Introduction
Malignant ascites frequently occur in patients with
ovarian, uterine, gastrointestinal tract (stomach and intestines),
breast and pancreatic cancers, in which fluid containing cancer
cells accumulate in the abdomen cavity. Without treatment, those
patients would have a poor prognosis with a survival period of less
than 2 months (1). Invasive therapy
(e.g., paracentesis) is efficient for attenuation of the symptom,
but reiterating paracentesis may cause biochemical disturbances
in vivo with the associated complications. A more effective
therapy which could reduce ascites with lesser complications seems
necessary for improving quality of life (QOF) of the patients and
prolonged survival of patients (2,3).
Continuous circulatory hyperthermic intraperitoneal
perfusion chemotherapy (CHIPC) is a novel adjuvant therapy for
peritoneal carcinomatosis with a mechanical scavenging effect on
peritoneal suspension cancer cells (4–8). Warm
chemotherapy liquid containing high-doses of anti-cancer drugs is
administered into peritoneal cavity to react with tumor metastases.
It has benefits of lessen drugs entering circulation and systemic
toxic side effects, and a greater cytotoxic effect of hyperthermia
on cancer (9–12).
A great clinical efficacy on the treatment of
malignant ascites using CHIPC has been demonstrated (13–17).
During traditional CHIPC, the perfusion catheter is traditionally
placed through an invasive laparotomy which requires general
anesthesia or epidural anesthesia (18–23).
Although the procedure has been improved recently with the
development of minimally invasive surgical techniques (MIS)
(1,13–16),
the laparoscope-assisted CHIPC requires special laparoscope devices
(usually expensive) with expertise in the field, which limits its
development and clinical application. In addition, many
laparoscopic surgeons may also have a difficulty in prevention and
treatment of seeding/metastasis of the punctured hole(s) on
abdominal wall (13,14,17).
B ultrasound is non-invasive, less expensive,
repeatable in use, and has a high specificity in the diagnosis for
ascites and liquid estimation in the peritoneal cavity. It also has
been widely used by clinicians for guiding intraperitoneal
punctuation (24,25) and intraperitoneal drainage
construction (26,27). Therefore, it seems rational to
replace laparoscope with B ultrasound during the procedure of
CHIPC. We have, for the first time, introduced B ultrasound to
guide CHIPC for the treatment of malignant ascites induced by solid
cancers. The clinical efficacy, side effects and prognosis of our
improved CHIPC procedure were investigated.
Materials and methods
Inclusion criteria and clinical data
Thirty-two ovarian cancer (OC), gastric cancer (GC),
colorectal cancer (CRC) and pancreatic cancer patients complicated
with malignant ascites patients (Table
I) administered in our hospital from 2007 to 2011 were included
in this study. The ascites volumes ranged from 4000 to 9000 ml
estimated by B volume of intraperitoneal drainage and the Karnofsky
performance scale (KPS) of QOF scored 40–70. The origin tumors of
these patients were diagnosed and confirmed by laparotomy
exploration and/or graphology or fiber endoscope examination and/or
serum tumor biomarker examination (CA125, CEA and CA199) or ascites
cytology examination. The existence and size of ascites were
examined by B ultrasound and/or computed tomography (CT) scan.
Diagnosis of ascites related to unresectable carcinomatosis was
made preoperatively in all cases by standard clinical and
radiological assessments (cytology and CT scan). Peritoneal
carcinomatosis was confirmed by PET-CT and/or other graphology
examination or detection of peritoneal suspension tumor cells.
Patients with extensive abdomen surgical procedures and/or with
insignificant ascites were excluded from this study. Prior to the
therapy, all participates were informed on the palliative role of
the procedure, any risks may be involved, possible complications
and expected benefits, and then consents were obtained as clinical
research guidelines. This study was approved by Medical Ethics
Committee of Cancer Hospital of Guangzhou Medical College.
 | Table ICharacteristics of patients undergoing
B-mode ultrasound guided CHIPC (n=32). |
Table I
Characteristics of patients undergoing
B-mode ultrasound guided CHIPC (n=32).
| n (%)/mean ± SD |
|---|
| Gender |
| Male | 13 (41) |
| Female | 19 (59) |
| Age (years) | 59.41±10.54 |
| Cancer types and
treatment before CHIPC |
| Ovarian cancer | 5 (15.63) |
| Peritoneal
carcinomatosis post ovarian cancer cytoreductive surgery | 6 (18.75) |
| Gastric cancer | 6 (18.75) |
| Peritoneal
carcinomatosis post gastric cancer resection | 4 (12.5) |
| Colorectal
cancer | 3 (9.38) |
| Peritoneal
carcinomatosis post colorectal colorectal cancer resection | 6 (18.75) |
| Pancreatic
cancer | 2 (6.25) |
| Disease courses
(days) | 18.91±10.57 |
| Ascite volume
(ml) | 4925.81±821.37 |
| Cases of free cancer
cells in ascites | 18 (56.25) |
B ultrasound-guided placement of
catheters for CHIPC
In a standard operating theater, patients were
placed in a supine position. Pethidine hydrochloride (75 mg) and
promethazine hydrochloride (25 mg) were administered by
intramuscular injection prior to placing CHIPC catheters. Propofol,
as an anesthetic agent, was given intravenously via a continuous
vein pump with dose (3–8 ml/h) adjusted according to patient
status. B ultrasound examinations on all 4 abdominal quadrants were
performed to choose puncture point which should be in the region of
a larger ascites together with no adhesion between abdominal wall
and the tissues of peritoneal cavity without original abdominal
incision or tumor. A 1.2-cm incision was made by a Hasson trocar
(1.2 cm in diameter) at the punctuation point after administering
0.5% lidocaine (anesthetic agent) locally before the infusion and
outflow catheters with multiple side holes (inner diameter 0.8 cm,
diameter 1.0 cm, 100 cm in length) were placed into intraperitoneal
cavity. The infusion catheters were sited in the left and right
upper quadrants of the intraperitoneal cavity with an inside length
of 40–80 cm, and the outflow catheters were placed in the pelvic
cavity of the left and right lower quadrants with a same length as
that of the infusion catheters. All port sites were fixed to
abdominal wall by cutaneous sutures as shown in Fig. 1.
CHIPC procedures
CHIPC was performed by our self-developed ‘BR-TRG-II
type high-precision hyperthermic intraperitoneal perfusion
treatment system’ with a precise of ±0.15°C on temperature control
and of ±5% on flow control, which couples with an automatic cooling
function. The devices, the only ones of this kind, have been
approved by State Food Drug Administration Firearms (SFDA) of China
(approval no. 2009-3260924) (Fig.
2).
Our CHIPC therapy consisted of three sessions, the
first was completed in the operating room in original status of
anesthesia post-placement of catheters/infusion tubes guided by
B-ultrasound on the same day, and the second and third sessions
were followed on in the intensive care unit (ICU) on the first and
the following days, respectively. Saline solution (0.9%)
(equivalent to the volume of cavity, i.e., 4500–6000 ml) was added
into the tailor-made infusion bag and delivered via infusion tubes
over 90 min with a velocity of 450–600 ml/min and an inflow
temperature of 43°C in attempt to achieve an interior abdominal
temperature of 41.5–42.5°C. The hyperthermic intraperitoneal
chemotherapeutic agents spiked in the perfusion fluid were: i)
cisplatin (50 mg/m2 of body surface) and doxorubicin (50
mg/m2 of body surface), and ii) mitomycin-C (12.5
mg/m2 of body surface) for the ascites originated
respectively from ovarian cancer, and from rectal colon or gastric
or pancreatic cancer, which were used equally in all 3 sessions of
CHIPC. Once the third session had been completed, the peritoneal
perfusion liquid and ascites were drained out. Two infusion and one
outflow catheters were then pulled out, keeping one outflow
catheter as a drainage catheter for 1–3 days.
Evaluation and determination of
efficacy
B ultrasonic and/or CT examinations were performed
at least fortnightly to assess the therapeutic efficacy on ascites
status/remission and tumor progression. KPS scores for QOL of all
participates prior to and after 2 weeks post-CHIPC procedure were
used for the evaluation. Clinical efficacy on all participates was
classified into three grades according to our previous modified WHO
criteria on efficacy assessment in malignant tumors (13): i) complete remission (CR): ascites
are completely absorbed after treatment sustained over 4 weeks; ii)
partial remission (PR): ascites are reduced by 50%; this is
sustained over 4 weeks; iii) no consequence (NC): ascites are not
reduced obviously or increased after treatment.
Statistical analysis
Overall survival and survival in OC, GC and CRC
groups were analyzed and compared by the Kaplan-Meier method with
the use of GraphPad Prism software, version 5.01 (GraphPad, San
Diego, CA, USA). Differences in survival were determined by the
log-rank test for statistical significance. P<0.05 was
considered statistically significant. Additional data analysis for
KPS scores was done by Student’s t-test for paired data.
Results
Clinical efficacy
All 32 participated patients were successfully
administered CHIPC guided by B ultrasound with an average time of
35 min for placement of catheters. The average KPS scores were
significantly elevated by 40% from 54.06 before treatment to 77.19
after CHIPC (p<0.001). Clinical CR of ascites was achieved in 26
out of 32 patients (81.25%), PR was achieved in 4 patients (12.5%),
and no consequence was observed in 2 patients (6.25%). Thus the
total objective remission rate (ORR, ORR = CR+PR) of this study was
93.75%, which demonstrated a significant achievement on clinical
efficacy with our modified CHIPC (Tables II and III).
 | Table IIClinical efficacy of patients with
malignant ascites secondary to peritoneal carcinomatosis undergoing
CHIPC guided by B ultrasound. |
Table II
Clinical efficacy of patients with
malignant ascites secondary to peritoneal carcinomatosis undergoing
CHIPC guided by B ultrasound.
| Overall | OC patients | GC patients | CRC patients |
|---|
| KPS mark |
| Before CHIPC | 54.06±9.46 | 59.09±9.44 | 49±7.38 | 52.22±9.72 |
| After CHIPC | 77.19±10.23 | 82.73±7.86 | 72±11.35 | 74.44±8.82 |
| p-values | <0.01 | <0.01 | <0.01 | <0.01 |
| Therapeutic outcome,
n (%) |
| CR | 26 (81.25%) | 11 (100%) | 6 (60%) | 7 (77.78%) |
| PR | 4 (12.5%) | - | 3 (30%) | 1 (11.11%) |
| NC | 2 (6.25%) | - | 1 (10%) | 1 (11.11%) |
| Median survival
(months) | 9 | 13 | 5.5 | 8 |
 | Table IIIComparison of clinical efficacy of
patients with malignant ascites treated by B ultrasound-guided and
laparoscopically-assisted CHIPC. |
Table III
Comparison of clinical efficacy of
patients with malignant ascites treated by B ultrasound-guided and
laparoscopically-assisted CHIPC.
| Clinical efficacy
(CR+PR) | Median survival time
(months) | Metastasis of
puncture hole (%) |
|---|
| B ultrasound-guided
(n=32) | 93.75% | 9 | 18.75% |
|
Laparoscopically-assisted (n=33) | 93.90% | 8 | 18.18% |
| p-values | >0.05 | >0.05 | >0.05 |
Side effect
During the CHIPC, the participating patients showed
no significant variation in vital signs or discomforts except
transient fever, abdominal distension, and/or abdominal pain. There
were 7 cases of first to second degree of bone marrow suppression
and 3 cases of mild gastrointestinal reaction. All symptoms were
alleviated after the treatment. No intraperitoneal infection or
adhesive intestinal obstruction or other complications occurred due
to the procedure. Therefore, B-mode ultrasound guided CHIPC can
serve as a safe paradigm for malignant ascites.
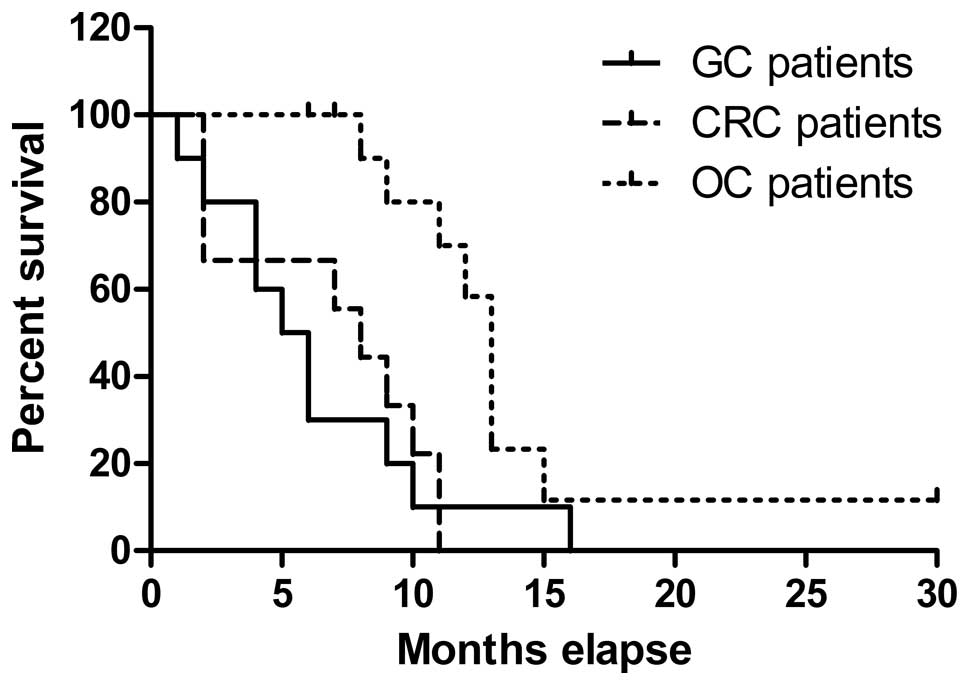
Follow-up and prognosis
The time periods of follow-up after CHIPC in this
study ranged from 3 to 30 months with survival periods ranging from
2 to 30 months (a median survival period of 9 months). Eleven
patients with ovarian cancer and peritoneal carcinomatosis
post-ovarian cancer cytoreductive surgery have survival time from 7
to 30 months with a median survived time of 13 months; 10 patients
with gastric cancer and peritoneal carcinomatosis post-gastric
cancer resection have survival time from 2 to 16 months with a
median survived time of 5.5 months; and 9 patients with colorectal
cancer and peritoneal carcinomatosis post colorectal cancer
resection have survival time ranged 5 to 17 months with a median
survival time of 8 months (Table
II). Furthermore, there was a significant difference in
survival time of patients with different types of cancers
(p<0.01) (Fig. 7). In addition,
CT scans showed a small fluid accumulation in the omental bursa and
in the pelvis in 2 and 1 patients, respectively.
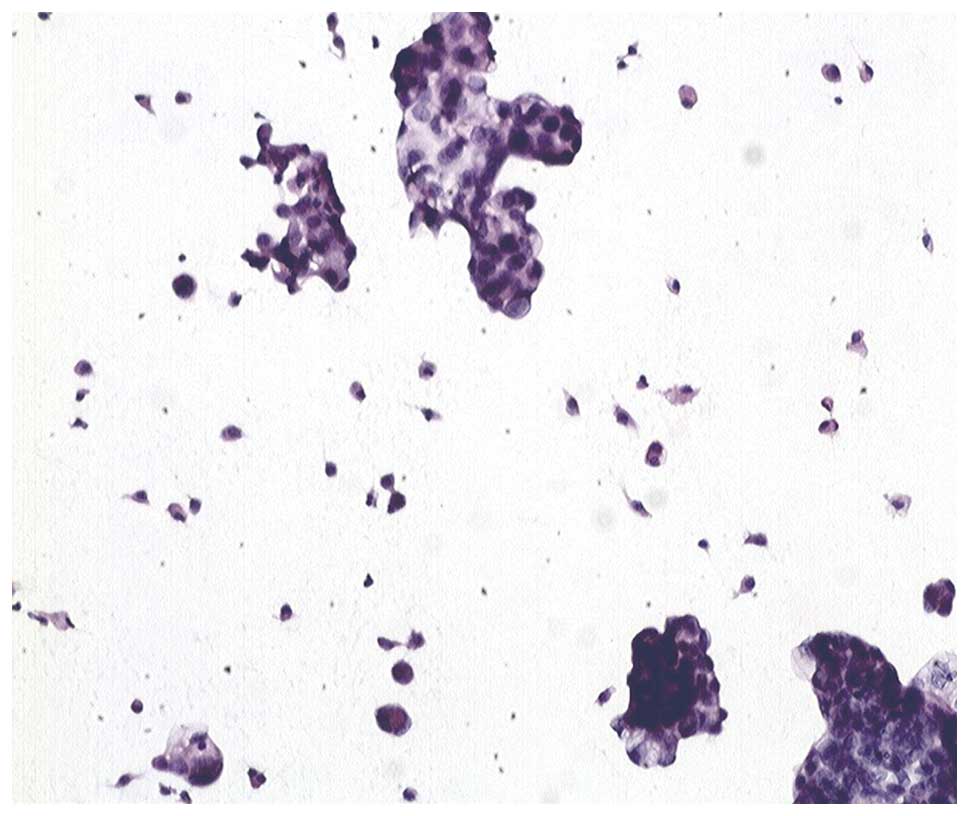
Case study: a 67-year-old woman with 4500 ml ascites
containing a great number of free cancer cells (Fig. 3) had a serum CA125 result of 530
IU/ml without peritoneal mass determined by image examinations. She
was diagnosed with ovarian cancer with normal size ovary and
malignant ascites. After 3 sessions of CHIPC, free cancer cells in
her ascites progressively disappeared and were replaced with
necrosis (Figs. 4–6) and serum CA125 values returned to
normal four weeks post CHIPC. After receiving two circles systemic
chemotherapy post CHIPC 35 days, she was performed exploratory
laparotomy. Tumor mass and tumor metastasis were found in her
bilateral ovary and uterus, but not in other organs. Biopsy tissue
showed her right ovarian tumor to be an ovarian cancer and a total
hysterectomy, bilateral adnexectomy and greater omentectomy were
performed. After surgery, she has alive for over 30 months in good
health.
Discussion
Since the 1980s, CHIPC therapeutic approach in the
treatment of peritoneal metastatic carcinoma has been applied in
the treatments of peritoneal carcinomatosis originated from
gastric, colorectal and ovarian cancers, and pseudomyxoma peritonei
world widely in challenging difficulties of the therapy regime
(2,3). It has achieved satisfactory
therapeutic effects (13,15,18,19).
The tradition CHIPC used a laparoscope or perform
open operation to assist the placement of catheters, which could
result in large traumatic wounds and required costly instruments
and expertise. We have, for the first time, introduced B ultrasound
in CHIPC to guide the placement of catheters in abdominal wall in
the treatment of malignant ascites. This approach largely avoids
injury of visceral organs caused by using laparoscopy and the
associated complications, in addition to its relatively low cost in
operation and less technical and clinical skills required. It also
has a greater advantage of choosing a puncture point with Hasson
trocars at the region with larger ascites as well as smaller
puncture points of 1.0 to 1.2 cm diameter for catheters. Both
B-mode ultrasound guided and laparoscope assisted CHIPC were not
only efficient in improving the patients’ QOF, but also obtained
equivalent efficacy in prolonging the survival time of patients
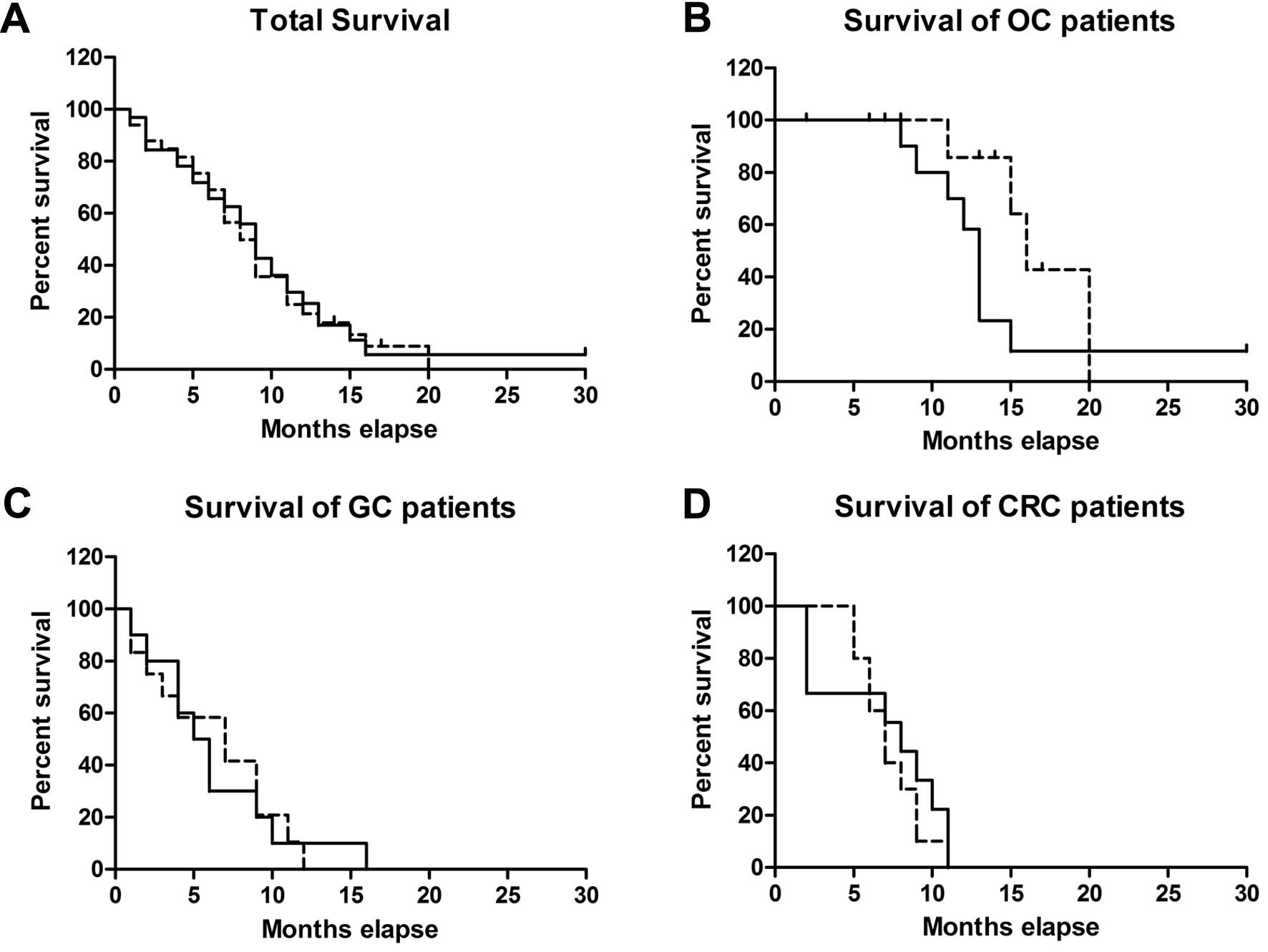
(p=0.83) (Fig. 8A). Therefore,
since 2008, this treatment method has become a standard treatment
strategy for patients with ovarian carcinoma with massive ascites
in our institution. In this study, we achieved an ORR of 93.75% for
the ultrasound guided treatment of ascites symptoms with an
improved median survival period of 9 months (from 2 to 30 months),
compared to 93.90% ORR and 8-month survival time (from 2 to 20
months) of patients who had received laparoscope assisted CHIPC
(p>0.05) (Table III). As to
patients of different cancers, the survival time was not different
either (p=0.54, 0.92 and 0.12) for CRC, GC and OC patients treated
with B ultrasound guided and laparoscope assisted CHIPC,
respectively (Fig. 8B-D). There
were fewer complications observed during sessions of CHIPC guided
by ultrasound. Our clinical results demonstrated that B
ultrasound-guided CHIPC is a safe, effective and feasible approach
for patients suffering from a large mount of ascites with lesser
complications.
We also found that, with the treatment of ascites,
the prognosis seems to be closely associated with the origins of
cancers. Patients with ovarian, colorectal and gastric cancers,
respectively, have the best, better and moderate prognosis
demonstrated by the length of survival period and improved QOF.
This might suggest that the differences in prognosis among these
patients could be related to the dissimilarities in: i) efficacy of
the anti-cancer drugs used, ii) reactivity of the therapeutic
reagents to the cancer tissues, and iii) the molecular and/or
pathophysiological nature of tumors. Further investigation on the
underlying mechanism is warranted.
Admittedly, laparoscopy has an advantage of
detecting occult peritoneal carcinomatosis over B ultrasound, CT,
MRI or PET-CT for evaluation of the stage of peritoneal malignant
tumor in order to avoid any unnecessary surgery (1,13,15,18,19,28).
Therefore, for those patients with unknown primary lesion status of
peritoneal cancer without surgical operation, especially with
ovarian cancer, an exploratory laparotomy should be performed as
soon as possible after the CHIPC together with new adjuvant
chemotherapy for a better prognosis. Another issue worth
considering during laparoscopic surgery is port-site
seeding/metastasis. Unfortunately, the ultrasound guided CHIPC
failed to minimize the rate of port-site seeding (Table III), although this may not due to
the manner of placing catheters.
In conclusion, we demonstrated a novel approach of
using B ultrasound guided CHIPC in the treatment of malignant
ascites with satisfactory outcomes. The approach took full
advantage of MIS, and improved the patient’s quality of life and
prolong survival time. It could be potentially applied in other
clinical applications.
Acknowledgements
This study was supported by The Funds for
Breakthroughs in Key Areas of Guangdong and Hong Kong Project (no.
2006Z1-E6041) and Guangdong Provincial Science and Technology
Program Project Funds (no. 2009A030301013). The study protocol was
approved by Cancer Hospital of Guangzhou Medical College (Guangzhou
510095, China).
References
|
1
|
Patriti A, Cavazzoni E, Graziosi L, et al:
Successful palliation of malignant ascites from peritoneal
mesothelioma by laparoscopic intraperitoneal hyperthermic
chemotherapy. Surg Laparosc Endosc Percutan Tech. 18:426–428. 2008.
View Article : Google Scholar
|
|
2
|
Becker G, Galandi D and Blum HE: Malignant
ascites: systematic review and guideline for treatment. Eur J
Cancer. 42:589–597. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woopen H and Sehouli J: Current and future
options in the treatment of malignant ascites in ovarian cancer.
Anticancer Res. 29:3353–3359. 2009.PubMed/NCBI
|
|
4
|
Aarts F, Hendriks T, Boerman OC, Koppe MJ,
Oyen WJ and Bleichrodt RP: A comparison between radioimmunotherapy
and hyperthermic intraperitoneal chemotherapy for the treatment of
peritoneal carcinomatosis of colonic origin in rats. Ann Surg
Oncol. 14:3274–3282. 2007. View Article : Google Scholar
|
|
5
|
Kusamura S, Younan R, Baratti D, et al:
Cytoreductive surgery followed by intraperitoneal hyperthermic
perfusion: analysis of morbidity and mortality in 209 peritoneal
surface malignancies treated with closed abdomen technique. Cancer.
106:1144–1153. 2006. View Article : Google Scholar
|
|
6
|
Levine EA, Stewart JH IV, Russell GB,
Geisinger KR, Loggie BL and Shen P: Cytoreductive surgery and
intraperitoneal hyperthermic chemotherapy for peritoneal surface
malignancy: experience with 501 procedures. J Am Coll Surg.
204:943–953. 2007. View Article : Google Scholar
|
|
7
|
Spratt JS, Adcock RA, Muskovin M, Sherrill
W and McKeown J: Clinical delivery system for intraperitoneal
hyperthermic chemotherapy. Cancer Res. 40:256–260. 1980.PubMed/NCBI
|
|
8
|
Zanon C, Bortolini M, Chiappino I, et al:
Cytoreductive surgery combined with intraperitoneal
chemohyperthermia for the treatment of advanced colon cancer. World
J Surg. 30:2025–2032. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Shammaa HA, Li Y and Yonemura Y:
Current status and future strategies of cytoreductive surgery plus
intraperitoneal hyperthermic chemotherapy for peritoneal
carcinomatosis. World J Gastroenterol. 14:1159–1166. 2008.
View Article : Google Scholar
|
|
10
|
Benoit L, Cheynel N, Ortega-Deballon P,
Giacomo GD, Chauffert B and Rat P: Closed hyperthermic
intraperitoneal chemotherapy with open abdomen: a novel technique
to reduce exposure of the surgical team to chemotherapy drugs. Ann
Surg Oncol. 15:542–546. 2008. View Article : Google Scholar
|
|
11
|
Huh JW, Kim YJ and Kim HR: Complete
peritonectomy and intraperitoneal chemotherapy for recurrent rectal
cancer with peritoneal metastasis. World J Gastroenterol.
15:756–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spratt JS, Adcock RA, Sherrill W and
Travathen S: Hyperthermic peritoneal perfusion system in canines.
Cancer Res. 40:253–255. 1980.PubMed/NCBI
|
|
13
|
Ba MC, Cui SZ, Lin SQ, et al: Chemotherapy
with laparoscope-assisted continuous circulatory hyperthermic
intraperitoneal perfusion for malignant ascites. World J
Gastroenterol. 16:1901–1907. 2010. View Article : Google Scholar
|
|
14
|
Facchiano E, Scaringi S, Kianmanesh R, et
al: Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC)
for the treatment of malignant ascites secondary to unresectable
peritoneal carcinomatosis from advanced gastric cancer. Eur J Surg
Oncol. 34:154–158. 2008. View Article : Google Scholar
|
|
15
|
Ferron G, Gesson-Paute A, Classe JM and
Querleu D: Feasibility of laparoscopic peritonectomy followed by
intra-peritoneal chemohyperthermia: an experimental study. Gynecol
Oncol. 99:358–361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garofalo A, Valle M, Garcia J and
Sugarbaker PH: Laparoscopic intraperitoneal hyperthermic
chemotherapy for palliation of debilitating malignant ascites. Eur
J Surg Oncol. 32:682–685. 2006. View Article : Google Scholar
|
|
17
|
Gesson-Paute A, Ferron G, Thomas F, de
Lara EC, Chatelut E and Querleu D: Pharmacokinetics of oxaliplatin
during open versus laparoscopically assisted heated intraoperative
intraperitoneal chemotherapy (HIPEC): an experimental study. Ann
Surg Oncol. 15:339–344. 2008. View Article : Google Scholar
|
|
18
|
Di Giorgio A, Naticchioni E, Biacchi D, et
al: Cytoreductive surgery (peritonectomy procedures) combined with
hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment
of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer.
113:315–325. 2008.
|
|
19
|
Elias D, Lefevre JH, Chevalier J, et al:
Complete cytoreductive surgery plus intraperitoneal
chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of
colorectal origin. J Clin Oncol. 27:681–685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujimoto S, Shrestha RD, Kokubun M, et al:
Intraperitoneal hyperthermic perfusion combined with surgery
effective for gastric cancer patients with peritoneal seeding. Ann
Surg. 208:36–41. 1988. View Article : Google Scholar
|
|
21
|
Helm CW, Bristow RE, Kusamura S, Baratti D
and Deraco M: Hyperthermic intraperitoneal chemotherapy with and
without cytoreductive surgery for epithelial ovarian cancer. J Surg
Oncol. 98:283–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scaringi S, Kianmanesh R, Sabate JM, et
al: Advanced gastric cancer with or without peritoneal
carcinomatosis treated with hyperthermic intraperitoneal
chemotherapy: a single western center experience. Eur J Surg Oncol.
34:1246–1252. 2008. View Article : Google Scholar
|
|
23
|
Shido A, Ohmura S, Yamamoto K, Kobayashi
T, Fujimura T and Yonemura Y: Does hyperthermia induce peritoneal
damage in continuous hyperthermic peritoneal perfusion? World J
Surg. 24:507–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inadomi J, Cello JP and Koch J:
Ultrasonographic determination of ascitic volume. Hepatology.
24:549–551. 1996.
|
|
25
|
Ozkan O, Akinci D, Gocmen R, Cil B, Ozmen
M and Akhan O: Percutaneous placement of peritoneal port-catheter
in patients with malignant ascites. Cardiovasc Intervent Radiol.
30:232–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaushik N, Khalid A, Brody D and McGrath
K: EUS-guided paracentesis for the diagnosis of malignant ascites.
Gastrointest Endosc. 64:908–913. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nguyen PT and Chang KJ: EUS in the
detection of ascites and EUS-guided paracentesis. Gastrointest
Endosc. 54:336–339. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Knutsen A, Sielaff TD, Greeno E and Tuttle
TM: Staged laparoscopic infusion of hyperthermic intraperitoneal
chemotherapy after cytoreductive surgery. J Gastrointest Surg.
10:1038–1043. 2006. View Article : Google Scholar
|