33.4) for the TACE-PEI
group. No significant difference was found between the two groups.
However, the pattern of recurrence was significantly different
(P<0.05). During follow-up, the detection of a local residual
lesion was observed in 1 of 13 nodules (7.6%) in the TACE-PEI group
and in 6 of 13 nodules (46.1%) in the PEI-alone group. No local
residual tumor was detected after 2 years of follow-up in the
TACE-PEI group. On the other hand, new nodular recurrences were
observed in 8 of 13 patients (61.5%) in the TACE-PEI group and in 3
of 14 patients (21.4%) in the PEI-alone group.
Secondary endpoint
Death
Of the 13 patients (61.5%), 8 treated with TACE-PEI
and 6 of the 14 patients (44%) treated with PEI alone succumbed to
the disease during the follow-up period. In the TACE-PEI group,
causes of death included development of HCC in 2 patients,
variceral bleeding in 3 patients and hepatic failure in 3 patients.
In the PEI-alone group, causes of death were development of HCC in
2 patients, hepatic failure in 3 patients and other diseases
(tuberculosis) in 1 patient. The cumulative survival curves of the
two groups are shown in Fig. 2. The
mean patient survival time of the TACE-PEI group was 42.4 months
(95% CI 29.2–55.6) and that of the PEI-alone group was 57.2 months
(95% CI 37.2–77.2). No significant difference was noted between the
two groups.
Adverse events
In all 30 cases, serious adverse effects or
complications, such as acute liver failure, liver infarction,
abscess, cholecystitis, gastrointestinal mucosal lesions, pulmonary
embolism, variceral bleeding, iatrogenic dissection or perforation
of the celiac artery and its branches, were not related to
treatment with TACE and/or PEI.
Discussion
This RCT study failed to show the anticipated
efficacy of TACE-PET therapy compared to PEI treatment alone on
survival time for patients with relatively small HCCs of 2–4 cm in
diameter. Together with the previous RCT result by Koda et
al, it was found that a tumor size smaller than 2 cm may be
critical in obtaining significant effectiveness by combining TACE
therapy to PEI (19).
The present study showed marked differences in
recurrence patterns after initial treatment. Our results indicate
that TACE-PEI is superior to PEI therapy alone regarding local
tumor control. The addition of TACE, however, evoked new tumors in
different lesions other than the original tumor. We believe that
the induction of growth factors such as VEGF and HGF (20–23),
due to ischemia by TACE, are involved in the development of new
nodules. A liver with HCCs larger than 2 cm in diameter may be
prone to develop HCCs in whole liver lesions. Stimulation by TACE
may enhance the progression of small nodules that are not detected
by CT examination. When the stage of HCC is evaluated using more
sensitive methods, such as CT during arterial portography and/or
superparamagnetic iron oxide-enhanced gradient-recalled echo MRI
(24–29), extremely small focal nodules can be
detected.
The main causes of patient death in the present
study were related to hepatic failure and not to tumor progression
in either group. Although it is reported that TACE improves tumor
control in large-size HCCs, our data suggest that the prognosis of
patients with HCCs of 2–4 cm in diameter depends on residual liver
function, and not on tumor progression. No statistical significance
was found in the present study which showed that the mean patient
survival time was shorter in the TACE-PEI group than that in the
PEI-alone group. Therefore, local tumor control may not directly
contribute to patient survival time.
A number of limitations should be noted. Although
patients were enrolled at different sites, a relatively small
number of patients was unable to participate, and the follow-up
period was short. All but two tumors were virus-related HCCs.
Recently, the incidence of HCC from non-alcoholic steatohepatitis
has been on the increase and its characteristics are reportedly
different from HCCs resulting from HCV and HBV (30). However, this study used random
assignment, providing us with important information regarding the
treatment of relatively small-size virus-related HCCs. For HCCs of
2–4 cm in diameter, the additional TACE to PEI did not markedly
improve patient survival. Moreover, the additional TACE treatment
appeared to shorten the patient survival time as the treatment did
not (at least notably) damage residual liver function and
stimulated new tumor growth in lesions other than the primary one.
Additionally, other modalities, such as radio frequency ablation
(RFA), are available. Such treatment modalities are considered to
be superior to PEI in local tumor control and attack tumors in a
pin-point manner (31–35). Thus, our data suggest that RFA alone
as well as PEI may be recommended in the treatment of relatively
small HCCs of 2–4 cm in diameter.
Acknowledgements
We would like to thank Dr Hidetsugu Saitou who
supervised the study.
References
|
1
|
Okita K: Clinical aspects of
hepatocellular carcinoma in Japan. Intern Med. 45:229–233. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Livraghi T, Festi D, Monti F, Salmi A and
Vettori C: US-guided percutaneous alcohol injection of small
hepatic and abdominal tumors. Radiology. 161:309–312. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohoto M, Ebara M, Watanabe Y, et al:
Percutaneous ethanol injection (PEI) therapy for small
hepatocellular carcinoma. Evaluation of its utility on the basis of
tumor-images and survival after therapy. Jpn J Med Imaging.
7:25–33. 1988.
|
|
4
|
Livraghi T, Giorgio A, Marin G, et al:
Hepatocellular carcinoma and cirrhosis in 746 patients: long-term
results of percutaneous ethanol injection. Radiology. 197:101–108.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kotoh K, Sakai H, Sakamoto S, Nakayama S,
Satoh M, Morotomi I and Nawata H: The effect of percutaneous
ethanol injection therapy on small solitary hepatocellular
carcinoma is comparable to that of hepatectomy. Am J Gastroenterol.
89:194–198. 1994.PubMed/NCBI
|
|
6
|
Castells A, Bruix J, Bru C, et al:
Treatment of small hepatocellular carcinoma in cirrhotic patients:
a cohort study comparing surgical resection and percutaneous
ethanol injection. Hepatology. 18:1121–1126. 1993.
|
|
7
|
Koda M, Murawaki Y, Mitsuda A, et al:
Predictive factors for intrahepatic recurrence after percutaneous
ethanol injection therapy for small hepatocellular carcinoma.
Cancer. 88:529–537. 2000. View Article : Google Scholar
|
|
8
|
Ishii H, Okada S, Nose H, et al: Local
recurrence of hepatocellular carcinoma after percutaneous ethanol
injection. Cancer. 77:1792–1796. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castellano L, Calandra M, del Vecchio
Blanco C and de Sio I: Predictive factors of survival and
intrahepatic recurrence of hepatocellular carcinoma in cirrhosis
after percutaneous ethanol injection: analysis of 71 patients. J
Hepatol. 27:862–870. 1997. View Article : Google Scholar
|
|
10
|
Ohnishi K, Yoshioka H, Ito S and Fujiwara
K: Prospective randomized controlled trial comparing percutaneous
acetic acid injection and percutaneous ethanol injection for small
hepatocellular carcinoma. Hepatology. 27:67–72. 1998. View Article : Google Scholar
|
|
11
|
Doyon D, Mouzon A, Jourde AM, Regensberg C
and Frileux C: [Hepatic, arterial embolization in patients with
malignant liver tumours]. Ann Radiol. 17:593–603. 1974.
|
|
12
|
Yamada R, Sato M, Kawabata M, Nakatsuka H,
Nakamura K and Takashima S: Hepatic artery embolization in 120
patients with unresectable hepatoma. Radiology. 148:397–401. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi BI, Kim HC, Han JK, et al:
Therapeutic effect of transcatheter oily chemoembolization therapy
for encapsulated nodular hepatocellular carcinoma: CT and
pathologic findings. Radiology. 182:709–713. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruix J, Llovet JM, Castells A, et al:
Transarterial embolization versus symptomatic treatment in patients
with advanced hepatocellular carcinoma: results of a randomized,
controlled trial in a single institution. Hepatology. 27:1578–1583.
1998. View Article : Google Scholar
|
|
15
|
Kobayashi S, Nakanuma Y, Terada T and
Matsui O: Postmortem survey of bile duct necrosis and biloma in
hepatocellular carcinoma after transcatheter arterial
chemoembolization therapy: relevance to microvascular damage of
peribiliary capillary plexus. Am J Gastroenterol. 88:1410–1415.
1993.
|
|
16
|
Chung JW, Park JH, Han JK, Choi BI, Han
MC, Lee HS and Kim CY: Hepatic tumors: predisposing factors for
complications of transcatheter oily chemoembolization. Radiology.
198:33–40. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakamoto I, Aso N, Nagaoka K, et al:
Complications associated with transcatheter arterial embolization
for hepatic tumors. Radiographics. 18:605–619. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka K, Okazaki H, Nakamura S, et al:
Hepatocellular carcinoma: treatment with a combination therapy of
transcatheter arterial embolization and percutaneous ethanol
injection. Radiology. 179:713–717. 1991. View Article : Google Scholar
|
|
19
|
Koda M, Murawaki Y, Mitsuda A, et al:
Combination therapy with transcatheter arterial chemoembolization
and percutaneous ethanol injection compared with percutaneous
ethanol injection alone for patients with small hepatocellular
carcinoma: a randomized control study. Cancer. 92:1516–1524. 2001.
View Article : Google Scholar
|
|
20
|
Li X, Feng GS, Zheng CS, Zhuo CK and Liu
X: Expression of plasma vascular endotherial growth factor in
patients with hepatocellular carcinoma and effect of transcatheter
arterial chemoembolization therapy of plasma vascular endothelial
growth factor level. World J Gastroenterol. 10:2878–2882. 2004.
|
|
21
|
Suzuki H, Mori M, Kawaguchi C, Adachi M,
Miura S and Ishii H: Serum vascular endothelial growth factor in
the course of transcatheter arterial embolization of hepatocellular
carcinoma. Int J Oncol. 14:1087–1090. 1999.PubMed/NCBI
|
|
22
|
Kobayashi N, Ishii M, Ueno Y, Kisara N,
Chida N, Iwasaki T and Toyota T: Coexpression of Bcl-2 protein and
vascular endothelial growth factor in hepatocellular carcinomas
treated by chemoembolization. Liver. 19:25–31. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gupta S, Kobayashi S, Phongkitkarun S,
Broemeling LD and Kan Z: Effect of transcatheter hepatic arterial
embolization on angiogenesis in an animal model. Invest Radiol.
4:516–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HC, Kim TK, Sung KB, et al: CT during
hepatic arteriography and portography: an illustrative review.
Radiographics. 22:1041–1051. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heiken JP, Weyman PJ, Lee JK, Balfe DM,
Picus D, Brunt EM and Flye MW: Detection of focal hepatic masses:
prospective evaluation with CT, delayed CT, CT during arterial
portography, and MR imaging. Radiology. 171:47–51. 1989. View Article : Google Scholar
|
|
26
|
Yu JS, Kim KW, Lee JT and Yoo HS: MR
imaging during arterial portography for assessment of
hepatocellular carcinoma: comparison with CT during arterial
portography. Am J Roentgenol. 170:1501–1506. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanematsu M, Hoshi H and Murakami T:
Detection of hepatocellular carcinoma in patients with cirrhosis:
MR imaging versus angiographically assisted helical CT. Am J
Roentgenol. 169:1507–1515. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murakami T, Oi H, Hori M, et al: Helical
CT during arterial portography and hepatic arteriography for
detecting hypervascular hepatocellular carcinoma. Am J Roentgenol.
169:131–135. 1997. View Article : Google Scholar
|
|
29
|
Kanematsu M, Hoshi H, Imaeda T, Murakami
T, Inaba Y, Yokoyama R and Nakamura H: Detection and
characterization of hepatic tumors: value of combined helical CT
hepatic arteriography and CT during arterial portography. Am J
Roentgenol. 168:1193–1198. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Page JM and Harrison SA: NASH and HCC.
Clin Liver Dis. 13:631–647. 2009. View Article : Google Scholar
|
|
31
|
Shiina S, Teratani T, Obi S, et al: A
randomized controlled trial of radiofrequency ablation with ethanol
injection for small hepatocellular carcinoma. Gastroenterology.
129:122–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin SM, Lin CJ, Lin CC, Hsu CW and Chen
YC: Radiofrequency ablation improves prognosis compared with
ethanol injection for hepatocellular carcinoma ≤4 cm.
Gastroenterology. 127:1714–1723. 2004.
|
|
33
|
Lencioni RA, Allgaier HP, Cioni D, et al:
Small hepatocellular carcinoma in cirrhosis: randomized comparison
of radio-frequency thermal ablation versus percutaneous ethanol
injection. Radiology. 228:235–240. 2003. View Article : Google Scholar
|
|
34
|
Livraghi T, Goldberg SN, Lazzaroni S,
Meloni F, Solbiati L and Gazelle GS: Small hepatocellular
carcinoma: treatment with radio-frequency ablation versus ethanol
injection. Radiology. 210:655–661. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brunello F, Veltri A, Carucci P, et al:
Radiofrequency ablation versus ethanol injection for early
hepatocellular carcinoma: a randomized controlled trial. Scand J
Gastroenterol. 43:727–735. 2008. View Article : Google Scholar
|