Introduction
Primary liver cancer is one of the most common
malignancies that occurs worldwide, and the vast majority of
primary liver cancers are hepatocellular carcinoma (HCC) (1). Numerous studies have examined survival
in patients with HCC treated by transcatheter arterial
chemoembolization (TACE), with chemoembolization showing no clear
benefit to survival (2–5). However, patients receiving
chemoembolization in these studies included cases with unresectable
HCC and poor liver function.
Solitary HCC with good liver function is usually
treated by hepatic resection, but not chemoembolization. However, a
small number of studies have described the results of
chemoembolization for patients with resectable HCC and good liver
function (6).
Findings of this study showed survival rates for
patients with resectable HCC who received chemoembolization in
comparison to those of HCC patients who underwent hepatic
resection. To reduce selection bias from our database, patients
selected had solitary HCC and liver function of Child-Pugh A or B
and were stratified according to the Cancer of the Liver Italian
Program (CLIP) (7), the
International Union Against Cancer (UICC) T factor (8) and the Milan criteria (9).
Materials and methods
Patients
A total of 1,387 patients with newly diagnosed HCC,
admitted to three hospitals and treated from July 1990 to October
2001, were studied. According to this database, patients treated
with hepatic resection or chemoembolization were recruited.
Inclusion criteria were: i) solitary HCC; ii) Child-Pugh class A;
and iii) UICC stage T1-3N0M0 (8). T
factors in this study were defined as: T1, solitary tumor without
vascular invasion; T2, solitary tumor with vascular invasion or
multiple tumors, none of which were >5 cm in maximum diameter;
T3, multiple tumors of >5 cm or tumors involving a major branch
of the portal or hepatic vein; and T4, tumors with direct invasion
of adjacent organs other than the gallbladder or with perforation
of visceral peritoneum. The degree of portal vein involvement was
classified as: Vp0, no involvement of the portal vein; Vp1,
involvement of the third or more distal branch of the left or right
portal vein; Vp2, involvement of the second branch of the portal
vein; and Vp3, involvement of the first branch or trunk of the
portal vein.
The subjects were divided into three groups
according to portal vein involvement (Vp0-1, Vp2 and Vp3). Subjects
comprised 187 men and 64 women, with a mean age of 63 years (range
21–84). A total of 164 patients were hepatitis C virus-positive
(65%) and 43 patients were hepatitis B virus-positive (17%).
Hepatitis B and C were positive in 2 patients (1%) and negative in
42 patients (17%). HCC was diagnosed based on findings obtained
from ultrasonography, biphasic dynamic computed tomography (CT),
dynamic magnetic resonance imaging (MRI) and angiography, and/or
pathologically by biopsy specimens. Serum α-fetoprotein or protein
induced by vitamin K absence or antagonist-II (PIVKAII) was also
determined. The mean tumor size was 3.7 cm (range 1–9.7). Informed
consent was obtained from all patients after information was
provided concerning the HCC and the two treatments
(chemoembolization and hepatic resection). As a result, 149
patients received hepatic resection and 102 patients, who declined
hepatic resection, received chemoembolization. Age, gender, size of
HCC and background patient characteristics did not differ
significantly between the hepatic resection and TACE groups
(Tables I and II).
 | Table ICharacteristics of the HCC
patients. |
Table I
Characteristics of the HCC
patients.
|
Characteristics | Treatment | P-value |
|---|
|
| |
|---|
| Hepatic
resection | TACE | |
|---|
| No. of
patients | 149 | 102 | |
| Age, years | | | 0.1000 |
| Range | 21–84 | 37–82 | |
| Mean | 63.8 | 61.9 | |
| Gender | | | 0.4500 |
| Male/female | 108/41 | 79/23 | |
| TNM classification,
n (%) | | | 0.9283 |
| I | 28 (19) | 19 (19) | |
| II | 103 (69) | 69 (68) | |
| III | 18 (12) | 14 (13) | |
| Tumor size, n
(%) | | | 0.1805 |
| <2 cm | 24 (16) | 15 (15) | |
| 2–5 | 91 (61) | 53 (52) | |
| 5–10 | 34 (23) | 34 (33) | |
| Portal vein
involvement, n (%) | | | 0.8705 |
| Vp0 | 131 (88) | 92 (90) | |
| Vp1 | 12 (8) | 6 (6) | |
| Vp2 | 4 (3) | 2 (2) | |
| Vp3 | 2 (1) | 2 (2) | |
| CLIP score, n
(%) | | | 0.5452 |
| 0 | 83 (56) | 52 (51) | |
| 1 | 54 (36) | 40 (39) | |
| 2 | 10 (7) | 10 (10) | |
| 3 | 2 (1) | 0 (0) | |
 | Table IIComparison of the two groups of
hepatic resection and TACE in demographics. |
Table II
Comparison of the two groups of
hepatic resection and TACE in demographics.
| Hepatic resection
(n=149) | TACE (n=102) | P-value |
|---|
| Etiology |
| Hepatitis B | 25 | 18 | |
| Hepatitis C | 101 | 63 | |
| Hepatitis B and
C | 1 | 1 | |
| Non-B, non-C | 22 | 20 | |
| Platelet count
(×10,000) | 13.7 (2.8–123) | 13.7
(3.8–35.9) | 0.9700 |
| Albumin (g/dl) | 3.9 (2.5–5.1) | 3.9 (1.7–6.7) | 0.9148 |
| Prothrombin time
(sec) | 86 (43–108) | 86 (59–108) | 0.7698 |
| Tumor size
(cm) | 3.6 (1.0–8.5) | 3.9 (1.0–9.7) | 0.1109 |
Chemoembolization (10)
Hepatic arteriography was performed using
Seldginger’s method. After arterial access, diagnostic
arteriography was performed to evaluate hepatic arterial and portal
venous anatomy. Following the study of CT during arterial
portography to assess whether the liver tumor was solitary,
superselective catheterization was performed in tumor-feeding
vessels. The coaxial catheter system was used to perform
chemoembolization (Tracker-18 infusion catheter or Renegade; Boston
Scientific, Fremont, CA, USA). The chemotherapeutic agent
(epirubicin; Kyowa Hakko Kogyo, Tokyo, Japan) was dissolved in a
solution of non-ionic water-soluble contrast medium and saline
solution and mixed with lipiodol (Laboratoire Andre Guerbet, Paris,
France). The dose of iodized oil and anticancer drugs was
determined on the basis of tumor size, hepatic function, renal
function and blood chemistry data. After the microcatheter tip was
placed in the tumor-feeding vessel without stopping blood inflow,
the chemotherapeutic agent was injected. Following confirmation of
little or no visualization of tumor staining on arteriography,
gelfoam particles (Gelfoam; Upjohn, Kalamazoo, MI, USA) were
injected into tumor vessels as an embolizing agent (6,11–16).
CT was performed at 7–10 days and 1 month after treatment, and
subsequently every 2–3 months. If recurrent lesions appeared on the
follow-up CT, chemoembolization was repeated.
Hepatic resection
Hepatic resection was performed for 149 patients.
Methods of hepatic resection were: subsegmentectomy, 111 patients;
segmentectomy, 25 patients; lobectomy, 11 patients; and extended
lobectomy, 2 patients. No patients succumbed to or presented with
complications related to the hepatic resection.
Statistical analysis
The main end-point (survival from initial treatment)
was evaluated for the hepatic resection and chemoembolization
groups using the Kaplan-Meier method and compared statistically by
log-rank testing. According to UICC, CLIP scores and Milan
criteria, patients were stratified, and survival rates were
compared between the treatment groups according to each
stratification. Statistical analysis was carried out using the
Student’s t-test for continuous variables and a Chi-square test for
categorical variables with commercially available software packages
(MedCalc Version 9.5.1.0; MedCalc Software, Mariakerke, Belgium). A
two-tailed P-value (P<0.05) was considered significant.
Results
Survival analysis of total hepatic
resection and chemoembolization groups
By October 2001, 79 of the 149 patients with hepatic
resection treatment and 54 of the 102 patients with
chemoembolization were deceased. No significant difference in the
causes of death was noted between the two treatment groups, with
the majority of deaths resulting from liver failure, including
hepatic encephalopathy and spontaneous bacterial peritonitis, varix
bleeding or progression of the tumor itself. During the follow-up
period of chemoembolization, radiofrequency ablation (RFA) or
percutaneous ethanol injection therapy (PEIT) was performed in 4
patients (3%) and repeated chemoembolization was performed in 24
patients (24%). During the follow-up period of hepatic resection,
chemoembolization and PEIT were performed in 45 patients (30%; 40
and 5 patients, respectively).
In the chemoembolization group, 1 patient was lost
to follow-up and was censored, while no patients were lost to
follow-up in the hepatic resection group. The median duration of
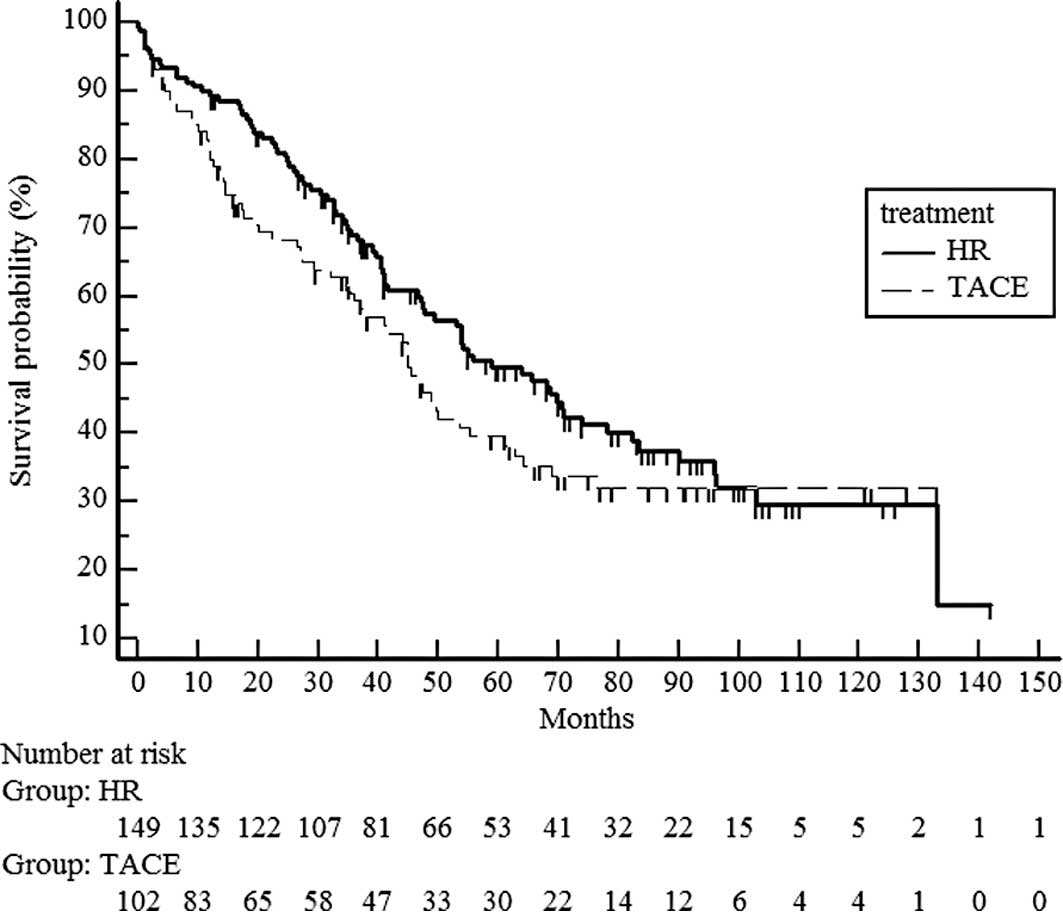
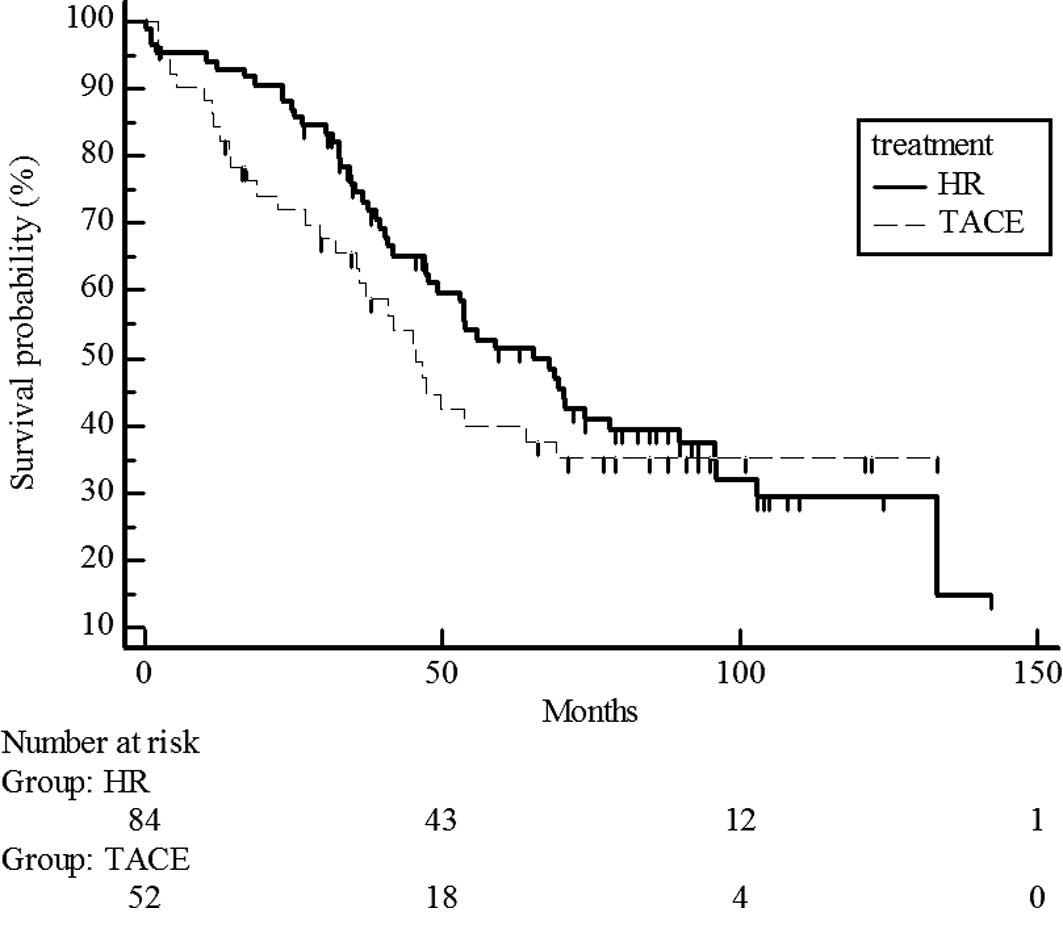
follow-up was 47.4 months (Fig. 1).
Median survival time was 51 months in the hepatic resection group
and 41 months in the chemoembolization group. No significant
difference in survival was noted between the hepatic resection and
chemoembolization groups (median survival time 58.9 vs. 45 months)
(P=0.1697) (Fig. 1).
Subgroup survival analysis of hepatic
resection and chemoembolization groups
UICC T stage
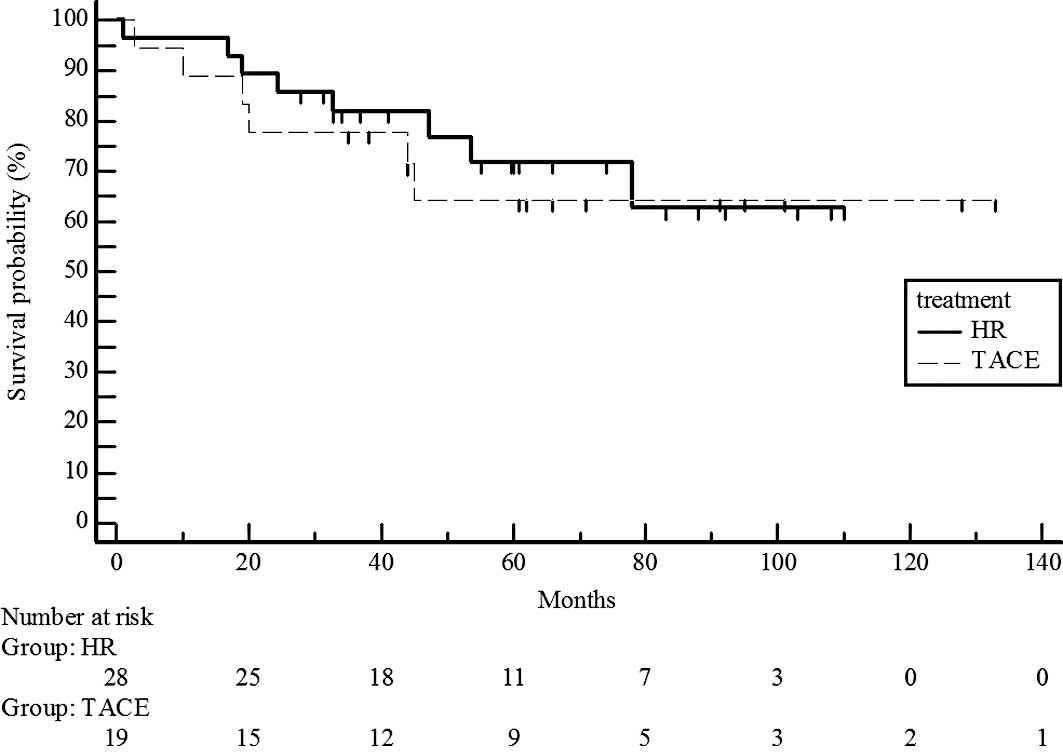
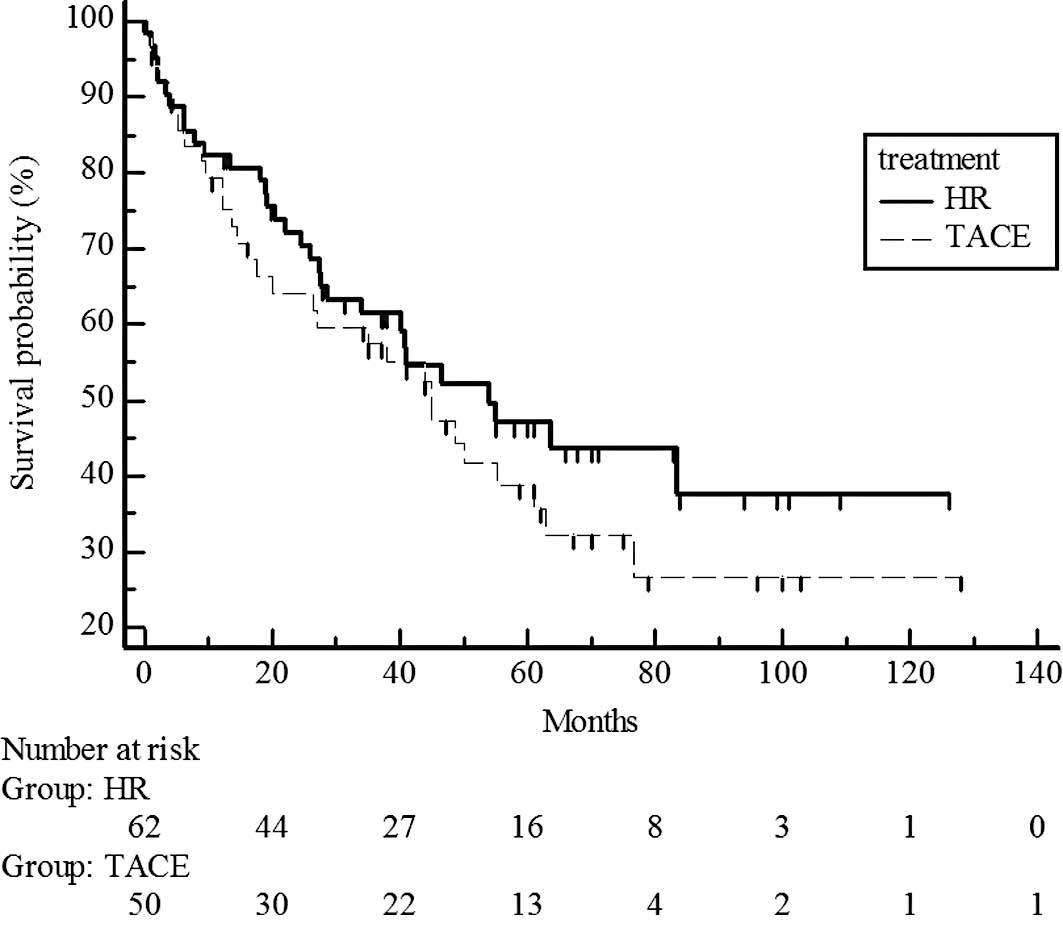
Survival rates did not differ significantly for UICC
T1 stage patients in the hepatic resection and chemoembolization
groups (P=0.7329; estimated 5-year survival rate, 70 vs. 65%)
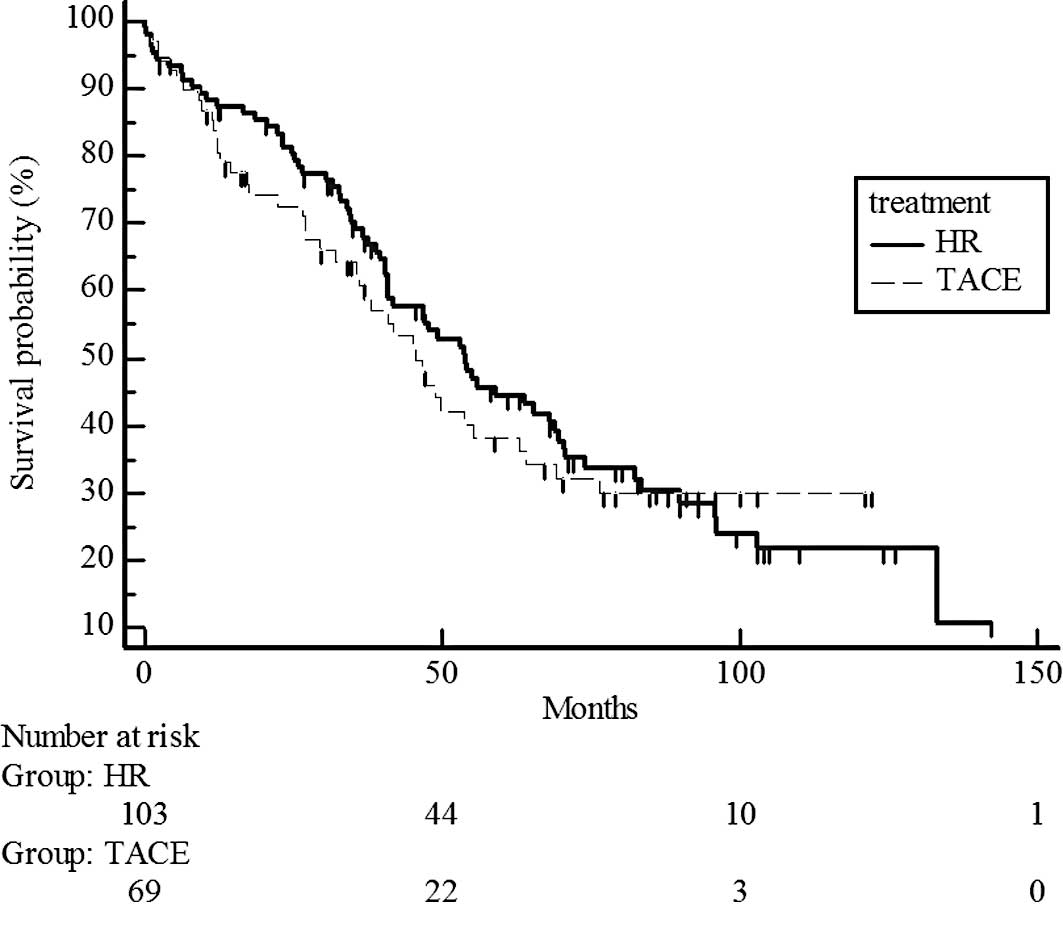
(Fig. 2) and T2 (P=0.5741;
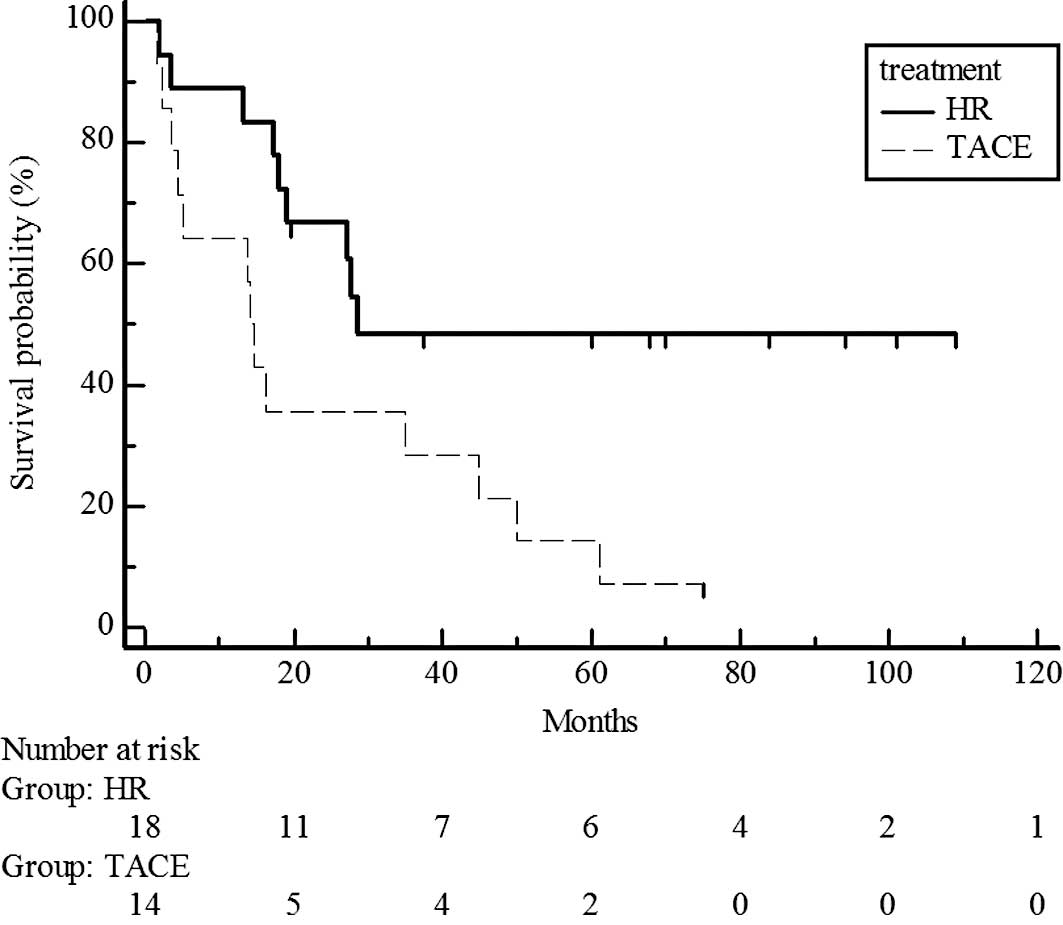
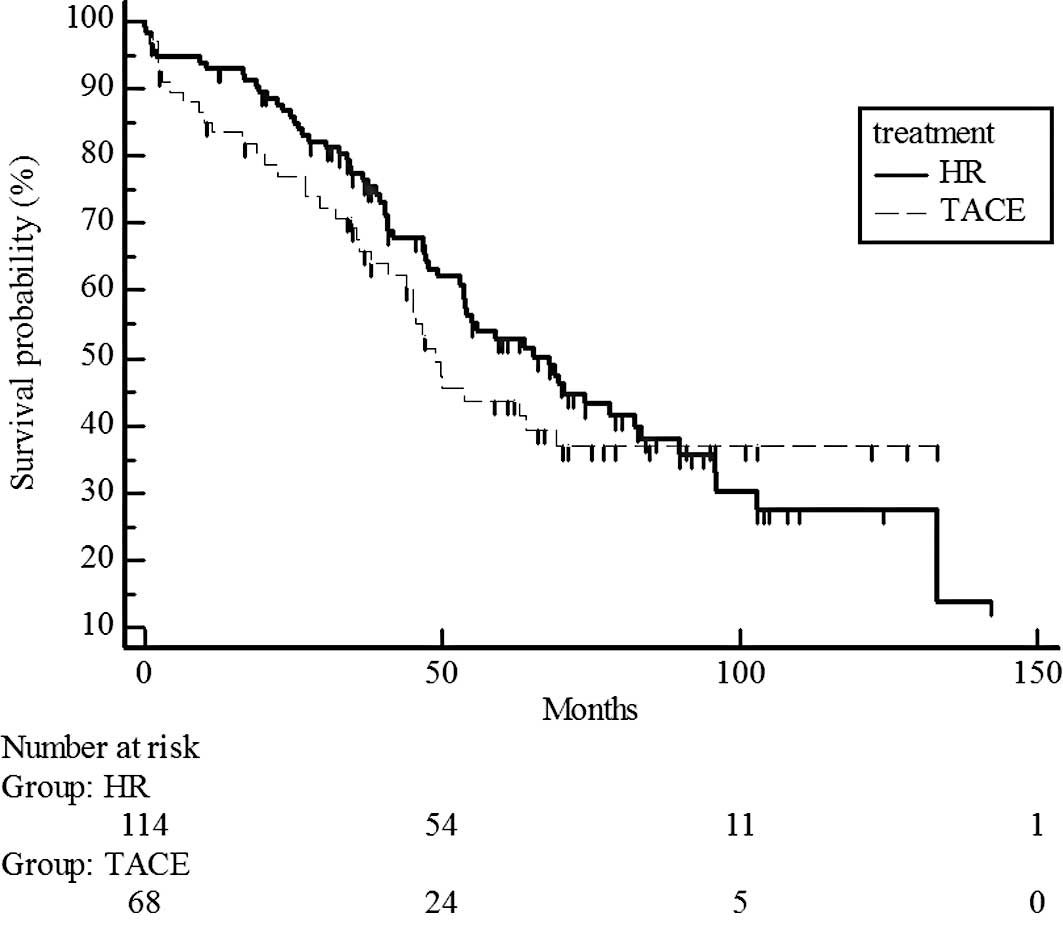
estimated 5-year survival rate, 44 vs. 38%) (Fig. 3). However, survival rates were
significantly different for UICC T3, with higher rates in the
hepatic resection group than in the chemoembolization group
(P=0.017; estimated 5-year survival rate, 48 vs. 14%) (Fig. 4).
CLIP score
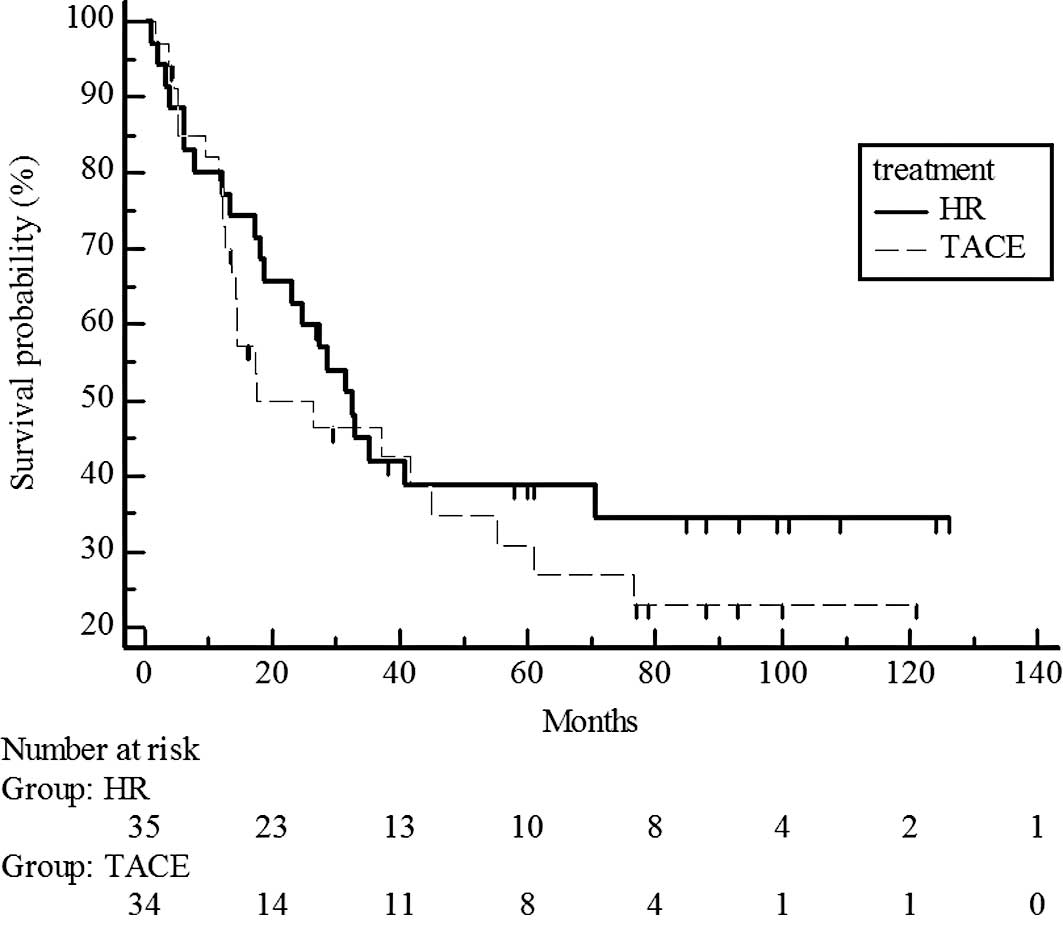
Survival rates did not differ significantly between
the hepatic resection and chemoembolization groups in CLIP 0
(P=0.3593; estimated 5-year survival rate, 51 vs. 40%) (Fig. 5) and in the CLIP 1–2 groups
(P=0.3287; estimated 5-year survival rate, 47 vs. 39%) (Fig. 6).
Milan criteria
Survival rates did not differ significantly between
the hepatic resection and chemoembolization groups within Milan
criteria (>5 cm, P=0.4429; estimated 5-year survival rate, 53
vs. 43%) (Fig. 7) and beyond Milan
criteria (≤5 cm, P=0.4; estimated 5-year survival rate, 39 vs. 30%)
(Fig. 8).
Patient complications
In the chemoembolization group, no relevant
post-embolization complication, including death related to
chemoembolization, was reported. Post-embolization syndrome,
including mild abdominal pain and fever, was common and treated
with anti-inflammatory drugs. On the other hand, four patients
succumbed (2.7%) within 30 days after surgery in the hepatic
resection group. However, it was not evident that there was a
direct relationship between hepatic resection and death.
Discussion
Previous reports showed that the treatment
modalities offering a cure of HCC include surgical resection
(17,18), PEIT (19), RFA (20) and liver transplantation (21). However, chemoembolization has yet to
be considered as a curative treatment of choice for HCC. The reason
for this is that numerous studies have been unable to demonstrate
any improvement in survival for the chemoembolization treatment of
HCC (2,22).
Chemoembolization involves mixing iodized oil and
one or more anticancer drugs, such as doxorubicin hydrochloride,
epirubicin hydrochloride, mitomycin C, cisplatin, neocarzinostatin
or floxuridine; injecting the mixture into tumor-feeding vessels;
and embolizing the vessels with gelatin sponges (3,6,11,14–16,23–33).
In our series, the main anticancer drug used in chemoembolization
was epirubicin hydrochloride. According to the latest nationwide
report by the Liver Cancer Study Group of Japan, anticancer drugs
used for chemoembolization in Japan include doxorubicin
hydrochloride, epirubicin hydrochloride and cisplatin (33). However, strict dose criteria for
anticancer drugs and lipiodol have yet to be determined. The
objective of chemoembolization is to accumulate lipiodol in the
liver tumor as compactly as possible (4,6,13,15,24,34).
Almost all patients in our study undertook CT approximately 1 week
after chemoembolization. When the accumulation of lipiodol in the
tumor was insufficient, additional chemoembolization was performed.
Lee et al reported favorable survival rates for patients
with HCC who received chemoembolization when lipiodol was compactly
retained (6).
Chemoembolization has been used as a palliative
therapy for unresectable HCC. Previous reports showed that eligible
candidates for chemoembolization are patients with unresectable HCC
and poor liver function, multiple liver tumors (>3) or large
tumor size (>10 cm) (4,5,16,22,30,32,35).
An initial randomized controlled trial of
chemoembolization did not identify superior survival by
chemoembolization compared to palliative therapies (2,22,36,37).
However, previous randomized control trials showed that
chemoembolization is superior to symptomatic treatment in terms of
the 2-year survival rate (38,39).
Resected specimens following chemoembolization have
shown a high correlation between complete retention of lipiodol in
the tumor and pathological necrosis (11,14,24,34,40).
In those studies, patients had good liver function and a solitary
liver tumor. Therefore, the possibility of a favorable prognosis
exists after chemoembolization in selected patients with operable
HCC. Choi et al (24) stated
that chemoembolization was performed to reduce the possibility of
tumor recurrence and to decrease tumor size in operable cases of
HCC. Takayasu et al (15)
reported a correlation between lipiodol accumulation in the HCC and
survival rate. These authors showed that survival rates of 1, 2 and
3 years after TACE were 93.3, 77.1 and 77.1%, respectively. In
comparison, our results demonstrated that survival rates of 1, 2
and 3 years after TACE were 80.9, 68.2 and 59.3%, respectively.
However, Takayasu et al used the inclusion criterion for
chemoembolization of one main lesion (<5 cm) associated with no
more than two lesions (<3 cm) (15), and patients with solitary HCC
underwent surgery. In addition, few studies have compared hepatic
resection and chemoembolization in patients with solitary HCC and
good liver function (6).
Numerous criteria have been proposed for an HCC
staging system (41–46). Among these criteria, the most
frequently used are the Okuda staging system (47), the Child-Pugh staging system
(48), tumor node metastasis (TNM)
staging (8) and CLIP score
(7). Although a number of obstacles
and limitations exist with these proposed criteria, Georgiades
et al (42) reported
Child-Pugh staging as the most accurate of 12 liver staging systems
for predicting results in unresectable HCC patients. We applied the
Child-Pugh staging system, TNM staging and CLIP score as liver
staging systems for our patients.
The present results showed that the survival rate
did not differ significantly between the hepatic resection and
chemoembolization groups for UICC T1-2 HCC, while a significant
difference was apparent for UICC T3 HCC. UICC T3 indicates multiple
tumors larger than 5 cm or a tumor involving a major branch of the
portal or hepatic veins. Previous reports indicated tumor size,
number of tumors, serum α-fetoprotein levels, liver function and
portal vein involvement as prognostic factors of HCC (16). In our study, no significant
differences in the number of tumors and liver function were noted
between the two groups. However, portal vein involvement was not
thoroughly considered in the T3 HCC subgroup between treatments.
Portal vein involvement may thus be one source of survival
bias.
The Milan criteria are used in patient selection for
liver transplantation (9), which is
considered to be the optimal treatment of small HCC. Bridge
treatments, including hepatic resection, TACE and RFA, are
necessary for patients anticipating organ transplantation (50,51).
Roayaie et al (51) reported
that patients with HCC measuring more or equal to 5 cm achieve
long-term survival after liver transplantation combined with TACE.
Belghiti et al (50)
reported that liver resection prior to liver transplantation does
not increase the morbidity nor impair long-term survival following
liver transplantation in patients with Milan criteria. Our results
showed that there was no significant difference between
chemoembolization and hepatic resection in patients both within
(<5 cm) and beyond (>5 cm) Milan criteria. We suggest that
patients eligible for liver transplantation should be managed by
hepatic resection or TACE until such time organ transplantation
occurs.
Limitations were noted in this study. The study
design was retrospective and showed selection bias. Furthermore,
the backgrounds of chronic liver damage varied. The majority of
background disease was hepatitis B or C, but the two diseases
exhibit different characteristics (41,45).
The background bias should therefore be considered. In conclusion,
chemoembolization appears to be as effective as hepatic resection
in treating solitary HCC and in subpopulations with UICC T1-2N0M0
or CLIP 0-2 HCC with adequate liver function. However, hepatic
resection is preferable for treating the subgroup of patients with
UICC T3N0M0 HCC.
References
|
1
|
Okuda K: Hepatocellular carcinoma. J
Hepatol. 32:225–237. 2000. View Article : Google Scholar
|
|
2
|
: A comparison of lipiodol
chemoembolization and conservative treatment for unresectable
hepatocellular carcinoma: Groupe d’ Etude et de Traitement du
Carcinome Hepatocellulaire. N Engl J Med. 332:1256–1261.
1995.PubMed/NCBI
|
|
3
|
Achenbach T, Seifert JK, Pitton MB, Schunk
K and Junginger T: Chemoembolization for primary liver cancer. Eur
J Surg Oncol. 28:37–41. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dumortier J, Chapuis F, Borson O, et al:
Unresectable hepatocellular carcinoma: survival and prognostic
factors after lipiodol chemoembolisation in 89 patients. Dig Liver
Dis. 38:125–133. 2006.PubMed/NCBI
|
|
5
|
Mondazzi L, Bottelli R, Brambilla G, et
al: Transarterial oily chemoembolization for the treatment of
hepatocellular carcinoma: a multivariate analysis of prognostic
factors. Hepatology. 19:1115–1123. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee HS, Kim KM, Yoon JH, et al:
Therapeutic efficacy of transcatheter arterial chemoembolization as
compared with hepatic resection in hepatocellular carcinoma
patients with compensated liver function in a hepatitis B
virus-endemic area: a prospective cohort study. J Clin Oncol.
10:4459–4465. 2002.
|
|
7
|
The Cancer of the Liver Italian Program. A
new prognostic system for hepatocellular carcinoma: a retrospective
study of 435 patients. Hepatology. 28:751–755. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sobin LH and Wittekind Ch; International
Union Against Cancer (UICC). Liver TNM Classification of Malignant
Tumours. 6th edition. John Wiley & Sons, Inc; New York:
2002
|
|
9
|
Mazzaferro V, Regalia E, Doci R, et al:
Liver transplantation for the treatment of small hepatocellular
carcinoma in patients with cirrhosis. N Engl J Med. 334:693–699.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown DB, Gould JE, Gervais DA, et al:
Transcatheter therapy for hepatic malignancy: standardization of
terminology and reporting criteria. J Vasc Interv Radiol.
18:1469–1478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Higashihara H and Okazaki M: Transcatheter
arterial chemoem bolization of hepatocellular carcinoma: a Japanese
experience. Hepatogastroenterology. 28:72–78. 2002.
|
|
12
|
Matsuo N, Uchida H, Nishimine K, et al:
Segmental transcatheter hepatic artery chemoembolization with
iodized oil for hepatocellular carcinoma: antitumor effect and
influence on normal tissue. J Vasc Interv Radiol. 4:543–549. 1993.
View Article : Google Scholar
|
|
13
|
Miyayama S, Matsui O, Yamashiro M, et al:
Ultraselective transcatheter arterial chemoembolization with a 2-f
tip microcatheter for small hepatocellular carcinomas: relationship
between local tumor recurrence and visualization of the portal vein
with iodized oil. J Vasc Interv Radiol. 18:365–376. 2007.
View Article : Google Scholar
|
|
14
|
Nakamura H, Liu T, Hori S, et al: Response
to transcatheter oily chemoembolization in hepatocellular carcinoma
3 cm or less: a study in 50 patients who underwent surgery.
Hepatogastroenterology. 3:6–9. 1993.PubMed/NCBI
|
|
15
|
Takayasu K, Muramatsu Y, Maeda T, et al:
Targeted transarterial oily chemoembolization for small foci of
hepatocellular carcinoma using a unified helical CT and angiography
system: analysis of factors affecting local recurrence and survival
rates. Am J Roentgenol. 176:681–688. 2001. View Article : Google Scholar
|
|
16
|
Ueno K, Miyazono N, Inoue H, Nishida H,
Kanetsuki I and Nakajo M: Transcatheter arterial chemoembolization
therapy using iodized oil for patients with unresectable
hepatocellular carcinoma: evaluation of three kinds of regimens and
analysis of prognostic factors. Cancer. 172:1574–1581. 2000.
View Article : Google Scholar
|
|
17
|
Ikai I, Arii S, Kojiro M, et al:
Reevaluation of prognostic factors for survival after liver
resection in patients with hepatocellular carcinoma in a Japanese
nationwide survey. Cancer. 101:796–802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto J, Kosuge T, Saiura A, et al:
Effectiveness of hepatic resection for early-stage hepatocellular
carcinoma in cirrhotic patients: subgroup analysis according to
Milan criteria. Jpn J Clin Oncol. 37:287–295. 2007. View Article : Google Scholar
|
|
19
|
Sung Y, Choi D, Lim H, et al: Long-term
results of percutaneous ethanol injection for the treatment of
hepatocellular carcinoma in Korea. Korean J Radiol. 7:187–192.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tateishi R, Shiina S, Teratani T, et al:
Percutaneous radiofrequency ablation for hepatocellular carcinoma.
An analysis of 1000 cases. Cancer. 103:1201–1209. 2005.PubMed/NCBI
|
|
21
|
Imvrios G, Papanikolaou V, Vrochides D, et
al: Liver transplantation outcomes in patients with cirrhosis and
hepatocellular carcinoma: experience of a single center in a viral
hepatitis endemic area. Transplant Proc. 39:1508–1510. 2007.
View Article : Google Scholar
|
|
22
|
Bruix J, Llovet J, Castells A, et al:
Transarterial embolization versus symptomatic treatment in patients
with advanced hepatocellular carcinoma: results of a randomized,
controlled trial in a single institution. Hepatology. 27:1578–1583.
1998. View Article : Google Scholar
|
|
23
|
Chen MS, Li JQ, Zhang YQ, et al: High-dose
iodized oil transcatheter arterial chemoembolization for patients
with large hepatocellular carcinoma. World J Gastroenterol.
8:74–78. 2001.PubMed/NCBI
|
|
24
|
Choi B, Kim H, Han J, et al: Therapeutic
effect of transcatheter oily chemoembolization therapy for
encapsulated nodular hepatocellular carcinoma: CT and pathologic
findings. Radiology. 182:709–713. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eurvilaichit C: Outcome of transcatheter
oily chemoem bolization in patients with hepatocellular carcinoma.
Hepatogastroenterology. 51:20–24. 2004.PubMed/NCBI
|
|
26
|
Hashimoto N, Kawai S, Mikuriya S, et al:
Effects of transcatheter arterial chemoembolization with oral
chemotherapy on hepatic neoplasms. Cancer Chemother Pharmacol.
23:S21–S25. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamada K, Nakanishi T, Kitamoto M, et al:
Long-term prognosis of patients undergoing transcatheter arterial
chemoembolization for unresectable hepatocellular carcinoma:
comparison of cisplatin lipiodol suspension and doxorubicin
hydrochloride emulsion. J Vasc Interv Radiol. 12:847–854. 2001.
View Article : Google Scholar
|
|
28
|
Maeda S, Shibata J, Fujiyama S, et al:
Long-term follow-up of hepatic arterial chemoembolization with
cisplatin suspended in iodized oil for hepatocellular carcinoma.
Hepatogastroenterology. 50:809–813. 2003.PubMed/NCBI
|
|
29
|
Okusaka T, Okada S, Ueno H, et al:
Transcatheter arterial embolization with zinostatin stimalamer for
hepatocellular carcinoma. Oncology. 62:228–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ono Y, Yoshimasu T, Ashikaga R, et al:
Long-term results of lipiodol-transcatheter arterial embolization
with cisplatin or doxorubicin for unresectable hepatocellular
carcinoma. Am J Clin Oncol. 23:564–568. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimamura Y, Gunven P, Takenaka Y, et al:
Combined peripheral and central chemoembolization of liver tumors.
Experience with lipiodol-doxorubicin and gelatin sponge (L-TAE).
Cancer. 61:238–242. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stuart K, Stokes K, Jenkins R, Trey C and
Clouse M: Treatment of hepatocellular carcinoma using
doxorubicin/ethiodized oil/gelatin powder chemoembolization.
Cancer. 72:3202–3209. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takayasu K, Arii S, Ikai I, et al:
Prospective cohort study of transarterial chemoembolization for
unresectable hepatocellular carcinoma in 8510 patients.
Gastroenterology. 131:461–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takayasu K, Arii S, Matsuo N, et al:
Comparison of CT findings with resected specimens after
chemoembolization with iodized oil for hepatocellular carcinoma. Am
J Roentgenol. 175:699–704. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O’Suilleabhain CB, Poon RT, Yong JL, Ooi
GC, Tso WK and Fan ST: Factors predictive of 5-year survival after
transarterial chemoembolization for inoperable hepatocellular
carcinoma. Br J Surg. 90:325–331. 2002.
|
|
36
|
Pelletier G, Ducreux M, Gay F, et al:
Treatment of unresectable hepatocellular carcinoma with lipiodol
chemoembolization: a multicenter randomized trial. Groupe CHC. J
Hepatol. 29:129–134. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pelletier G, Roche A, Ink O, et al: A
randomized trial of hepatic arterial chemoembolization in patients
with unresectable hepatocellular carcinoma. J Hepatol. 11:181–184.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Llovet J, Real M, Montana X, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: a
randomised controlled trial. Lancet. 18:1734–1739. 2002. View Article : Google Scholar
|
|
39
|
Lo CM, Ngan H, Tso WK, et al: Randomized
controlled trial of transarterial lipiodol chemoembolization for
unresectable hepatocellular carcinoma. Hepatology. 35:1164–1171.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takayasu K, Moriyama N, Muramatsu Y, et
al: Hepatic arterial embolization for hepatocellular carcinoma.
Comparison of CT scans and resected specimens. Radiology.
150:661–665. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kudo M, Chung H, Haji S, et al: Validation
of a new prognostic staging system for hepatocellular carcinoma:
the JIS score compared with the CLIP score. Hepatology.
40:1396–1405. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Georgiades C, Liapi E, Frangakis C, et al:
Prognostic accuracy of 12 liver staging systems in patients with
unresectable hepatocellular carcinoma treated with transarterial
chemoembolization. J Vasc Interv Radiol. 17:1619–1624. 2006.
View Article : Google Scholar
|
|
43
|
Kudo M, Chung H and Osaki Y: Prognostic
staging system forhepatocellular carcinoma (CLIP score): its value
and limitations, and a proposal for a new staging system, the Japan
Integrated Staging Score (JIS score). J Gastroenterol. 38:207–215.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leung T, Tang A, Zee B, et al:
Construction of the Chinese University Prognostic Index for
hepatocellular carcinoma and comparison with the TNM staging
system, the Okuda staging system, and the Cancer of the Liver
Italian Program staging system: a study based on 926 patients.
Cancer. 94:1760–1769. 2002. View Article : Google Scholar
|
|
45
|
Ueno S, Tanabe G, Nuruki K, et al:
Prognosis of hepatocellular carcinoma associated with Child class B
and C cirrhosis in relation to treatment: a multivariate analysis
of 411 patients at a single center. J Hepatobiliary Pancreat Surg.
9:469–477. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ueno S, Tanabe G, Sako K, et al:
Discrimination value of the new western prognostic system (CLIP
score) for hepatocellular carcinoma in 662 Japanese patients.
Cancer of the Liver Italian Program. Hepatology. 34:529–534. 2001.
View Article : Google Scholar
|
|
47
|
Okuda K, Ohtsuki T, Obata H, et al:
Natural history of hepatocellular carcinoma and prognosis in
relation to treatment. Study of 850 patients. Cancer. 56:918–928.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pugh R, Murray-Lyon I, Dawson J, Pietroni
M and Williams R: Transection of the oesophagus for bleeding
oesophageal varices. Br J Surg. 60:646–669. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Majno PE, Sarasin FP, Mentha G and
Hadengue A: Primary liver resection and salvage transplantation or
primary liver transplantation or primary liver transplantation in
patients with single, small hepatocellular carcinoma and preserved
liver function: an outcome-oriented decision analysis. Hepatology.
31:899–906. 2000. View Article : Google Scholar
|
|
50
|
Belghiti J, Cortes A, Abdalla EK, et al:
Resection prior to liver transplantation for hepatocellular
carcinoma. Ann Surg. 238:885–892. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roayaie S, Frischer JS, Emre SH, et al:
Long-term results with multimodal adjuvant therapy and liver
transplantation for the treatment of hepatocellular carcinomas
larger than 5 centimeters. Ann Surg. 235:533–539. 2002. View Article : Google Scholar : PubMed/NCBI
|