Introduction
GBM (WHO-grade IV) is the most malignant and
frequent brain tumor and has the worst prognosis of any cancer.
Despite advances in treatment by surgery combined with radiotherapy
or chemotherapy, patients with GBM have a mean survival of only
14.6 months (1). Therefore, new
therapeutic targets are urgently needed. A formidable difficulty in
treating GBM is that tumor cells diffuse and infiltrate the normal
peripheral tissue, therefore it is not possible to completely
dissect out and remove the tumor. Degradation of extracellular
matrix (ECM) is the defining step in tumor cell invasion and
members of the matrix metalloproteinase family (MMPs) have crucial
roles in regulating this process (2–4). Thus,
understanding and blocking the invasive process may be an effective
strategy for treating GBM.
The recent discovery of miRNAs is a major advance.
Accumulating evidence suggests that miRNAs are involved in multiple
biological functions, including cell invasion, by altering the
expression of multiple target genes. They bind to the 3′
untranslated region (UTR) of target messenger RNAs (mRNAs) to
suppress translation or induce degradation of these mRNAs. The
roles different miRNAs play have been recently expounded in
numerous human tumors as detailed below.
Accumulated evidence shows that downregulation of
miR-218 can enhance tumor cell invasion and proliferation in
several kinds of solid tumors (5,6). In
this study, we have identified that miR-218 is downregulated in GBM
tissues and has a considerable involvement in GBM cell invasion.
Ectopic expression of miR-218 can decrease the invasive ability of
a GBM cell line. Conversely, inhibiting miR-218 expression can
increase this ability. Our data shown an inverse correlation in GBM
tissue between levels of miR-218 and MMP mRNAs (MMP-2, -7 and -9).
These members of the MMP family are downstream effectors of the Wnt
pathway (7,8). Using sophisticated algorithms from
target prediction databases and in vitro tests, we found
that LEF1, a nuclear transducer involved in the Wnt/β-catenin
pathway, is a candidate target of miR-218. MiR-218 regulates cell
invasive ability by targeting LEF1, resulting in reduced synthesis
of MMPs. Furthermore, ectopic expression of miR-218 can reduce
protein levels of LEF1 and MMP-9 in GBM cell line and inhibiting
miR-218 expression can increase their protein expression that was
detected by western blotting. More importantly, LEF1 siRNA can
imitate the role of miR-218. Luciferase reporter assay further
confirmed the direct interaction between miR-218 and the 3′ UTR of
LEF1 mRNA. Our previous work has shown that MMP-9 is associated
with GBM recurrence and poor patient prognosis (9). However, the reason for the increased
expression of MMP-9 in GBM tissues remains poorly understood.
Wnts are a family of secreted glycoproteins with
diverse roles in tumor development, including regulation of cell
invasion. Wnt signaling stabilizes β-catenin protein and directly
targets the MMP promoters (MMP-2, -9 and -7) through the LEF/TCF
complex (7,8). Additionally, our previously
unpublished data, obtained by miRNA microarray and gene expression
profiling, showed that the mRNA levels of MMP-9 and -7 were
inversely related to the expression of miR-218 in 60 GBM tissues.
These data suggest that upregulation of miR-218 can decrease MMP-7,
and-9 expression by targeting LEF1 and may be an efficient strategy
in preventing glioma cell invasion.
Materials and methods
Clinical samples
Tumor specimens (GBM) were obtained from patients
who underwent positive debulking surgery in the Neurosurgery
Department of Beijing Tiantan Hospital from 2006 to 2009. The
diagnosed gliomas were re-reviewed in histological slides by the
experiential neuropathologist according to the 2007 WHO
classification. Normal brain tissue samples were obtained from
internal decompression of patients with cerebral injury and
temporal lobe resection for epilepsy. Tissue samples were fixed by
formalin, embedded by paraffin and tissue microarray blocks
comprising a total of 38 tissue cores (4 normal, 8 low grade, 8 AA
and 18 GBM) were constructed with a tissue microarrayer (Beecher
Instruments, USA). The tissue microarrays were stored at 4°C until
analysis for IHC and FISH. The study complied with the requirements
of the local ethics committee. Individual informed consent was
obtained from all participants.
Cell lines and transfection
The human GBM cell lines U251, U87, SNB19 and LN229,
obtained from the Institute of Biochemistry and Cell Biology,
Chinese Academy of Science, were used in this study. Cells were
maintained in DMEM containing 10% FBS, 50 U/ml penicillin G, and
250 μg/ml streptomycin in a humidified atmosphere containing 5%
CO2 at 37°C. Transfections with miR-218 were performed
in serum-free medium 24 h after plating, using Lipofectamine 2000
(Invitrogen). After 6 h, cells were placed in complete medium and
maintained at 37°C in 5% CO2. The miR-218 mimic sequence
used was 5′-UUGU GCUUGAUCUAACCAUGUAU GGUUAG AUCAAGCACAAUU-3′.
Inhibitor sequence: 5′-ACAUGGUUA GAU CAAG CACAA-3′, LEF1-1589
siRNA: 5′-CAUCCCGA GAACAUCAAAUTTAUUUGAUGUUCUCGGGAUGTT-3′ (Gima Biol
Engineering Inc., Shanghai, China).
Western bloting
Cells were lysed 1% Nonidet P-40 lysis buffer 48 h
following exposure to LY294002 or vehicle. Homogenates were
clarified by centrifugation at 20,000 × g for 15 min at 4°C, and
protein concentrations were determined with a bicinchoninic acid
protein assay kit (Pierce Biotechnology). SDS-PAGE was performed on
40 μg of protein from each sample, gels were transfered to PVDF
membranes (Millipore) and incubated with primary antibodies
detecting LEF1, MMP-9 (Cell Signaling Technology; 1:1000 dilution),
TIMP-1 (Santa Cruz; 1:1000 dilution) followed by incubation with an
HRP-conjugated secondary antibody (Zymed, San Diego, CA; 1:1000
dilution). The specific protein was detected using a SuperSignal
protein detection kit (Pierce, USA). Membranes were stripped and
reprobed with a primary antibody against GAPDH (Santa Cruz; 1:1000
dilution).
Immunohistochemistry (IHC) and
immunofluorescence (IF)
IHC was performed on a glioma tissue array by the
avidin-biotin-complex (ABC) method as previously described
(10). Briefly, the sections were
incubated with primary antibody (1:100 dilution) overnight at 4°C,
then incubated with a biotinylated secondary antibody (1:200
dilution) at room temperature for 1 h, followed by the incubation
with ABC-peroxidase reagent (1:200 dilution, Vector, USA) for an
additional 1 h. After washing with Tris-buffer, the sections were
stained with DAB (3,3 diaminobenzidine, 30 mg dissolved in 100 ml
Tris-buffer containing 0.03% H2O2) for 5 min,
rinsed in water and counterstained with hematoxylin. The antibodies
used in this study were those to LEF1 and MMP-9 (Cell Signaling
Technology). Negative controls were obtained by substituting
primary antibodies with non-immune serum. The proportion of
positively stained tumor cells was graded as follows: 0, no
positive tumor cells; 1, <5% positive tumor cells; 2, 5–20%
positive tumor cells; and 3, >20% positive tumor cells (11,12).
The intensity of staining was recorded on a scale of 0 (no
staining), 1 (weak staining, light yellow), 2 (moderate staining,
yellowish brown) and 3 (strong staining, brown). The staining index
was calculated as follows: staining index = staining intensity ×
proportion of positively stained tumor cells.
IF was performed on LN229 cell line as previously
described (13). Cells were
incubated with LEF1 antibody (1:100 dilution) for 1 h at room
temperature. TRITC-labeled secondary antibodies were added at 1:100
dilution, and the cells were then incubated for another 30 min.
Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI;
Invitrogen).
Fluorescence in situ hybridization
(FISH)
FISH was subsequently performed on the same tissue
array using a miR-218 Probe Kit (Boster Co., Wuhan, China)
according to the manufacturer’s instructions. The sequence of
miR-218: 5′-ACATGGT TAGATCAAGCACAA-3′. The expression of miR-218 in
each spot was estimated by an epifluorescence microscope (Olympus,
Tokyo, Japan) and graded according to a previous method. In three
separate regions in each spot ≥100 cells was calculated. The degree
of fluorescence was determined by combining the proportion of
positively stained tumor cells and the intensity of staining. The
proportion of positively stained tumor cells was graded as follows:
0, <5% positive tumor cells; 1, 5–30% positive tumor cells; and
2, 30–70% positive tumor cells. The intensity of staining was
recorded on a scale of 0 (no light), 1 (weak light), 2 (moderate
light) and 3 (strong light). The staining index was calculated as
follows: staining index = staining intensity + proportion of
positively stained tumor cells.
Transwells
Transwell filters (Costar, USA) were coated with
Matrigel (3.9 μg/μl, 60–80 μl) on the upper surface of the
polycarbonic membrane (diameter 6.5 mm, pore size 8 μm). Following
30-min incubation at 37°C, Matrigel solidified and served as the
extracellular matrix for tumor cell invasion analysis. Harvested
cells (1×105) in 100 μl of serum-free DMEM were added
into the upper compartment of the chamber. The experimental
procedure was as previously described (13).
Scratch assay
LN229 and U87 cells were grown in 6-well plates with
complete medium. After 90% confluence was reached, the medium was
replaced with FBS-free media for 24 h. Wound was created by a
germ-free 100 μl pipette tip in the monolayer. The cells were
washed with PBS and grown in FBS-free media for 36 h. The wounds
were observed under a phase contrast microscope (IX81, Olympus).
The images were analysed by drawing lines at the wound edges. The
width of the scratch was measured at 0 and 36 h post-treatment. The
migration distance in the wound was calculated according to the
following formula: cell-free area at 0 h - cell-free area at 36 h.
Experiments were repeated thrice in duplicate with comparable
results.
Quantitative real-time polymerase chain
reaction (qRT-PCR)
qRT-PCR analysis of miR-218 expression in 4 GBM cell
lines was performed as previously described (14). Briefly, total RNA from cells was
extracted by TRIzol (Invitrogen) and subjected to reverse
transcription using a first-strand cDNA synthesis kit (Invitrogen)
according to the manufacturer’s instructions. The quantitative
analysis of the change in expression levels was calculated by
real-time PCR machine (7500 ABI, USA). For detection of miR-218,
the TaqMan MicroRNA assay kit (Applied Biosystems) was used
according to the manufacturer’s instructions. U6 was used as an
internal control to normalize variances.
Luciferase assay
Cells were seeded in 96-well plates and culture for
24 h. The reporter plasmid was purchased from GenScript (Jiangsu,
China). The 3′-UTR binding sequence was subcloned into a firefly
luciferase-based reporter construct immediately downstream of
Luc coding sequence. The luciferase reporter plasmids
containing wild or mutant 3′-UTRs of LEF1 were transfected into
cells together with miR-218 mimics, pGL3-control-wild LEF1:
5′-AAATGTAAAAGCACATGAGAAT-3′; pGL3-control-mutant LEF1:
5′-AAAGTACGGATCCGTGAGAAT-3′. The luciferase reporter assay was
carried out as previously described. Three independent experiments
were performed and the data are presented as means ± SD.
Statistical analysis
All tests were done using SPSS Graduate Pack 13.0
statistical software (SPSS, Chicago, IL). Descriptive statistics
including the mean ± SE along with one-way ANOVAs were used to
determine significant differences. Non-parametric test was
performed in the grading system. P<0.05 was considered
significant.
Results
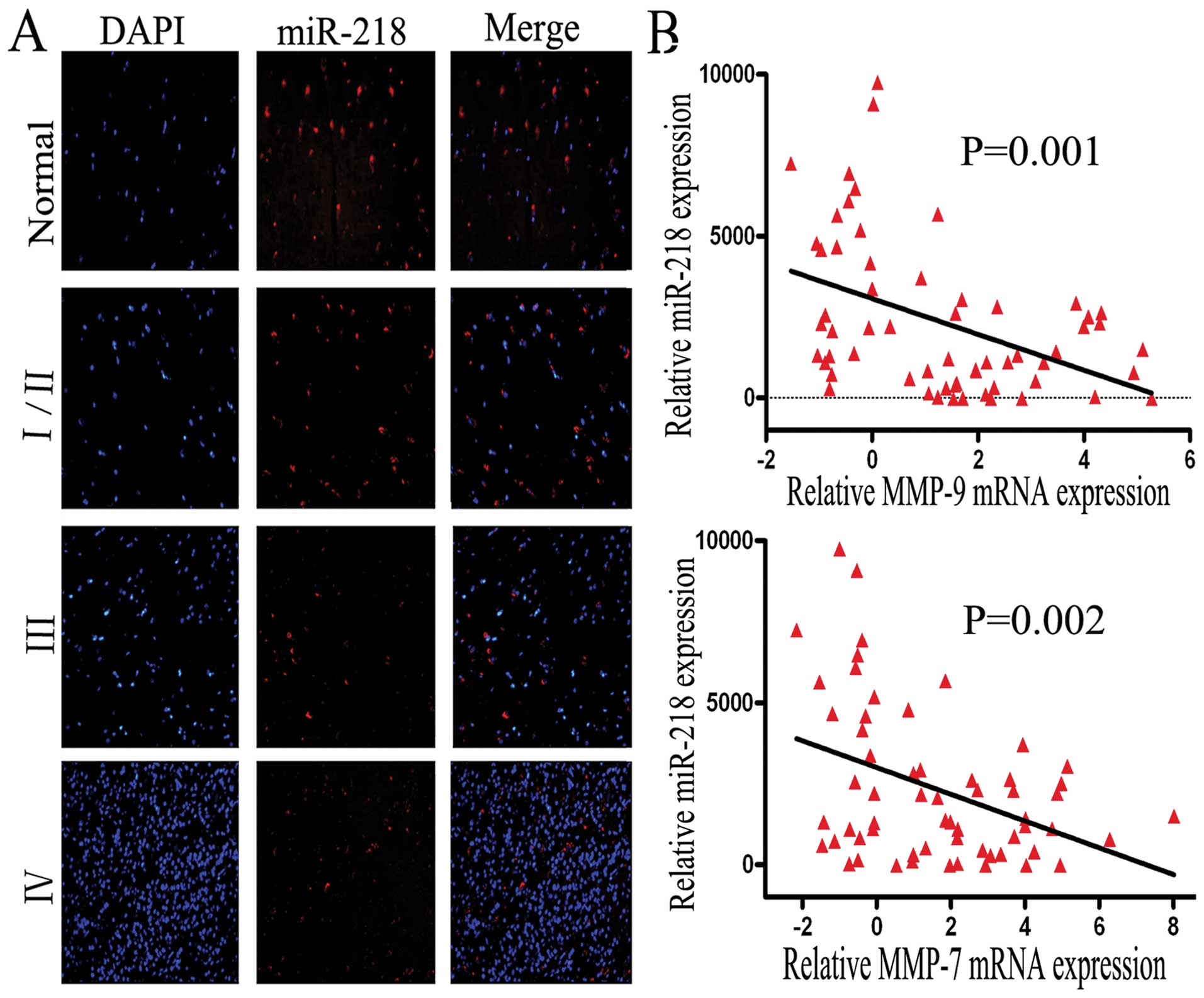
MiR-218 is downregulated in glioma
tissues and inversely related to MMP-9 and MMP-7 mRNA expression in
GBM tissues
Previous studies have reported that miR-218 is
downregulated in cervical cancer, lung cancer and gastric cancer
(5,15,16).
Similarly, in both AA and GBM, miR-218 is also expressed at a low
level, compared to normal brain tissue; P=0.009 or P≤0.01 (17,18).
To identify whether the expression of miR-218 is downregulated in
gliomas of Chinese patients, FISH was performed using a tissue
array that included 38 samples (4 normal tissues, 8 low grade, 8
grade III and 18 grade IV samples). Consistent with previous
observations, we observed that the expression level of miR-218 was
significantly decreased in glioma tissues, especially in grade
III/IV tissues (P=0.012/0.003), compared to normal brain tissue
(Fig. 1A). Interestingly, this
result shows that miR-218 expression decreases markedly from normal
brain tissue to low grade to GBM tissue (I/II VS III or IV, P=0.021
or 0.001).
For tumor cell invasion, obvious candidates for
signaling molecules are members of the MMP family and the
relationship between MMP-9 levels and GBM recurrence over a short
time period, or poor patient prognosis was reported in our previous
study (9). Our previously
unpublished data, obtained by miRNA microarray and mRNA expression
profiling, showed that mRNA expression of MMP-9 (P=0.001) and MMP-7
(P=0.002) is inversely related to the expression of miR-218 in 60
GBM tissues (Fig. 1B). Given the
above data, we suggest that the invasive function of miR-218 may be
carried out by MMP-7 and -9. Due to the common function of MMP-9
and -7 and the lack of specific reagents, MMP-7 was not
investigated further in this study.
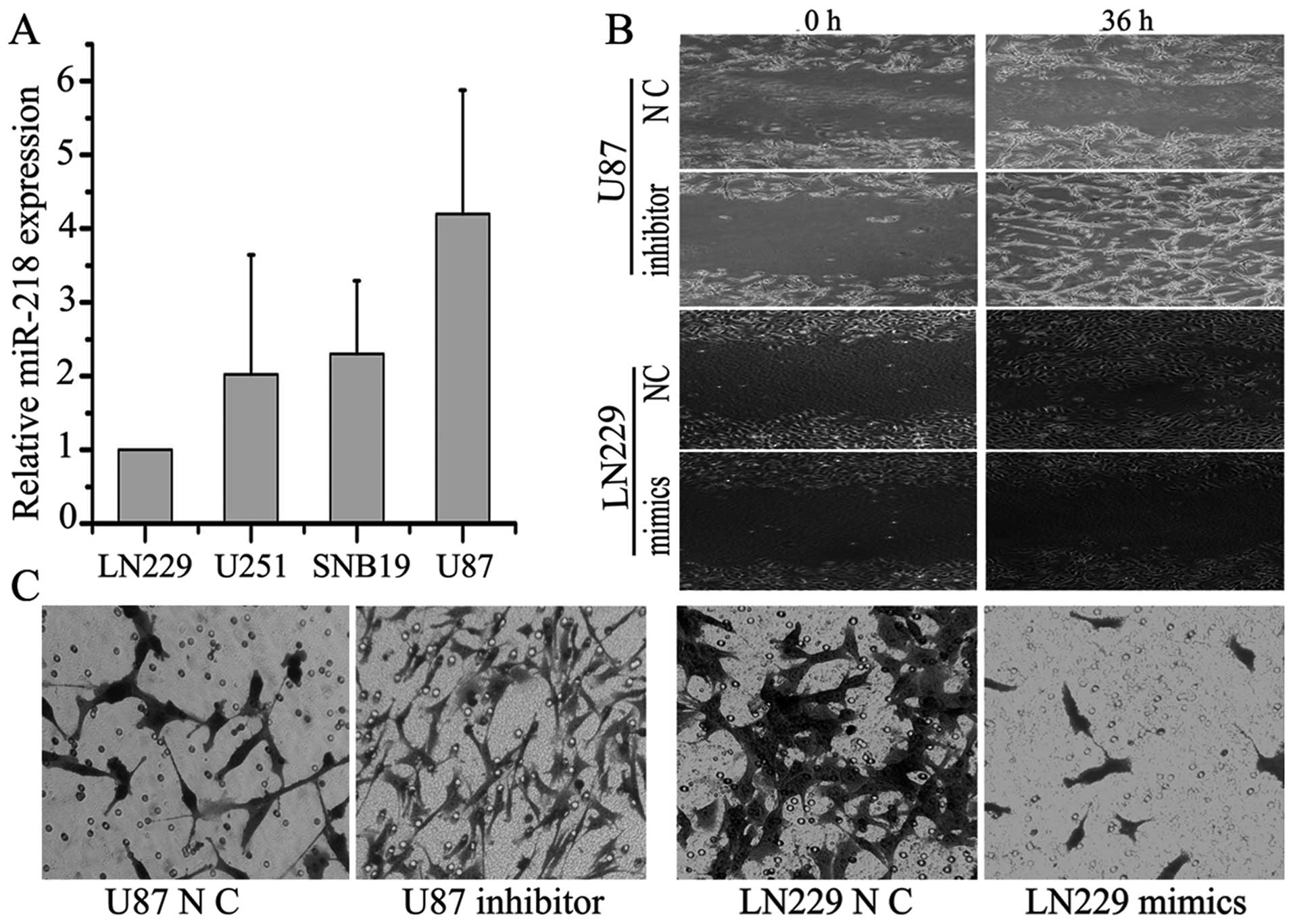
Upregulation of miR-218 inhibits GBM cell
invasion and inhibiting miR-218 expression enhanced this
ability
The role of miRNAs in tumor cell invasion has been
intensively studied in recent years. To identify the invasive
function of miR-218, four GBM cell lines (LN229, SNB19, U251 and
U87) were screened for miR-218 expression levels by qRT-PCR. U87
cells had the highest level of miR-218 expression, while LN229
cells had the lowest (Fig. 2A).
Cells were then assayed in a transwell invasion assay. LN229 and
U87 cells transfected with specific miR-218 mimics and inhibitor,
respectively. The processed cells were incubated in 6-well plates
for 24 h and then plated on the upper Matrigel plugs of the
transwells. The transwell assay showed that overexpression of
miR-218 significantly suppressed the invasive ability of LN229
cells by ~3–4-fold, while inhibiting miR-218 expression enhanced
this ability by ~6-fold (P<0.05) (Fig. 2C). Indeed, a similar effect of
miR-218 inhibiting cell invasion and of the miR-218 inhibitor
promoting invasion were also observed in the scratch assay
(Fig. 2B). Migration ability was
inhibited by ~2-fold by overexpression of miR-218 compared to
negative controls.
This finding indicates that miR-218 can suppress the
invasive ability of GBM cell lines in vitro. However,
whether or how the effectors MMP-9 and MMP-7 were regulated by
miR-218 remains unclear.
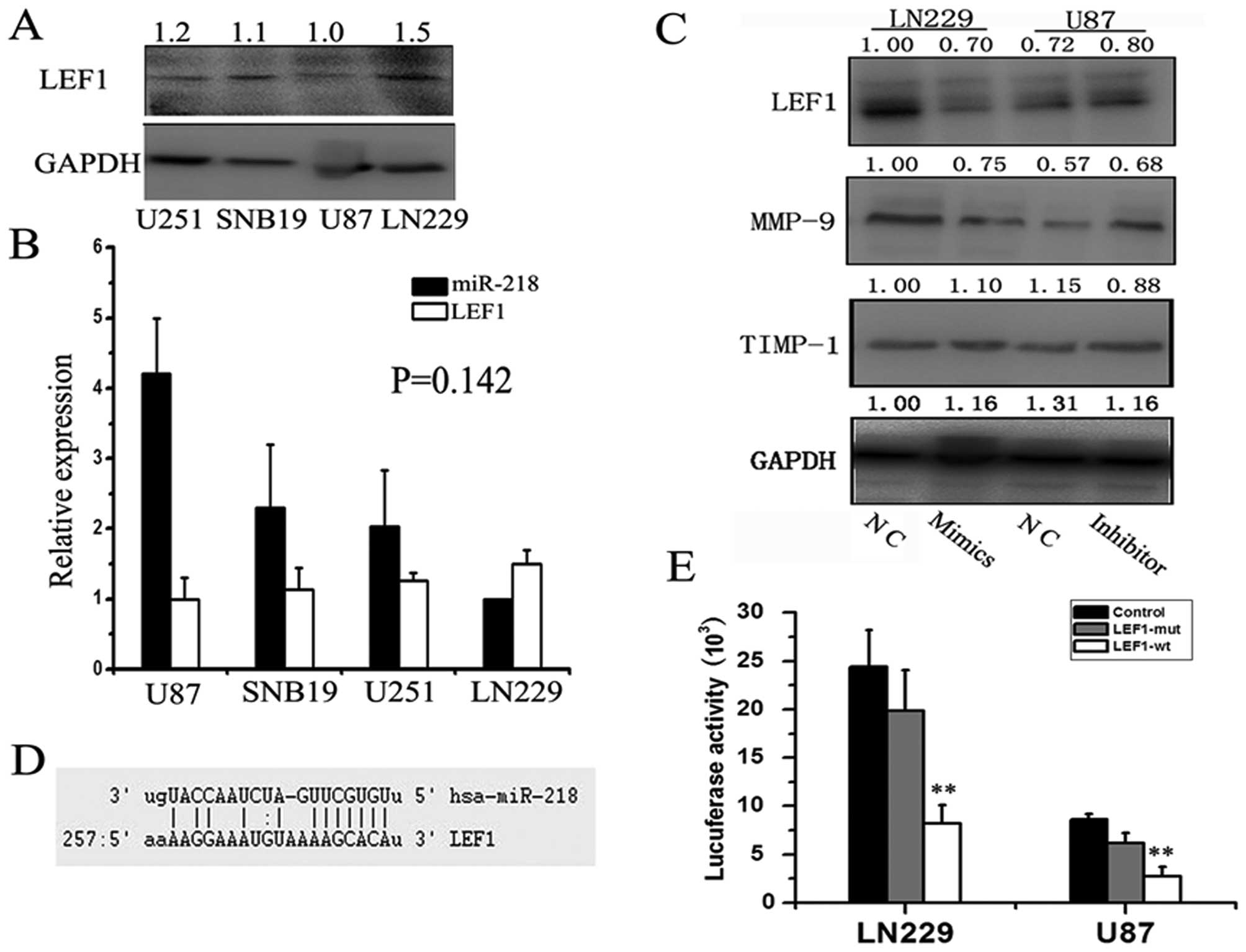
Mature miR-218 directly targets LEF1 and
regulates MMP-9 expression
The Wnt/β-catenin/LEF1 pathway is one of the best
known signaling pathways and it correlates with tumorigenesis,
especially with tumor cell invasion and adhesion. Increasing MMP-9
expression is associated with dysfunctions of Wnt signaling
(19); therefore, we conceived a
biological transport chain: the miR-218-intermediary molecule-MMP-9
axis. MMP-9 is a key molecule downstream of Wnt activation and is
responsible for increased rates of neural stem cell (NSC)
proliferation and migration in 1% O2(19). MMP-7 is also downstream effector in
the Wnt pathway; therefore, we hypothesized that miR-218 can
regulate the expression of MMP-9 and -7 by targeting transcription
factors involved in the Wnt pathway. Using sophisticated algorithms
in miRanda (http://www.microrna.org/), we
identified LEF1 as a candidate target of miR-218 (Fig. 3D). To validate this, we detected
LEF1 protein levels in the same four cell lines and normalized
these levels to the level of GAPDH (Fig. 3A). As expected, an inverse
relationship between miR-218 and LEF1 protein was confirmed by
qRT-PCR and western blot analysis (P=0.142) (Fig. 3B). Although the P-value was not
significant due to the limited number of cell lines, the
relationship was quite clear. To explore this functional axis:
miR-218-LEF1-MMP-9, we re-expressed miR-218 in LN229 cells by
transfection of miR-218 mimics and knocked down miR-218 in U87
cells using its inhibitor. We then detected changes in levels of
LEF1 protein. Western blotting confirmed a 1.43-fold decrease in
levels of LEF1 48 h after re-expression of miR-218 mimics in LN229
cells, whereas significantly increased expression was observed 48 h
after transfection of miR-218 inhibitor compared to negative
controls (Fig. 3C). Consistent with
the expression of LEF1, MMP-9 expression was reduced 1.33-fold when
miR-218 mimics were transfected into LN229 cells and was
significantly increased when U87 cells were transfected with
miR-218 inhibitor compared to negative controls. A luciferase
reporter assay further confirmed the direct interaction between
miR-218 and the 3′ UTR of LEF1 mRNA (Fig. 3E). The luciferase activity for the
wild-type 3′ UTR of LEF1 was significantly inhibited by
co-transfection with miR-218 mimics compared to constructs
containing mutated 3′ UTRs (LN229, P=0.000; U87, P=0.000). This
experiment demonstrated that LEF1 is a direct target of
miR-218.
In addition, we also detected the protein levels of
the tissue inhibitor of metalloproteinase-1 (TIMP-1, an endogenous
inhibitor of MMP-9) (20) and found
no marked difference between either TIMP-1 and miR-218 or TIMP-1
and LEF1. These data suggest that miR-218 plays a critical role in
GBM cell invasion by directly targeting and downregulating LEF1,
thereby decreasing MMP-9 expression, but not through TIMP-1.
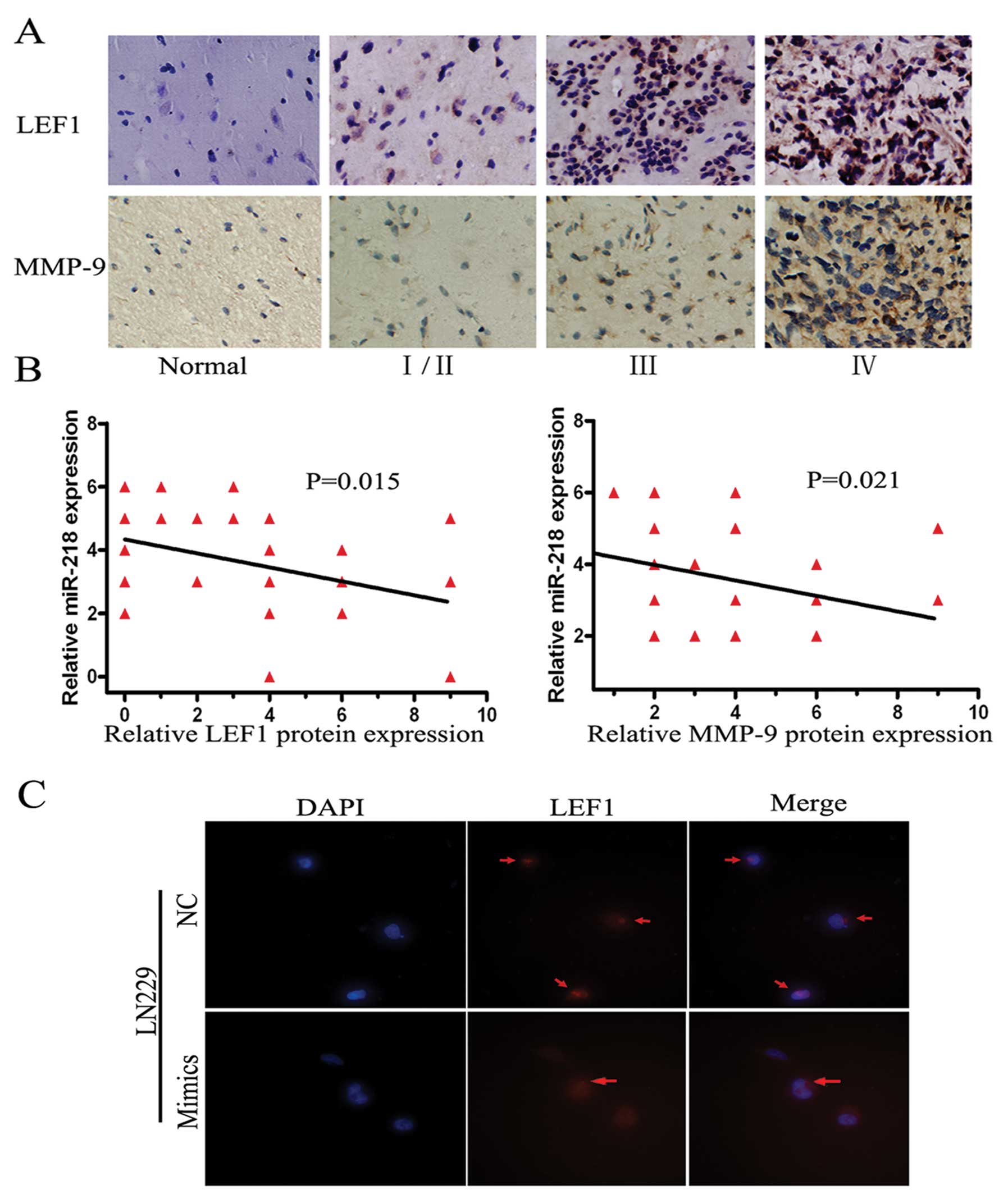
The protein levels of LEF1 and MMP-9 are
low and inversely related to miR-218 expression in glioma tissues
and miR-218 targets LEF1 mRNA resulting in downregulating LEF1
protein in LN229 cells
Biological functions are ultimately carried out by a
diversity of functional proteins in vivo. Therefore, we
detected LEF1 and MM-9 protein levels in the aforementioned 38
tissues, including one GBM tissue which was damaged during antigen
retrieval, by IHC. Both proteins were at high levels in high grade
gliomas and at low levels in low grade gliomas or normal brain
tissue (Fig. 4A). The degree of
staining was graded by a previously described scoring system. The
inverse relationship between miR-218 and LEF1 (P=0.021) or MMP-9
(P=0.015) was confirmed in these tissues by IHC and FISH (Fig. 4B). Additionally, immune fluorescence
was performed using the highest expression cell lines of LEF1
protein, LN229. The signal intensity of LEF1 was markedly decreased
when miR-218 was transfected into LN229 cells (Fig. 4C). As a result, the biological
transport axis, miR-218-LEF1-MMP-9, was demonstrated in the
invasive pathway.
LEF1 siRNA can imitate the role of
miR-218 in U87
To further verify this axis, LEF1 siRNA was
constructed to imitate the functions of miR-218. It was
co-transfected into U87 cells with the miR-218 inhibitor. As
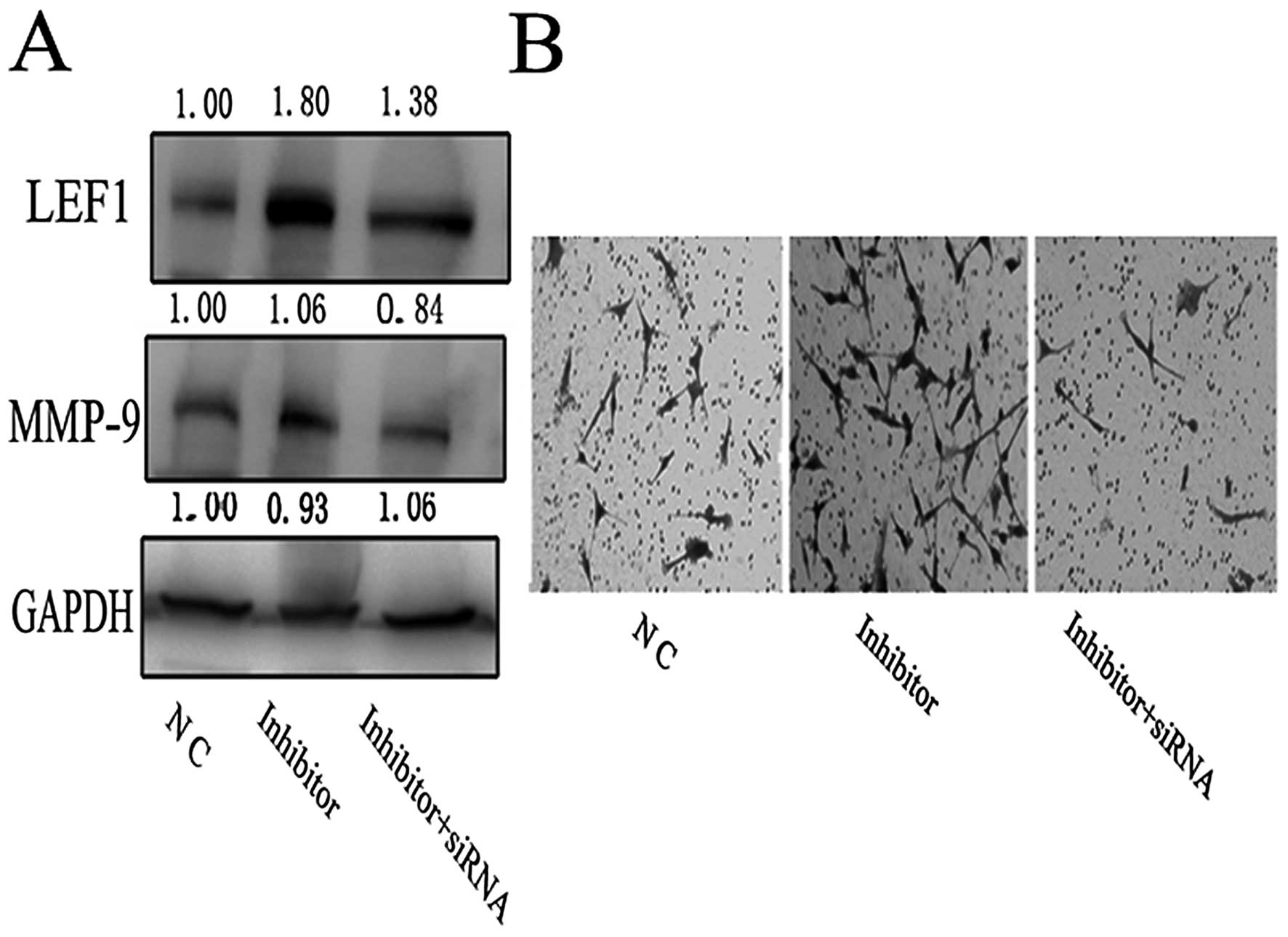
expected, the upregulation of MMP-9 protein due to knockdown of
miR-218 was significantly reduced by the specific LEF1 siRNA
(Fig. 5A). This result was
corroborated using the transwell invasion assay. The enhanced
invasive ability of miR-218 inhibitor-transfected U87 cells
declined ~4-fold when LEF1 siRNA was co-transfected with miR-218
inhibitor (Fig. 5B).
Discussion
GBM is the most common malignant neoplasm of the
human brain. Despite advances in treatment strategies in recent
years, surgery plus radiotherapy or chemotherapy, which has been
used for decades, is still commonly prescribed. Combined treatment
of radiotherapy plus temozolomide resulted in a slight survival
rate improvement from 12.1 to 14.6 months (1). The search for new treatment methods is
an urgent clinical challenge for neuroscientists. Recurrence is the
most difficult problem in the treatment of GBM. Diffuse
infiltration of normal peripheral tissue is a major obstacle to
deciding the extent of resection. Therefore, inhibiting tumor cell
invasion by blocking the invasive pathway may be beneficial in
treating GBM. There are several signaling pathways that contribute
to GBM pathogenesis. The AKT pathway contributes to tumor cell
proliferation and apoptosis (21),
the NF-κB pathway is involved in cell survival, inflammation and
immune regulation, while the MAPK/ERK pathway is involved in growth
and differentiation. The Notch, Toll-like receptor and TGF-β
signaling pathways also play roles. All these pathways interact to
form a biological network regulating a variety of biological
behavior participating in tumorigenesis. Stepwise accumulation of
genetic alterations in these pathways results in tumor development.
Recent reports show that aberrant expression of miRNAs contributes
to many human tumors: miR-199a/b-3p in hepatocellular carcinoma
(22), miR-301 in breast cancer
(23), miR-99 family in prostate
cancer (24), miR-200a in
meningiomas (25) and miR-21 in
glioblastoma (26). MiRNAs are
endogenous non-coding RNAs that bind to the 3′ UTR of target mRNAs
to suppress translation or induce mRNA degradation. These miRNAs
can regulate multiple signaling molecules belonging to different
signaling pathways.
In this study, we found that miR-218 can directly
target LEF1 to regulate MMP-9 expression in the Wnt pathway.
Published reports and our previous studies have demonstrated that
the Wnt signaling pathway significantly correlated with the
invasion and proliferation of tumor cells (27). Wnts are a family of secreted
glycoproteins that signal by binding the Frizzled family of
receptors. This activates the modular protein disheveled, resulting
in the accumulation of cytosolic β-catenin and subsequent formation
of the β-catenin/LEF1 complex in the nucleus. This DNA binding
complex activates a large number of downstream target genes
(including MMP-7, 9, Axin-2, cyclin D1 and Myc) (28,29)
causing invasion, migration, adhesion and proliferation.
Dysregulation of Wnt/β-catenin signaling was identified for the
initiation of colorectal cancer development (30), but its involvement in gliomas it is
not currently clear. LEF-1, a member of the LEF1/TCF transcription
factor family involved in the Wnt pathway, is a DNA binding
transcription factor that functions by recruiting β-catenin to Wnt
target genes for regulation. It has recently been reported that
levels of LEF1 are markedly correlated with tumor cell invasion and
patient prognosis (31–34). Certain members of the MMP family
(MMP-2, -7, -9 and -26) have been identified as downstream
target-genes of the LEF1/TCF complex. Moreover, in silico
analysis revealed 4 putative LEF1/TCF binding sites in the MMP-9
proximal promoter (8).
MiR-218 is significantly downregulated and plays a
critical role in the progression of many human cancers as a tumor
suppressor (5,6,15). The
expression of miR-218 is also downregulated in gliomas but is
specifically expressed or greatly enriched in normal brain tissue
(35). We found a significant
inverse relationship between miR-218 expression and expression of
some members of the MMP family, including MMP-2, -7 and -9 in 60
GBM tissues. A single miRNA can target multiple transcripts, named
a ‘targetome’, to regulate gene expression (36). ROBO1, BIRC5 and GJA1 in the
SLIT-ROBO pathway (6), and ECOP and
IKK-β in the NF-κB pathway (37)
were identified as direct targets of miR-218 in the regulation of
cell proliferation and invasion. In this study, LEF1 was identified
as an important new target of miR-218 in the conventional
prediction website (http://www.microrna.org). We detected the expression
levels of miR-218, LEF1 and MMP-9 in 38 tissues, consisting of
normal brain tissue, low and high grade glioma tissues by FISH and
IHC and found the expression of miR-218 was always inverse to that
of LEF1 (P=0.021) and MMP-9 proteins (P=0.015). Importantly, the
LEF1 and MMP-9 protein levels were significantly decreased 48 h
after transfection of miR-218 mimics in LN229 cells, whereas it was
increased after transfection of miR-218 inhibitor. Therefore, we
suggest that miR-218 can regulate the expression of MMPs by
directly targeting LEF1. The interaction between miR-218 and LEF1
mRNA was confirmed by luciferase assays. Transwell assays and
scratch tests showed that upregulation of miR-218 significantly
suppressed the invasive ability of LN229 cells in vitro,
while inhibiting miR-218 expression enhanced this ability.
Moreover, LEF1 siRNA can rescue the invasive ability of the cells
that was enhanced by exogenous expression of miR-218 inhibitor.
Therefore, we suggest that miR-218 can suppress invasion by
targeting LEF1 and indirectly regulating MMP-9 expression.
For the treatment of GBM, inhibiting any element of
the miR-218-LEF1-MMPs axis would be an effective strategy and would
extend the recurrence periods. For example, the MMP inhibitor, α
lipoic acid, blocked T cell migration into the spinal cord
(38). In addition, RNA
interference (RNAi) strategies include the use of small interfering
RNAs (siRNAs) and miRNAs have the potential to selectively inhibit
gene expression by blocking the translation of target mRNAs. They
have been used as genetic tools in higher eukaryotes, and are one
of the most promising therapeutic modalities for the future.
However, delivering these RNAs to specific cells presents a
significant challenge that requires traversing the circulatory
system while avoiding kidney filtration, degradation by
endonucleases, aggregation with serum proteins, and uptake by
phagocytes (39). Dose-dependent
toxicity has not been definitively determined in mammals. Grimm
et al reported fatal side effects from abundant RNAi
expression in the liver of adult mice (40). Moreover, non-specific delivery may
cause side effects, including the activation of immune responses.
The safety of the transfection reagent and the long-term efficacy
need to be further explored.
The upstream steps of miRNA expression should be
discussed here. By analysis of the Sanger miR database, Alajez
et al found that miR-218 primary transcripts (hsa-miR-218-1
and hsa-miR-218-2) were embedded in the intronic regions of SLIT2
(4p15.31) and SLIT3 (5q35.1), respectively (6). They detected that miR-218 expression
is increased concordant with SLIT2 and SLIT3 expression when cells
were treated with the demethylation drug, 5-Aza-2-deoxycytidine.
Narayan et al identified a high frequency of promoter
hypermethylation in SLIT1, SLIT2 and SLIT3 in cervical cancer
tumors (41). Thus it can be seen
that downregulation of miR-218 results from SLIT gene promoter
hypermethylation. A new therapeutic protocol for the treatment of
GBM may be to test available demethylation drugs. However, to
determine whether these genes are hypermethylated in gliomas
require further research.
In conclusion, our previous experiments and this
study show that miR-218 is also correlated with GBM cell
proliferation; however, we have not extended these studies further
because of the lack of specific reagents. We have demonstrated for
the first time the existence of the miR-218-LEF1-MMPs axis and that
it is involved in GBM cell invasion. MiR-218 binds to the 3′ UTR of
LEF1, reducing the binding of LEF1 to the promoter of MMP-9,
resulting in decreased expression of MMP-9 protein. Inhibiting any
element of this axis may be an effective therapeutic strategy.
Acknowledgements
We wish to thank Yuling Yang for the tissue sample
collection and clinical data retrieval. This study was supported by
grants from the National Key Project of Science and Technology
Supporting Programs of China (no. 2007BAI05B08), National Basic
Research Program of China (973 Program) (no. 2011CB707804), China
National Natural Scientific Found (30971136), Program for New
Century Excellent Talents in University (NCET-07-0615), and the
Natural Science Foundation of Tianjin Municipal Science and
Technology Commission (10SYSYJC28800, 09JZCD17600).
Abbreviations:
|
GBM
|
glioblastoma multiforme
|
|
AA
|
anaplastic astrocyteoma
|
|
miRNA
|
microRNA
|
|
LEF1
|
lymphocyte enhancer-binding
factor-1
|
|
MMP-9
|
a member of matrix metalloproteinases
family
|
|
Wnt
|
Wnt proteins form a family of highly
conserved secreted signaling molecules that regulate cell-to-cell
interactions during embryogenesis
|
|
IHC
|
immunohistochemistry
|
|
FISH
|
fluorescence in situ
hybridization
|
|
qRT-PCR
|
quantitative real-time polymerase
chain reaction
|
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sameshima T, Nabeshima K, Toole BP, et al:
Glioma cell extracellular matrix metalloproteinase inducer
(EMMPRIN) (CD147) stimulates production of membrane-type matrix
metalloproteinases and activated gelatinase A in co-cultures with
brain-derived fibroblasts. Cancer Lett. 157:177–184. 2000.
View Article : Google Scholar
|
|
3
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martinez I, Gardiner AS, Board KF, Monzon
FA, Edwards RP and Khan SA: Human papillomavirus type 16 reduces
the expression of microRNA-218 in cervical carcinoma cells.
Oncogene. 27:2575–2582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alajez NM, Lenarduzzi M, Ito E, et al:
MiR-218 suppresses nasopharyngeal cancer progression through
downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res.
71:2381–2391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crawford HC, Fingleton BM, Rudolph-Owen
LA, et al: The metalloproteinase matrilysin is a target of
beta-catenin transactivation in intestinal tumors. Oncogene.
18:2883–2891. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu B, Crampton SP and Hughes CC: Wnt
signaling induces matrix metalloproteinase expression and regulates
T cell transmigration. Immunity. 26:227–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan W, Zhang W, Sun L, et al:
Identification of MMP-9 specific microRNA expression profile as
potential targets of anti-invasion therapy in glioblastoma
multiforme. Brain Res. 1411:108–115. 2011.PubMed/NCBI
|
|
10
|
Zhang J, Han L, Zhang A, et al: AKT2
expression is associated with glioma malignant progression and
required for cell survival and invasion. Oncol Rep. 24:65–72.
2010.PubMed/NCBI
|
|
11
|
Birner P, Toumangelova-Uzeir K, Natchev S
and Guentchev M: STAT3 tyrosine phosphorylation influences survival
in glioblastoma. J Neurooncol. 100:339–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Chen L, Bao Z, et al: Inhibition
of STAT3 reverses alkylator resistance through modulation of the
AKT and beta-catenin signaling pathways. Oncol Rep. 26:1173–1180.
2011.PubMed/NCBI
|
|
13
|
Debinski W and Gibo DM: Fos-related
antigen 1 modulates malignant features of glioma cells. Mol Cancer
Res. 3:237–249. 2005.PubMed/NCBI
|
|
14
|
Zhou X, Ren Y, Moore L, et al:
Downregulation of miR-21 inhibits EGFR pathway and suppresses the
growth of human glioblastoma cells independent of PTEN status. Lab
Invest. 90:144–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tie J, Pan Y, Zhao L, et al: MiR-218
inhibits invasion and metastasis of gastric cancer by targeting the
Robo1 receptor. PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yanaihara N, Caplen N, Bowman E, et al:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rao SA, Santosh V and Somasundaram K:
Genome-wide expression profiling identifies deregulated miRNAs in
malignant astrocytoma. Mod Pathol. 23:1404–1417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silber J, Lim DA, Petritsch C, et al:
miR-124 and miR-137 inhibit proliferation of glioblastoma
multiforme cells and induce differentiation of brain tumor stem
cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ingraham CA, Park GC, Makarenkova HP and
Crossin KL: Matrix metalloproteinase (MMP)-9 induced by Wnt
signaling increases the proliferation and migration of embryonic
neural stem cells at low O2 levels. J Biol Chem.
286:17649–17657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collier IE, Legant W, Marmer B, et al:
Diffusion of MMPs on the surface of collagen fibrils: the mobile
cell surface-collagen substratum interface. PLoS One. 6:e240292011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uddin S, Hussain AR, Siraj AK, et al: Role
of phosphatidylinositol 3′-kinase/AKT pathway in diffuse large
B-cell lymphoma survival. Blood. 108:4178–4186. 2006.
|
|
22
|
Hou J, Lin L, Zhou W, et al:
Identification of miRNomes in human liver and hepatocellular
carcinoma reveals miR-199a/b-3p as therapeutic target for
hepatocellular carcinoma. Cancer Cell. 19:232–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi W, Gerster K, Alajez NM, et al:
MicroRNA-301 mediates proliferation and invasion in human breast
cancer. Cancer Res. 71:2926–2937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun D, Lee YS, Malhotra A, et al: miR-99
family of MicroRNAs suppresses the expression of prostate-specific
antigen and prostate cancer cell proliferation. Cancer Res.
71:1313–1324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saydam O, Shen Y, Wurdinger T, et al:
Downregulated microRNA-200a in meningiomas promotes tumor growth by
reducing E-cadherin and activating the Wnt/beta-catenin signaling
pathway. Mol Cell Biol. 29:5923–5940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maiese K, Li F, Chong ZZ and Shang YC: The
Wnt signaling pathway: aging gracefully as a protectionist?
Pharmacol Ther. 118:58–81. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He TC, Sparks AB, Rago C, et al:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shtutman M, Zhurinsky J, Simcha I, et al:
The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway.
Proc Natl Acad Sci USA. 96:5522–5527. 1999. View Article : Google Scholar
|
|
30
|
Li TW, Ting JH, Yokoyama NN, Bernstein A,
van de Wetering M and Waterman ML: Wnt activation and alternative
promoter repression of LEF1 in colon cancer. Mol Cell Biol.
26:5284–5299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin AY, Chua MS, Choi YL, et al:
Comparative profiling of primary colorectal carcinomas and liver
metastases identifies LEF1 as a prognostic biomarker. PLoS One.
6:e166362011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mei JM, Borchert GL, Donald SP and Phang
JM: Matrix metalloproteinase(s) mediate(s) NO-induced dissociation
of beta-catenin from membrane bound E-cadherin and formation of
nuclear beta-catenin/LEF-1 complex. Carcinogenesis. 23:2119–2122.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rivat C, Le Floch N, Sabbah M, et al:
Synergistic cooperation between the AP-1 and LEF-1 transcription
factors in activation of the matrilysin promoter by the src
oncogene: implications in cellular invasion. FASEB J. 17:1721–1723.
2003.PubMed/NCBI
|
|
34
|
Kriegl L, Horst D, Reiche JA, Engel J,
Kirchner T and Jung A: LEF-1 and TCF4 expression correlate
inversely with survival in colorectal cancer. J Transl Med.
8:1232010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sempere LF, Freemantle S, Pitha-Rowe I,
Moss E, Dmitrovsky E and Ambros V: Expression profiling of
mammalian microRNAs uncovers a subset of brain-expressed microRNAs
with possible roles in murine and human neuronal differentiation.
Genome Biol. 5:R132004. View Article : Google Scholar
|
|
36
|
Selbach M, Schwanhausser B, Thierfelder N,
Fang Z, Khanin R and Rajewsky N: Widespread changes in protein
synthesis induced by microRNAs. Nature. 455:58–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao C, Zhang Z, Liu W, Xiao S, Gu W and Lu
H: Reduced microRNA-218 expression is associated with high nuclear
factor kappa B activation in gastric cancer. Cancer. 116:41–49.
2010.PubMed/NCBI
|
|
38
|
Marracci GH, Jones RE, McKeon GP and
Bourdette DN: Alpha lipoic acid inhibits T cell migration into the
spinal cord and suppresses and treats experimental autoimmune
encephalomyelitis. J Neuroimmunol. 131:104–114. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh S, Narang AS and Mahato RI:
Subcellular fate and off-target effects of siRNA, shRNA, and miRNA.
Pharm Res. 28:2996–3015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grimm D, Streetz KL, Jopling CL, et al:
Fatality in mice due to oversaturation of cellular microRNA/short
hairpin RNA pathways. Nature. 441:537–541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Narayan G, Goparaju C, Arias-Pulido H, et
al: Promoter hypermethylation-mediated inactivation of multiple
Slit-Robo pathway genes in cervical cancer progression. Mol Cancer.
5:162006. View Article : Google Scholar : PubMed/NCBI
|