Introduction
Lung cancer is the most frequently diagnosed major
cancer in the world and the most common cause of cancer-related
death, with a yearly growth in incidence (1). By virtue of its abundant blood supply,
the lung is also a common site for metastasis from tumors arising
at other sites in the body. The therapeutic strategies and
prognosis differ between different histopathological
classifications. However, making a definite diagnosis is not always
easy, even for experienced pathologists. In order to improve the
diagnostic and prognostic value, molecular markers are needed to
facilitate the classification. Napsin A is a recently described
aspartic protease, uniquely expressed in lung and kidney cells
(2–4). In normal lung it has been found to be
present in type II pneumocytes and to be involved in the maturation
of the biologically active surfactant protein B (SP-B) (5). Studies have shown its expression in
primary lung adenocarcinoma; thus, napsin A can be used in defining
primary adenocarcinoma of the lung (6,7).
Nevertheless, compared with other markers, there has been limited
research involving napsin A. Its usefulness in lung cancer has yet
to be clarified.
The present study aimed to examine the prevalence of
napsin A in lung cancer tissues, compared with another marker,
thyroid transcription factor-1 (TTF-1), which has recently been
recognized as a useful marker for lung adenocarcinoma (8,9), and
to evaluate their utilization in the identification of primary and
metastatic lung cancer. The association of their expression with
clinicopathological parameters is also evaluated in this study.
Materials and methods
Patient selection and tissue sample
collection
All patients with lung cancer in this study were
from Changhai Hospital, Shanghai, P.R. China, during the period
2007 and 2008. The pathological tissue specimen and clinical data
for each patient were colleted prior to treatment. The clinical
data included age, gender, smoking history, performance status
(PS), histopathological diagnosis, grade of tumor differentiation,
tumor stage, primary tumor size and nodal metastasis. Histological
subclassification was carried out according to the World Health
Organization (WHO) classification. PS was estimated using the
Eastern Cooperative Oncology Group (ECOG) scale. Tumor stage was
defined according to the International Union Against Cancer (UICC)
classification. In total, 324 patients with primary lung cancer and
27 patients with metastatic lung cancer met our study criteria.
Among the 324 patients, 222 cases were male and 102
cases, female (gender ratio, 2.2:1), and the median age was 61
years (range 30–84). A total of 145 patients (44.8%) had a history
of smoking and 264 patients (81.5%) had symptoms when first
diagnosed, such as cough, expectoration, hemoptysis, chest pain and
fever. Adenocarcinoma (n=212) was the most common tumor type in the
patients (65.4%), followed by squamous cell (26.2%), small-cell
(5.6%), adenosquamous cell (2.5%) and large-cell carcinoma (0.3%).
A total of 119 patients (36.7%) had stage I–II disease and the
remaining 205 patients (63.2%) had stage III–IV disease. There were
200 patients (61.7%) with nodal metastasis and 284 patients (87.7%)
with a primary tumor >3 cm when diagnosed.
Of the 27 cases with metastatic lung cancer, 8 cases
originally developed from mammary adenocarcinoma, 7 from colon
carcinoma, 3 from gastric carcinoma, 1 from thyroid adenocarcinoma,
2 from melanoma, 2 from hepatic cellular carcinoma, 2 from uterine
cervix cancer and 2 from suprarenal epithelioma.
Immunohistochemistry
All specimens were routinely fixed in 10% buffered
neutral formalin and embedded in paraffin. Each section (5.0 μm)
was stained with the standard hematoxylin and eosin method for
screening review by two experienced pathologists (L.H. and
D.L.M.).
Immunohistochemistry was performed using an EnVision
two-step method. Primary antibodies included anti-napsin A and
anti-TTF-1 antibody (both at a 1:200 dilution, mouse monoclonal
antibody; Dako, Denmark). The paraffin was removed from the slides
by xylene, and the tissue was rehydrated in various concentrations
of ethanol. Hydrogen peroxide (3%) was added to eliminate
endogenous peroxidase activity. The slides were then processed
using steam-heat retrieval for 30 min. The antibody was incubated
for 30 min at 37°C. The abovementioned steps were performed
automatically in a Dako automatic immunostainer. Negative controls
for napsin A and TTF-1 were carried out by omitting the primary
antibody. The positive controls were previously known napsin A- or
TTF-1-positive lung tissues.
Scoring
For napsin A, only a granular cytoplasmic staining
pattern was accepted as positive, while for TTF-1, only a nuclear
staining pattern was considered to be positive. The stained slides
were observed microscopically by two experienced pathologists.
Results were scored in accordance with the criteria of Ueno et
al (6) and expressed on a
plus-minus scale based on the proportion of tumor cells stained: −,
0–10%; ++, 10–50% and +++, 50–100%.
Statistical analysis
Univariate clinicopathological variables were
analyzed using the χ2-test. Variables with p<0.05 in
the univariate analysis were included in a Logistic model. The
correlation between napsin A and TTF-1 expression was evaluated
using Spearman’s correlation coefficient. P<0.05 was considered
statistically significant. Statistical tests were performed using
the software SPSS 13.0 version (SPSS Inc., Chicago, IL, USA).
Results
Napsin A expression in lung cancer
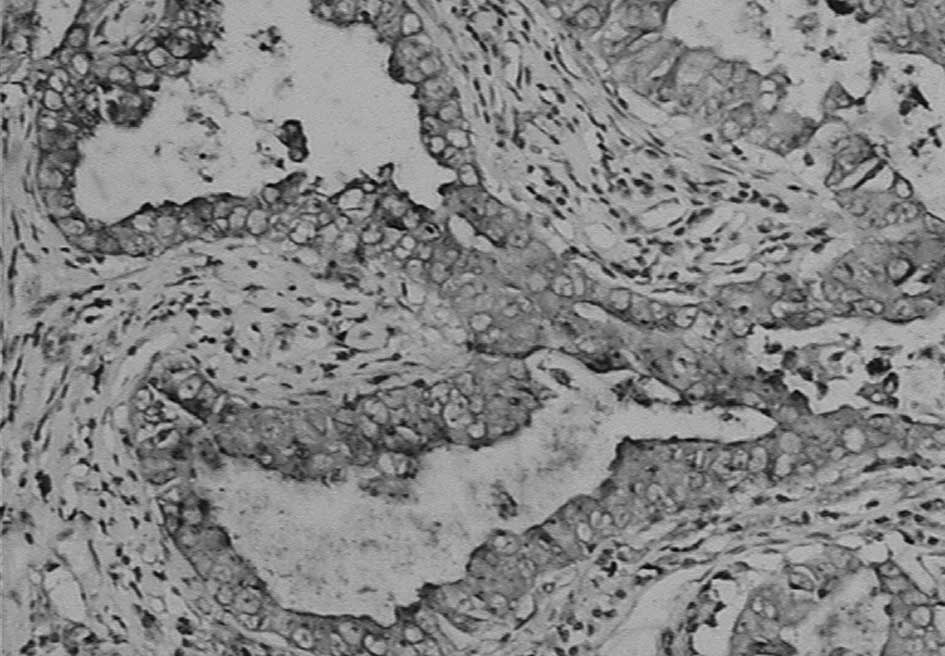
Immunohistochemistry showed that napsin A was
expressed in the cytoplasm with a granular staining pattern, but no
staining in the nucleus (Fig. 1).
As shown in Table I, napsin A
expression was positive in 84.9% of the lung adenocarcinomas, and
87.5% in the glandular component of adenosquamous cell carcinomas,
but negative in other types of primary lung cancer and metastatic
lung cancer cases. Napsin A had a high specificity of 93.8% to lung
adenocarcinomas. Comparatively, napsin A expression was also noted
in normal type II pneumocytes and some alveolar macrophages near
the neoplasms, while no expression was observed in type I
pneumocytes, bronchiolar epithelium, bronchial epithelium and
stromal cells.
 | Table IPositive expression of napsin A and
TTF-1 in lung cancer. |
Table I
Positive expression of napsin A and
TTF-1 in lung cancer.
| Histology | Napsin A | TTF-1 |
|---|
| PLC | 187/324 (57.7%) | 197/324 (60.8%) |
| AdC | 180/212 (84.9%) | 179/212 (84.4%) |
| SCC | 0/85 (0.0%) | 2/85 (2.4%) |
| SCLC | 0/18 (0.0%) | 12/18 (66.7%) |
| ASCC | 7/8 (87.5%) | 4/8 (50%) |
| LCC | 0/1 (0.0%) | 0/1 (0.0%) |
| MLC | 0/27 (0.0%) | 1a/27 (3.7%) |
TTF-1 expression in lung cancer
TTF-1 expression was located in the nucleus with no
staining on the membrane and in the cytoplasm (Fig. 2). A positive expression was observed
mostly in adenocarcinomas, small-cell carcinomas and the glandular
component of adenosquamous cell carcinomas. However, TTF-1
expression was negative in the squamous component of adenosquamous
cell carcinoma, large-cell carcinoma and most of the squamous cell
carcinoma specimens. Its sensitivity and specificity to
adenocarcinoma were 84.4 and 83.9%, respectively. Nevertheless,
positive nuclear staining was also observed in normal pulmonary
alveolar pneumocytes near the neoplastic cells, but it was not
noted in the stromal and infiltrating inflammatory cells. The
metastatic lung cancer specimens were TTF-1-negative except for one
adenocarcinoma from the thyroid.
Association of TTF-1 and napsin A
expression with clinicopathological parameters in primary lung
cancer
The expression of napsin A and TTF-1 increased
significantly in female and male patients with a history of smoking
(p<0.001). Their positive expression was also associated with
patients without symptoms (PS score, 0; p<0.01). In lung
adenocarcinoma, both napsin A and TTF-1 expression was positively
related to differentiation (p<0.001), since poorly
differentiated adenocarcinoma had a lower expression. An increased
expression of both napsin A and TTF-1 was noted in patients with a
primary tumor size <3 cm (p<0.01) and in those without nodal
metastasis (p<0.05). No significant relationships were observed
between napsin A and TTF-1 expression and age and TNM stage
(Table II).
 | Table IIExpression of napsin A and TTF-1 in
NSCLC and their association with clinicopathological variables. |
Table II
Expression of napsin A and TTF-1 in
NSCLC and their association with clinicopathological variables.
| Napsin A | TTF-1 |
|---|
|
|
|
|---|
| + | − | Positive rate
(%) | P-value | + | − | Positive rate
(%) | P-value |
|---|
| Age |
| <61 | 93 | 54 | 63.3 | | 90 | 57 | 61.2 | |
| ≥61 | 94 | 83 | 53.1 | 0.065 | 107 | 70 | 60.5 | 0.887 |
| Gender |
| Male | 107 | 115 | 48.1 | | 117 | 105 | 52.7 | |
| Female | 80 | 22 | 78.4 | <0.001 | 80 | 22 | 78.4 | <0.001 |
| Smoking |
| Never | 113 | 32 | 77.9 | | 114 | 31 | 78.6 | |
| Ever | 74 | 105 | 41.3 | <0.001 | 83 | 96 | 48.4 | <0.001 |
| Performance
status |
| 0 | 46 | 14 | 76.7 | | 47 | 13 | 78.3 | |
| 1,2 | 141 | 123 | 53.4 | <0.001 | 150 | 114 | 56.8 | 0.002 |
|
Differentiationa |
| Well | 173 | 18 | 90.6 | | 174 | 17 | 91.1 | |
| Poor | 7 | 14 | 33.3 | <0.001 | 6 | 15 | 28.6 | <0.001 |
| Tumor size |
| ≤3cm | 31 | 9 | | 77.5 | 35 | 5 | 87.5 | |
| >3cm | 156 | 128 | 54.9 | 0.007 | 162 | 122 | 57.0 | <0.001 |
| Nodal metastasis |
| No | 85 | 39 | 68.5 | | 84 | 40 | 67.7 | |
| Yes | 102 | 98 | 51.0 | 0.002 | 113 | 87 | 56.5 | 0.044 |
| TNM |
| I–II | 74 | 45 | 62.2 | | 74 | 45 | 62.2 | |
| III–IV | 113 | 92 | 55.1 | 0.215 | 123 | 82 | 60.0 | 0.698 |
| TTF-1 |
| + | 166 | 13 | | | | | | |
| − | 14 | 19 | | <0.001 | | | | |
Logistic multivariate analysis demonstrated that
napsin A and TTF-1 expression was associated with smoking history
(p<0.01) and pathological type (p<0.001). Expression rates
were higher in patients with adenocarcinoma or an absence of
smoking history (Table III).
Napsin A was correlated with gender (p=0.026) and TTF-1 with PS
(p=0.020).
 | Table IIILogistic regression analysis of
napsin A and TTF-1 expression in lung cancer. |
Table III
Logistic regression analysis of
napsin A and TTF-1 expression in lung cancer.
| Napsin A | TTF-1 |
|---|
|
|
|
|---|
| B | Significance | B | Significance |
|---|
| Gender | 0.836 | 0.026 | 0.640 | 0.060 |
| Smoking
history | −0.972 | 0.003 | −0.976 | 0.002 |
| Histology | −2.228 | 0.000 | −1.354 | 0.000 |
| Tumor size | −0.927 | 0.104 | 0.011 | 0.946 |
| Nodal
metastasis | −0.216 | 0.493 | 0.086 | 0.544 |
| Performance
status | −0.372 | 0.382 | −0.855 | 0.020 |
| Constant | 6.017 | 0.000 | 3.449 | 0.000 |
Correlation of napsin A and TTF-1
expression in lung adenocarcinoma
Table IV shows the
phenotype of lung adenocarcinoma by immunohistochemical staining.
Combining napsin A and TTF-1 resulted in sensitivity to lung
adenocarcinoma increasing to 91.0% (193/212), whereas the
specificity was 80.4% (90/112). Using Spearman’s correlation
coefficient analysis, the expression of napsin A significantly
correlated with TTF-1 in lung adenocarcinomas (r=0.510,
p<0.001), as well as in moderately and well-differentiated
adenocarcinomas (r=0.387, p<0.001). Napsin A expression also
positively correlated with TTF-1 in poorly differentiated
adenocarcinomas, but without significance (r=0.224, p=0.330).
 | Table IVNapsin A and TTF-1 expression in
primary lung adenocarcinoma. |
Table IV
Napsin A and TTF-1 expression in
primary lung adenocarcinoma.
|
Napsin+/TTF+ |
Napsin+/TTF− |
Napsin−/TTF+ |
Napsin−/TTF− |
|---|
| Adenocarcinoma | 166 | 14 | 13 | 19 |
| Well or moderately
differentiated | 163 | 10 | 10 | 8 |
| Poorly
differentiated | 3 | 4 | 3 | 11 |
Discussion
Napsin A is a new member of the aspartic protease
family, with a molecular weight of 35 kDa and an isoelectric point
of 5.29. In 1998, Tatnell et al (2) reported the naspin A gene for the first
time. Prior to this study, a pair of polypeptide markers for lung
adenocarcinoma, TAO1/TAO2, were described using two-dimensional gel
electrophoresis (10,11). TAO2 was later demonstrated to be
identical to napsin A (3,12). In our study, napsin A was expressed
in 84.9% of the primary lung adenocarcinomas with a granular
cytoplasmic pattern. No positive expression was noted in squamous
cell carcinoma, small-cell or large-cell carcinomas. Napsin A had a
high specificity of 93.8% to lung adenocarcinoma. In the
immunohistochemical staining, napsin A-positive cells were also
observed in the glandular component of the adenosquamous cell
carcinoma specimens, while cells in the squamous component were all
negative. To the best of our knowledge, napsin A expression in
adenosquamous cell carcinoma has never been examined. When analyzed
with clinical data, napsin A was observed more frequently in female
non-smokers who are susceptible to lung adenocarcinoma. We also
studied napsin A expression in 27 metastatic lung cancer cases,
including 19 metastatic lung adenocarcinomas. They were all
negative for napsin A staining. The above observation further
demonstrated the tissue-specific expression of napsin A in lung
adenocarcinoma. By virtue of its specificity in lung
adenocarcinoma, studies have focused on its value in the diagnosis
of primary and metastatic adenocarcinoma of the lung. Ueno et
al (6) examined the expression
of napsin in 118 lung tissues, including 16 metastases by in
situ hybridization. Napsin was expressed in the tumor cell
compartment in 33 of 39 lung adenocarcinomas (84.6%). Only one
metastasis of a renal carcinoma origin expressed napsin, while none
of the other types of tumors expressed napsin. Suzuki et al
(13) showed that napsin A was
expressed in almost all tumor cells in most primary lung
adenocarcinoma specimens (84.3%, 70/83). Its expression was not
observed in any of the 32 metastatic lung tumors (colon, stomach,
breast, uterus, thyroid and submandibular gland), nor in any of the
primary sites of adenocarcinoma of other organs (stomach, colon,
breast and thyroid). These results are largely in accordane with
our observations.
This study analyzed the association of napsin A with
clinicopathological parameters. The expression of napsin A was
significantly higher in patients without nodal metastasis or
symptoms, or with a primary tumor size <3 cm, compared with
their counterparts. It was also found to be associated with a high
degree of differentiation in adenocarcinoma. These findings
indicate napsin A may be important in the carcinogenesis of lung
cancer. A number of aspartic proteinases are reported to be
involved in cancer progression. However, few studies exist on the
role of napsin A. Nevertheless, napsin A was suggested to be
involved in the processing of proSP-B in type II pneumocytes in the
human lung. Knockdown of napsin A by small interfering RNA resulted
in decreased levels of mature SP-B (14). In this context, napsin A is a
significant enzyme in type II pneumocytes. In a recent study
(15), napsin A was re-expressed in
the tumorigenic HEK 293 kidney cell line. Cells expressing napsin A
showed a reduced capacity for anchorage-independent growth, and
formed tumors in SCID mice with a lower efficiency and slower onset
compared to vector-transfected control cells. On the other hand,
napsin A staining was associated with tumor differentiation. Taken
together, these observations suggest the crucial role napsin A
plays in lung cancer. The loss of napsin A expression may enhance
the aggressive behavior of lung cancer, influencing disease
prognosis. Napsin A may also be related to patient prognosis in a
yet unidentified manner.
Furthermore, the expression of napsin A was
significantly correlated with TTF-1 in lung adenocarcinoma. TTF-1
was identified as an independent prognostic factor for survival,
besides PS and TNM stage (16,17).
In the meta-analysis by Berghmans et al (18), TTF-1 positivity was associated with
a more favorable survival in non-small cell lung cancer, mainly in
early and locally advanced stages, and adenocarcinoma. Our study
showed that TTF-1, as well as napsin A, exhibit a higher expression
in patients with a PS of 0. Based on this observation, we
hypothesize that napsin A is also a prognostic factor in lung
cancer, although further investigations are needed to better define
its prognostic role in this malignancy.
In the 324 primary lung cancer samples, napsin A and
TTF-1 had a similar sensitivity, while napsin A had a much higher
specificity to lung adenocarcinoma compared with TTF-1 (93.8 vs.
83.9%). Napsin A expression was almost limited to adenocarcinoma,
while TTF-1 was not so specific. Although TTF-1 was expressed in
84.4% of the adenocarcinoma specimens, its expression was also
noted in a large proportion of small-cell lung carcinomas, as well
as some of the squamous cell carcinomas, in accordance with
previous studies (19,20). Furthermore, by virtue of its
tissue-specific exclusive expression in thyroid neoplasms, it would
be difficult to distinguish primary lung adenocarcinoma from
metastatic lung adenocarcinoma from the thyroid based on clinical
and morphological features. In contrast, napsin A is much more
specific to lung adenocarcinoma. Although certain renal cell
carcinomas have been reported to express napsin A, it is thought
that these false positives are likely due to the presence of
intrinsic biotin, which is readily detected on negative controls
(8). Subsequently, napsin A may be
an alternative to TTF-1 for the identification of primary
adenocarcinoma of the lung. False negatives exist in lung
adenocarcinomas when using either napsin A or TTF-1, but these may
be reduced by using the two markers together. In our clinical
practice, the combined use of napsin A and TTF-1, as well as
morphology and clinical information, may facilitate the diagnosis
and differentiation of lung cancer.
In conclusion, napsin A has a tissue-specific
expression in primary lung adenocarcinoma and may be involved in
the process of carcinogenesis. Napsin A compared with TTF-1 may be
more useful in the identification of primary lung
adenocarcinoma.
References
|
1
|
Weir HK, Thun MJ, Hankey BF, et al: Annual
report to the nation on the status of cancer, 1975–2000, featuring
the uses of surveillance data for cancer prevention and control. J
Natl Cancer Inst. 95:1276–1299. 1998.
|
|
2
|
Tatnell PJ, Powell DJ, Hill J, et al:
Napsins: new human aspartic proteinases. Distinction between two
closely related genes. FEBS Lett. 441:43–48. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chuman Y, Bergman A, Ueno T, et al: Napsin
A, a member of the aspartic protease family, is abundantly
expressed in normal lung and kidney tissue and is expressed in lung
adenocarcinomas. FEBS Lett. 462:129–134. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schauer-Vukasinovic V, Bur D, Kling D, et
al: Human napsin A: expression, immunochemical detection and tissue
localization. FEBS Lett. 462:135–139. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brasch F, Ochs M, Kahne T, et al:
Involvement of napsin A in the C- and N-terminal processing of
surfactant protein B in type-II pneumocytes of the human lung. J
Biol Chem. 278:49006–49014. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ueno T, Linder S and Elmberger G: Aspartic
proteinase napsin is a useful marker for diagnosis of primary lung
adenocarcinoma. Br J Cancer. 88:1229–1233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirano T, Gong Y, Yoshida K, et al:
Usefulness of TA02 (napsin A) to distinguish primary lung
adenocarcinoma from metastatic lung adenocarcinoma. Lung Cancer.
41:155–162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jagirdar J: Application of
immunohistochemistry to the diagnosis of primary and metastatic
carcinoma to the lung. Arch Pathol Lab Med. 132:384–396.
2008.PubMed/NCBI
|
|
9
|
Chang YL, Lee YC, Liao WY, et al: The
utility and limitation of thyroid transcription factor-1 protein in
primary and metastatic pulmonary neoplasms. Lung Cancer.
44:149–157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirano T, Franzén B, Uryu K, et al:
Detection of polypeptides associated with the histopathological
differentiation of primary lung carcinoma. Br J Cancer. 72:840–848.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirano T, Fujioka K, Franzén B, et al:
Relationship between TA01 and TA02 polypeptides associated with
lung adenocarcinoma and histocytological features. Br J Cancer.
75:978–985. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirano T, Auer G, Maeda M, et al: Human
tissue distribution of TA02, which is homologous with a new type of
aspartic proteinase, napsin A. Jpn J Cancer Res. 91:1015–1021.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suzuki A, Shijubo N, Yamada G, et al:
Napsin A is useful to distinguish primary lung adenocarcinoma from
adenocarcinomas of other organs. Pathol Res Pract. 201:579–586.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueno T, Linder S, Na CL, et al: Processing
of pulmonary surfactant protein B by napsin and cathepsin H. J Biol
Chem. 279:16178–16184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueno T, Elmberger G, Weaver TE, et al: The
aspartic protease napsin A suppresses tumor growth independent of
its catalytic activity. Lab Invest. 88:256–263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bruno MD, Bohinski RJ, Huelsman KM, et al:
Lung cell-specific expression of the murine surfactant protein A
(SP-A) gene is mediated by interactions between the SP-A promoter
and thyroid transcription factor-1. J Biol Chem. 270:6531–6536.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barlési F, Pinot D, Legoffic A, et al:
Positive thyroid transcription factor 1 staining strongly
correlates with survival of patients with adenocarcinoma of the
lung. Br J Cancer. 93:450–452. 2005.PubMed/NCBI
|
|
18
|
Berghmans T, Paesmans M, Mascaux C, et al:
Thyroid transcription factor 1 – a new prognostic factor in lung
cancer: a meta-analysis. Ann Oncol. 17:1673–1676. 2006.
|
|
19
|
Tan D, Li Q, Deeb G, et al: Thyroid
transcription factor-1 expression prevalence and its clinical
implications in non-small cell lung cancer: a high-throughput
tissue microarray and immunohistochemistry study. Hum Pathol.
34:597–604. 2003. View Article : Google Scholar
|
|
20
|
Kaufmann O and Dietel M: Expression of
thyroid transcription factor-1 in pulmonary and extrapulmonary
small cell carcinomas and other neuroendocrine carcinomas of
various primary sites. Histopathology. 36:415–420. 2000. View Article : Google Scholar : PubMed/NCBI
|