Introduction
Breast cancer is a common malignancy among women and
is a leading cause of cancer-related mortality, ranking 2nd after
lung cancer (1). The incidence of
breast cancer has drastically increased over the past several
decades. The treatment of breast cancer includes surgery, radiation
and drugs (hormone therapy and chemotherapy). Unfortunately, the
poor 5-year survival rate of patients with breast cancer has not
been improved by current treatments, primarily due to the
propensity of breast cancer cells to metastasize as well as their
resistance to chemotherapy and radiation. Thus, there is an
increased interest in finding new agents for breast cancer
treatment. Cancer gene therapy represents a new and promising
therapeutic modality for cancer. It was evaluated as one of the top
10 scientific discoveries in 2009 by Science (2). Adenovirus (Ad) is one of the most
promising vectors for cancer gene therapy. Adenoviral vectors
harboring therapeutic genes have been used successfully for gene
transfer in vitro and in vivo.
ING4, a member of the inhibitor of growth (ING)
family, may significantly induce tumor growth suppression via the
induction of cell cycle alteration, apoptosis and inhibition of
tumor angiogenesis (3–8). In a previous study, we demonstrated
that the Ad-mediated ING4 gene remarkably inhibited the growth of
MDA-MB-231 breast tumors in vitro and in vivo
(9). However, cancer is
characterized by a multistep process of genetic and molecular
changes in oncogenes and tumor suppressor genes, which limits the
efficacy of a single gene-mediated cancer therapy due to the
difficulty in finding a pivotal gene conferring its occurrence and
progression. Therefore, a multigene-based combination therapy may
prove to be effective in breast cancer gene therapy.
The other tumor suppressor, interleukin-24 (IL-24),
originally identified in human melanoma cells treated with
interferon-β and mezerein by subtraction hybridization, is a unique
cytokine-tumor suppressor belonging to the IL-10 family (10,11).
Extensive studies have shown that IL-24 displays ubiquitous
antitumor properties and tumor-specific killing activity in a broad
spectrum of cancer cells but not in normal cells (12). IL-24 may also inhibit tumor
angiogenesis by directly suppressing vascular endothelial cell
differentiation and migration (13,14),
indirectly downregulating the production of pro-angiogenic factors
(13,15,16)
and by repressing tumor cell invasion and migration via the
downregulation of the phosphatidylinositol 3-kinase, focal adhesion
kinase and matrix metalloproteinase-2 (17). Thus, ING4 and IL-24, as promising
tumor suppressors negatively modulate tumor growth via multiple
pathways.
At present, there are two main methods for multigene
therapy: the first one involves the target cells being transfected
or infected with multiple independent vectors carrying different
genes simultaneously, with the advantage of conveniently adjusting
the proportion of each expression-vector combination and
co-ordination of time and the disadvantage of the efficiency of
multigene co-expression being low and onerous (18,19).
The second method involves the co-expression of multiple genes in
one identical vector (20).
Compared with a number of independent vectors carrying different
genes to achieve co-expression, a multigene co-expression vector
may increase the efficiency of transfection and expression. Since
the low efficiency of gene transfer is the bottleneck in gene
therapy, we constructed the ING4/IL-24 bicistronic Ad-mediated gene
co-transfection vector (Ad-ING4-IL-24) (21).
The therapeutic potential of the conjunction of ING4
and IL-24 for cancer has not been reported in breast cancer. To
enhance the therapeutic efficacy and develop a novel combination
therapeutic modality for breast cancer, based on the antitumor
features of ING4 and IL-24, we hypothesized that the combination
treatment with ING4 and IL-24 tumor suppressors would elicit an
enhanced antitumor efficacy. Therefore, in this study, we
investigated the potential combined effect of the ING4 and IL-24
double tumor suppressor genes (Ad-ING4-IL-24) against MDA-MB-231
human breast cancer cells in vitro and in vivo in an
athymic nude mouse model and elucidated its underlying molecular
mechanism.
Materials and methods
Vectors, cell lines, reagents and
mice
The Ad-ING4-IL-24 and Ad replication-incompetent
adenoviral vectors were constructed and maintained in our
laboratory (21). The MDA-MB-231
human breast cancer cell line was purchased from the American Type
Culture Collection (Shanghai, China) and cultured in RPMI-1640
(Gibco, Shanghai, China) supplemented with 10% fetal bovine serum
(HyClone, Shanghai, China). The reverse transcription (RT)-PCR
detection kit was purchased from Invitrogen (Shanghai, China). The
MTT kit was purchased from Sigma (Shanghai, China). The Annexin
V-PE/7-AAD apoptosis detection kit was purchased from BD
Biosciences (Shanghai, China). The In Situ Cell Death
Detection kit was purchased from Roche Applied Science (Shanghai,
China). The polyclonal anti-ING4 antibody was purchased from Abcam
(Shanghai, China). The monoclonal anti-IL-24 antibody was purchased
from R&D Systems (Shanghai, China). The antibodies specific for
p21, p27, Bcl-2, Bax, survivin and β-actin were purchased from Cell
Signaling (Shanghai, China). The Ultrasensitive™ SP kit was
purchased from Fuzhou Maixin (Fuzhou, China). The athymic nude mice
were purchased from the Shanghai Experimental Animal Center
(Shanghai, China) and maintained in the animal facility at Soochow
University with the approval of the Animal Research Ethics
Committee of Soochow University. The human IL-24 enzyme-linked
immunosorbent assay (ELISA) kit was purchased from Boster (Wuhan,
China).
Reverse transcription (RT)-PCR
analysis
The apoptosis-related genes, Bax, Bcl-2 and
survivin, in MDA-MB-231 human breast cancer cells were determined
by RT-PCR analysis. Briefly, the MDA-MB-231 cells
(5×106) were infected with Ad-ING4, Ad-IL-24,
Ad-ING4-IL-24 or Ad used as a blank control at a multiplicity of
infection (MOI) of 100 as previously described (5), or without an Ad (phosphate-buffered
saline, PBS) control. After 48 h of treatment, the infected and
uninfected MDA-MB-231 tumor cells were collected, and total
cellular RNA was extracted with TRIzol for RT-PCR. The PCR was
carried out using cDNA as templates and primers as following:
5′-GGA TGC GTC CAC CAA GAA-3′ and 5′-GCA CTC CCG CCA CAA AGA-3′ for
Bax; 5′-TGT GGC CTT CTT TGA GTT CG-3′ and 5′-CTA CCC AGC CTC CGT
TAT CC-3′ for Bcl-2; 5′-GCA TGG GTG CCC CGA CGT TG-3′ and 5′-GCT
CCG GCC AGA GGC TCA A-3′ for survivin; 5′-TGA TGA CAT CAA GAA GGT
GGT GAA-3′ and 5′-TCC TTG GAG GCC ATG TGG GCC-3′ for human GAPDH.
The RT-PCR products were then analyzed by 1% agarose gel
electrophoresis.
Detection of cytotoxicity by crystal
violet staining
The MDA-MB-231 human breast cancer cells were
planted in a 24-well plate and 24 h later cells were infected with
Ad, Ad-ING4, Ad-IL-24 and Ad-ING4-IL-24, respectively at various
MOIs (0, 10, 25, 50, 100 and 200). After a continuous incubation
for 4 days at 37°C, the medium was removed and 500 μl crystal
violet solutions (2% crystal violet in 20% methanol) were added to
each well for staining; and 15 min later all wells were washed and
images were captured.
MTT assay
The in vitro cytotoxic effect of
Ad-ING4-IL-24 on MDA-MB-231 human breast cancer cells was evaluated
by MTT assay. Briefly, the MDA-MB-231 tumor cells were dispensed
into 96-well culture plates at 1×104 cells/well. After a
24-h incubation at 37°C, the MDA-MB-231 tumor cells were infected
with Ad-ING4, Ad-IL-24, Ad-ING4-IL-24 or Ad, used as a blank
control at 100 MOI or without Ad (PBS control) and cultured for the
indicated time periods (0–4 days). Before treatment and at
different time-points after treatment, the viability of the
MDA-MB-231 tumor cells was analyzed using an MTT kit according to
the manufacturer’s instructions. Inhibition ratios were calculated
using the following formula: inhibition ratio = [optical density
(OD)570 of control group - OD570 of
experimental group]/OD570 of control group.
Flow cytometric analysis of
apoptosis
The MDA-MB-231 human breast cancer cells
(1×106) were cultured with Ad-ING4, Ad-IL-24,
Ad-ING4-IL-24 or Ad at 100 MOI or without an Ad (PBS control),
respectively. After 48 h, the treated and untreated MDA-MB-231
tumor cells were harvested, washed in cold PBS and apoptosis was
assessed by flow cytometric analysis using the Annexin V-PE/7-AAD
apoptosis detection kit following the manufacturer’s instructions.
Briefly, the treated and untreated MDA-MB-231 tumor cells
(1×106) were incubated with 5 μl Annexin V-PE (early
apoptotic marker) and 5 μl 7-AAD (late apoptotic marker) in 100 μl
of 1X Annexin V binding buffer at room temperature. After a 15-min
incubation, 400 μl of 1X binding buffer were added and the
apoptotic cells were then analyzed by flow cytometry. Another
section of the cells in each group were also fixed with cold 70%
ethanol for 12 h and then the cell cycles in each group were
detected by flow cytometry after propidium iodide (PI)
staining.
ELISA analysis
The Ad-mediated secretory expression of vascular
endothelial growth factor (VEGF) in MDA-MB-231 human breast cancer
cells was detected by ELISA analysis. Briefly, the MDA-MB-231 tumor
cells (5×106) were infected with Ad-ING4, Ad-IL-24,
Ad-ING4-IL-24 or Ad used as a blank control at 100 MOI or without
an Ad (PBS control) in a 10-ml medium, respectively. After 24 h of
treatment, the cellular culture supernatants generated from
Ad-ING4, Ad-IL-24, Ad-ING4-IL-24 or Ad-infected and uninfected
MDA-MB-231 tumor cells were harvested, and the amount of VEGF in
the culture supernatants was analyzed by ELISA using a human VEGF
ELISA kit according to the manufacturer’s instructions.
Animal studies
The athymic nude mice (5/group) were subcutaneously
(s.c.) inoculated on their armpits of the right anterior limbs with
2×106 MDA-MB-231 human breast cancer cells, and then
monitored daily for tumor growth. Tumor volume was measured with a
caliper and calculated by the formula, tumor size =
ab2/2, where ‘a’ is the larger and ‘b’ is the smaller of
the 2 dimensions. When the tumors grew up to a mean tumor volume of
~0.1 cm3, the MDA-MB-231 human breast cancer xenografted
tumor-bearing mice were intratumorally (i.t.) injected with PBS
(PBS control) or 1×108 gene transfer unit (GTU) of
Ad-ING4, Ad-IL-24, Ad-ING4-IL-24 and Ad every other day for a total
of 6 times, respectively. Tumor progression and regression were
monitored and tumor volume was measured daily. In addition, the
tumor-bearing mice were sacrificed 12 days after the treatments and
the xenografted tumors were removed, weighed, fixed by 10% neutral
formalin and embedded in paraffin for hematoxylin & eosin
(H&E) staining and immunohistochemical analysis. The tumor
inhibition rate was calculated using the formula: inhibition rate =
(tumor weight of PBS group - tumor weight of therapy group)/tumor
weight of PBS group ×100%.
Immunohistochemistry analysis
The expression of p21, p27, Bcl-2, Bax, survivin,
VEGF and CD34 in MDA-MB-231 human breast cancer s.c. xenografted
tumors was determined by immunohistochemistry analysis using an
Ultrasensitive™ SP kit according to the manufacturer's
instructions. The presence of buffy or brown diaminobenzidine
precipitates is indicative of positive reactivity. The integral
optical density (IOD) of immunohistochemical intensity was then
calculated using Image-Pro Plus 6.0 software. Microvessel density
(MVD), detected by immunostaining for CD34, was determined as
previously described by Weidner (22). Any endothelial cell cluster
immunoreactive for CD34 clearly separated from adjacent
microvessels was considered as a single countable vessel. Each
value represents IOD or microvessels counted at a high-power view
(x400) by a microscope. The mean value represents the average
number derived from the 5 high-power fields of each case.
Evaluation of combinatorial
interaction
The interactive effects of ING4 and IL-24 by
Ad-mediated ING4 and IL-24 co-expression were evaluated by
calculating the Q-value using the formula (23), Q = F (A + B)/FA + (1 - FA) FB, where
F (A + B) represents the fraction affected by treatment with
Ad-ING4-IL-24 compared to the untreated control group, FA
represents the fraction affected by Ad-ING4 alone, and FB
represents the fraction affected by Ad-IL-24 alone. A value of
Q>1.15 indicates a synergistic effect between ING4 and IL-24,
Q<0.85 indicates an antagonistic effect and a vaule of Q between
0.85 and 1.15 indicates an additive effect.
Statistical analysis
All data are presented as the means ± standard
deviation (SD). The significance of the difference between the
groups was evaluated by one-way and two-way repeated measures
analysis of variance (ANOVA) and multiple comparisons with SPSS
10.0 software. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Cytotoxicity induced by tumor suppressor
genes
MDA-MB-231 human breast cancer cells infected with
Ad-ING4-IL-24 in the 24-well plate were more slightly stained by
crystal violet than the cells infected with Ad-GFP, Ad-ING4 or
Ad-IL-24 at a same MOI (Fig. 1).
The results indicated that Ad-ING4-IL-24 are more highly toxic to
MDA-MB-231 tumor cells than Ad-ING4, Ad-IL-24 or Ad-GFP. Moreover,
even at 100 MOI the blank virus vector of Ad-GFP had no obvious
cytotoxic effect on MDA-MB-231 tumor cells.
Enhanced tumor suppression by ING4 and
IL-24 co-expression
To investigate whether the ING4 plus IL-24
combination treatment elicits enhanced antitumor effects, we
co-expressed ING4 and IL-24 double tumor suppressor genes by the
ING4/IL-24 bicistronic Ad-mediated gene co-transfer and evaluated
the combined effect on MDA-MB-231 human breast cancer cells. The
MDA-MB-231 tumor cells were infected with Ad-ING4, Ad-IL-24,
Ad-ING4-IL-24 or Ad at 100 MOI, respectively. The in vitro
cell viability was examined daily for 4 days before and after
treatment using MTT assay. As shown in Fig. 2A, compared with the Ad and PBS
control group, Ad-mediated ING4 and/or the IL-24 expression
significantly suppressed MDA-MB-231 human breast cancer cell growth
in vitro in a time-dependent manner with a peak inhibition
on day 4 after infection (P<0.05). Interestingly, the
combination treatment of ING4 and IL-24 co-expression induced a
more significant and synergistic inhibition on the growth of
MDA-MB-231 tumor cells compared with the Ad-ING4- and
Ad-IL-24-treated group, with inhibition ratios of 32, 30 and 62% on
day 4 in the Ad-ING4, Ad-IL-24 and Ad-ING4-IL-24 groups,
respectively (P<0.05; Q=1.21). To further explore whether the
combination of ING4 with IL-24 results in enhanced antitumor
efficacy in vivo, the athymic nude mice (5 mice/group)
bearing MDA-MB-231 human breast cancer s.c. xenografted tumors were
i.t. injected with Ad-ING4, Ad-IL-24, Ad-ING4-IL-24 or Ad
(1×108 GTU) every other day for a total of 6 times. The
tumor volumes were monitored every other day (Fig. 2B) and xenografted tumors were
removed (Fig. 2C) at 12 days after
treatment and the tumor weight (Fig. 2D
and E) was measured. Compared with the Ad-ING4- and
Ad-IL-24-treated group, the growth of MDA-MB-231 human breast
cancer xenografted tumors in nude mice was more significantly and
synergistically retarded in the Ad-ING4-IL-24-treated group
(P<0.05; Qvolume=1.29), indicating that Ad-ING4-IL-24
administration may also remarkably suppress in vivo
MDA-MB-231 human breast cancer s.c. xenografted tumor growth in an
athymic nude mouse model with a synergistic effect.
 | Figure 2Ad-ING4-IL-24 enhanced tumor
suppression in MDA-MB-231 human breast cancer cells. (A) The in
vitro cytotoxic effect of Ad-ING4-IL-24 on MDA-MB-231 human
breast cancer cells. The MDA-MB-231 human breast cancer cells were
treated with Ad-ING4, Ad-IL-24, Ad-ING4-IL-24 or Ad-GFP used as an
Ad control at the optimal MOI of 100 or PBS as the control for the
indicated time periods (0–4 days), respectively. The survival cells
were evaluated at days 0, 1, 2, 3 and 4 after treatment by MTT
assay. *P<0.05 compared with the PBS and Ad-GFP
groups; #P<0.05 compared with the Ad-ING4 and
Ad-IL-24 groups (Q=1.21 on day 4 after treatment), two-way repeated
measures ANOVA and multiple comparisons, n=4 replicates/condition.
(B) The MDA-MB-231 human breast cancer xenografted tumor volume
before and after treatment. Xenografted tumors were removed at 12
days after treatment and measured. *P<0.05 compared
with the PBS and Ad-GFP groups; #P<0.05 compared with
the Ad-ING4 and Ad-IL-24 groups, one-way and two-way repeated
measures ANOVA and multiple comparisons, n=5 mice/condition. Data
shown are representative of 3 independent experiments. (C) Tumor
masses removed from each group. (D) The MDA-MB-231 human breast
cancer xenografted tumor weight was measured. *P<0.05
compared with the PBS and Ad-GFP groups; #P<0.05
compared with the Ad-ING4 and Ad-IL-24 groups. One-way and two-way
repeated measures ANOVA and multiple comparisons, n=5
mice/condition. Data shown are representative of 3 independent
experiments. (E) Tumor inhibition rate of MDA-MB-231 human breast
cancer xenografted tumors in each treatment group.
*P<0.05 compared with the Ad-ING4 and Ad-IL-24
groups, Q=1.29. One-way and two-way repeated measures ANOVA and
multiple comparisons, n=5 mice/condition. Data shown are
representative of 3 independent experiments. |
Enhanced apoptosis by ING4 and IL-24
co-expression
To explore the mechanism by which Ad-ING4-IL-24
synergistically inhibits MDA-MB-231 tumor cell growth, the
apoptosis of MDA-MB-231 human breast cancer cells treated with
Ad-ING4, Ad-IL-24, Ad-ING4-IL-24 or Ad (100 MOI) for 48 h was
analyzed using Annexin V-PE (early apoptotic marker) and 7-AAD
(late apoptotic marker) double staining by flow cytometry. As shown
in Fig. 3, Ad-ING4-IL-24 treatment
resulted in 39.18±5.08% of MDA-MB-231 tumor cell apoptosis, whereas
there was 2.89, 3.92, 22.63±3.16 and 15.8±3.12% of apoptotic
MDA-MB-231 tumor cells found in the cells grown in the medium with
PBS, Ad, Ad-ING4 and Ad-IL-24, respectively.
Ad-ING4-IL-24 co-operatively regulates
apoptotic pathways
In order to further address the underlying molecular
mechanism responsible for Ad-ING4-IL-24-promoting apoptosis, the
transcriptions and expression levels of the apoptosis-related genes
including Bax, Bcl-2, and survivin in Ad-ING4-IL-24-, Ad-ING4-,
Ad-IL-24- or Ad-treated and untreated MDA-MB-231 human breast
carcinoma cells were analyzed by RT-PCR (in vitro) and
immunohistochemistry for tumor tissues. The transcription and
expression of Bax in the Ad-ING4, Ad-IL-24 and Ad-ING4-IL-24 groups
were significantly increased, and the transcription and expression
of Bcl-2 in the Ad-ING4 and Ad-ING4-IL-24 groups were decreased.
The transcription and expression of survivin in the Ad-ING4,
Ad-IL-24 and Ad-ING4-IL-24 groups were decreased (Fig. 4).
 | Figure 4Ad-ING4-IL-24 modulates
apoptosis-related molecules. (A) The transcription of Bax, Bcl-2
and survivin in MDA-MB-231 human breast cancer cells was detected
by RT-PCR. Lane 1, PBS; lane 2, Ad-GFP; lane 3, Ad-ING4; lane 4,
Ad-IL-24; lane 5, Ad-ING4-IL-24. (B) Semiquantitative analysis of
these gene transcriptions, *P<0.05 compared with the
PBS and Ad-GFP groups; △P<0.05 compared with the
Ad-ING4 and Ad-IL-24 groups (Q=1.17), one-way repeated measures
ANOVA and multiple comparisons, n=3 replicates/condition. (C)
Representative immunohistochemical images for Bax, Bcl-2 and
survivin in MDA-MB-231 human breast carcinoma xenografted tumors.
The MDA-MB-231 human breast carcinoma s.c. xenografted tumors were
maintained in 10% neutral formalin and embedded in paraffin. Tissue
sections were then stained with Bax, Bcl-2 and survivin (ING4 and
IL-24 were maintained as the control) by immunohistochemistry using
an Ultrasensitive™ SP kit and examined under a microscope (x400).
(D) The IOD of Bax, Bcl-2 and survivin immunohistochemical
intensity was quantified by Image-Pro Plus 6.0 software.
*P<0.05 compared with the Ad-GFP and PBS groups;
#P<0.05 compared with the Ad-ING4 and Ad-IL-24
groups, one-way repeated measures ANOVA and multiple comparisons,
n=5 replicates/condition, n=5 observations/representative
section. |
Enhanced cell cycle arrest by ING4 and
IL-24 co-expression
To explore the potential mechanism by which
Ad-ING4-IL-24 suppresses tumor growth, the cell cycle conditions of
the MDA-MB-231 human breast carcinoma cells treated with PBS, Ad,
Ad-ING4, Ad-IL-24 and Ad-ING4-IL-24 for 48 h were further analyzed
using PI staining by flow cytometry. As shown in Fig. 5A and B, the G2/M phase percentage of
MDA-MB-231 tumor cells was 32.36±3.62% in group Ad-ING4-IL-24,
whereas the percentages were 11.61±1.26, 13.52±1.35, 22.52±3.28 and
26.24±2.86%, in the MDA-MB-231 tumor cells grown in medium with
PBS, Ad, Ad-ING4 and Ad-IL-24, respectively. To further address the
underlying molecular mechanism responsible for Ad-ING4-IL-24
inducing cell cycle arrest, the cell cycle-related proteins, p21
and p27, in the Ad-ING4-IL-24-, Ad-ING4-, Ad-IL-24- or Ad-treated
and untreated MDA-MB-231 human breast carcinoma cells were analyzed
by immunohistochemistry for tumor tissues. The expression of p21 in
the Ad-ING4, Ad-IL-24 and Ad-ING4-IL-24 groups, and the expression
levels of p27 in the Ad-IL-24 and Ad-ING4-IL-24 groups were
significantly increased (Fig. 5C and
D).
Ad-ING4-IL-24 additively reduces tumor
angiogenesis
Previous studies have provided evidence that either
ING4 or IL-24 may suppress tumor growth by inhibiting angiogenesis
(5,24). To examine the combined effect of
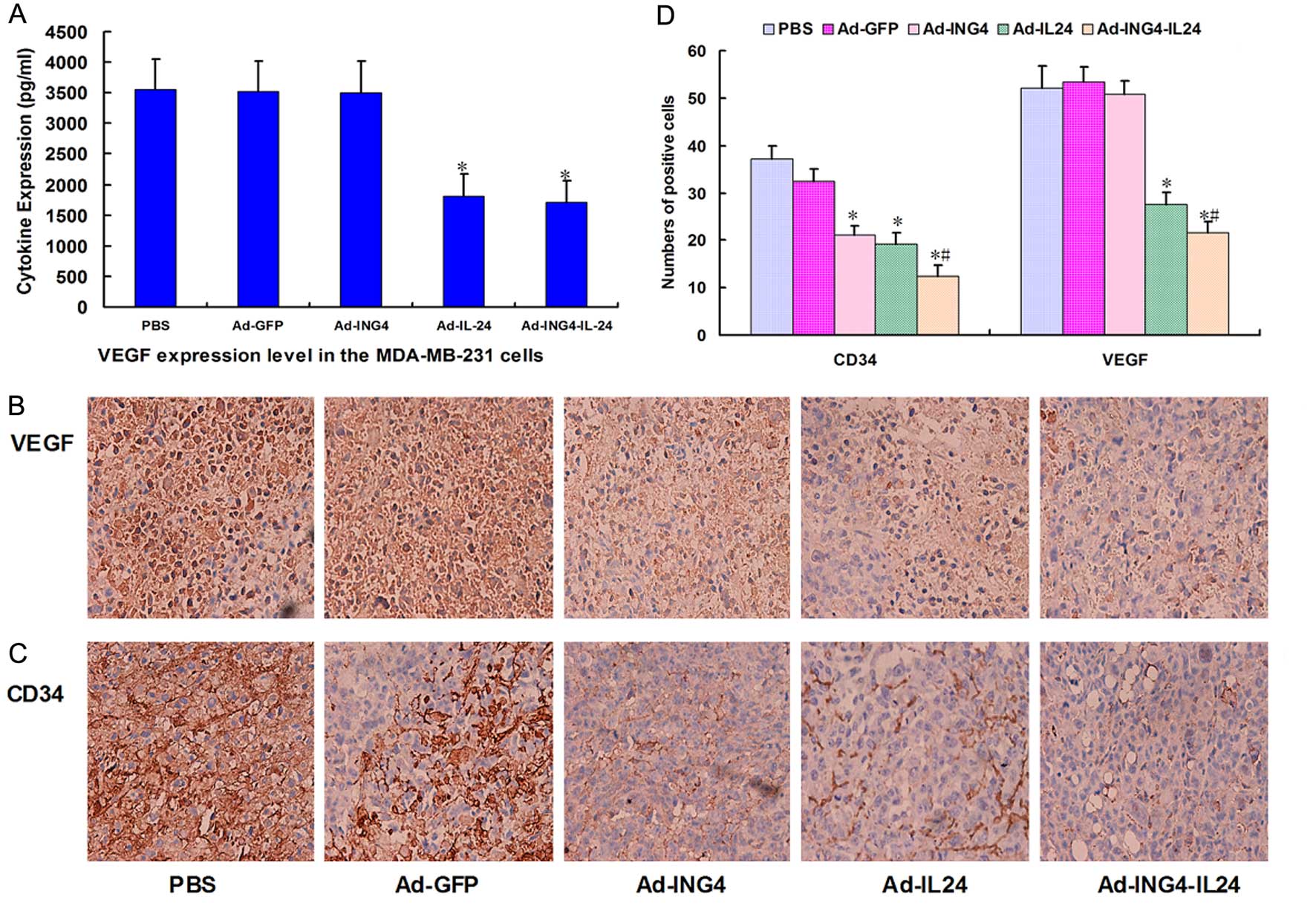
Ad-mediated ING4 and IL-24 co-expression on tumor angiogenesis, we
detected the concentrations of VEGF in the supernatants of
Ad-ING4-IL-24-, Ad-ING4-, Ad-IL-24- or Ad-treated and untreated
MDA-MB-231 human breast cancer cells by ELISA analysis. The
concentrations of VEGF in the Ad-IL-24 and Ad-ING4-IL-24 groups
also significantly decreased compared with the PBS and Ad groups
(Fig. 6A). The expression levels of
VEGF in the Ad-ING4-IL-24, Ad-ING4, Ad-IL-24 or Ad-treated and
untreated MDA-MB-231 human breast cancer s.c. xenografted tumors
were analyzed by immunohistochemical analysis. The expression
levels of VEGF in the Ad-IL-24 and Ad-ING4-IL-24 groups were
significantly decreased compared with the PBS and Ad groups
(Fig. 6B and D). The MVD in the
MDA-MB-231 human breast cancer s.c. xenografted tumors was
calculated on the basis of CD34 immunostaining. The CD34-positive
staining was mainly presented as brownish yellow or brownish
granules in vascular endothelial cells of MDA-MB-231 breast
carcinoma xenografted tumors (Fig.
6C). Compared with the PBS and Ad control groups, the CD34
expression of vascular endothelial cells in the Ad-ING4, Ad-IL-24
and Ad-ING4-IL-24 groups was weaker or less (Fig. 6C and D; P<0.05), indicating that
the Ad-mediated ING4 and/or IL-24 treatment downregulated the CD34
expression in MDA-MB-231 breast carcinoma xenografted tumor
vessels.
Discussion
Multigene-based combination therapy represents an
effective practice in cancer gene therapy, which may achieve
greater therapeutic benefits by targeting multiple pathways
(25). Recent studies have reported
that ING4 as a novel tumor suppressor plays an important role in
many cancer-related cellular processes including oncogenesis, cell
cycle regulation, apoptosis, DNA damage response, invasion and
migration, contact inhibition and tumor angiogenesis, implying that
it is a potent tumor suppressor for cancer therapy (8). Subsequent studies have demonstrated
that another tumor suppressor, IL-24, as a cytokine-tumor
suppressor can discriminate between normal and tumor cells, induce
apoptosis, inhibit tumor angiogenesis, stimulate immune responses,
promote bystander antitumor activity, and synergize with anticancer
drugs and radiation, suggesting that it is also an effective agent
for cancer treatment (10,11). However, the therapeutic effect of
the combination treatment of ING4 and IL-24 for cancer, remains
unreported. Based on the antitumor properties of ING4 and IL-24, we
speculated that the combination of ING4 and IL-24 double tumor
suppressors would exert enhanced tumor suppression. In this study,
we constructed an ING4/IL-24 biscistronic Ad harboring the ING4 and
IL-24 double tumor suppressor genes (Ad-ING4-IL-24) and evaluated
its combined therapeutic effect on MDA-MB-231 human breast cancer
cells in vitro and MDA-MB-231 human breast cancer s.c
xenografted tumors in vivo in an athymic nude mouse model
using Ad-mediated ING4 and IL-24 co-transfer. We demonstrated that
the combination treatment of Ad-mediated ING4 and IL-24
co-expression induced in vitro synergistic growth
suppression and apoptosis in MDA-MB-231 human breast cancer cells.
Moreover, Ad-ING4-IL-24 also synergistically inhibited MDA-MB-231
human breast cancer xenografted tumor growth in vivo in
athymic nude mice.
Genes regulating apoptosis may be divided into
apoptosis-related oncogenes and anti-oncogenes. The former promote
apoptosis (Bax), and the latter are anti-apoptotic (Bcl-2 and
survivin). The ratios between Bcl-2/Bax heterodimers and Bax/Bax
homodimers appear to be pivotal in deciding the life or death of a
cell (26). Bcl-2/Bax constitutes a
rheostat that sets the threshold of susceptibility to apoptosis
(27). Survivin inhibits apoptosis
by interacting with cyclin kinase CDK4, p34, CDC2 and blocking
apoptotic signal transduction. Survivin is rarely expressed in
normal adult tissues but displays a weak expression in the placenta
and thymus (28); however, it is
widely expressed in tumors. A high expression of survivin in cancer
is closely related to malignant progression, poor prognosis, tumor
recurrence and drug resistance (29). p21 and p27, important members of CDK
inhibitors belonging to the Cip/Kip family, inhibit cyclin E-CDK2,
cyclin A-CDK2, cyclin D-CDK4 and cyclin B1/CDC2 complexes leading
to G1 and G2/M arrest (30–33).
To elucidate the underlying mechanism involved in
Ad-ING4-IL-24-mediated synergistic antitumor activity, the in
vitro transcription and in vivo expression of
apoptosis-related proteins, such as Bcl-2, Bax and survivin in
MDA-MB-231 human breast cancer xenografted tumors were assessed by
RT-PCR and immunohistochemical analysis. Evidence from in
vitro and in vivo experiments demonstrated that
Ad-mediated ING4 and IL-24 co-expression elicited a co-operative
and overlapping effect on the upregulation of the apoptosis
promoting gene, Bax, and the downregulation of the anti-apoptotic
genes, Bcl-2 and survivin, in MDA-MB-231 human breast cancer cells
or tumor tissues. These results may closely account for the
Ad-ING4-IL-24-induced synergistic growth inhibition and apoptosis
in MDA-MB-231 tumor cells and xenografted tumors.
To uncover other mechanisms of tumor growth
inhibition by Ad-ING4-IL-24, the cell cycle conditions of
MDA-MB-231 human breast carcinoma cells in different groups were
analyzed by flow cytometry. The results showed an obvious G2/M cell
cycle arrest in the Ad-ING4 and Ad-IL-24 groups and an overlapping
effect in the Ad-ING4-IL-24 group. To explore the potential
molecular mechanism, the present study assessed the cell
cycle-related molecules, p21 and p27, in MDA-MB-231 human breast
cancer s.c. xenografted tumors by immunohistochemical analysis. The
results showed that the expression levels of p21 and p27 both
significantly increased in the Ad-ING4-IL-24 group, which indicated
that the Ad-mediated co-expression of ING4 and IL-24 induced G2/M
arrest and inhibited the cell cycle of MDA-MB-231 human breast
cancer cells by stimulating the expression of the cell cycle
inhibitors, p21 and p27.
In addition, the progressive growth and metastasis
of solid tumors are dependent on the process of angiogenesis. It
has been shown that ING4 suppresses tumor angiogenesis via the
downregulation of IL-8 and osteopontin pro-angiogenic factors by
inhibiting the activity of nuclear factor κB and hypoxia-inducible
factor-1α (8,24). It has also been reported that IL-24
inhibits tumor angiogenesis via directly interacting with the
IL-22R1/IL-20R2 heterodimeric receptor in vascular endothelial
cells (14) and indirectly reducing
pro-angiogenic factor production (13–15).
Animal experimental studies showed that the
Ad-mediated ING4 and IL-24 tumor suppressor gene co-transfer
reduced tumor volumes significantly, while the tumor inhibition
rate of the Ad-ING4-IL-24 group reached 76.5±7.4% (Q=1.29),
demonstrating the synergistic effect of ING4 and IL-24 in
vivo. In addition to the promotion of apoptosis and the
inhibition of the cell cycle, whether or not the suppression of
tumor angiogenesis is also an underlying mechanism involved in the
Ad-ING4-IL-24-mediated synergistic antitumor activity, remains
unclear. In order to clarify this, we detected the MVD of tumor
tissues in each group on the basis of CD34 immunostaining, and the
expression levels of VEGF, a key stimulator of vessel formation
(34), by ELISA in vitro and
immunostaining in vivo. We further found that Ad-ING4-IL-24
additively downregulated CD34 and VEGF expression and decreased MVD
in MDA-MB-231 human breast cancer xenografted tumors, which may be
another important mechanism involved in the Ad-ING4-IL-24-mediated
in vivo enhanced growth inhibition of MDA-MB-231 human
breast cancer xenografted tumors in an athymic nude mouse
model.
Acknowledgements
The current research was supported by grants from
the National Natural Science Foundation of China (nos. 81001016;
81101909).
Abbreviations:
|
Ad
|
adenovirus
|
|
GTU
|
gene transfer unit
|
|
IL-24
|
interleukin 24
|
|
ING4
|
inhibitor of growth 4
|
|
IOD
|
integral optical density
|
|
MOI
|
multiplicity of infection
|
|
MVD
|
microvessel density
|
|
OD
|
optical density
|
|
PBS
|
phosphate-buffered saline
|
|
RT
|
reverse transcription
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Cartier N, Hacein-Bey-Abina S, Bartholomae
CC, et al: Hematopoietic stem cell gene therapy with a lentiviral
vector in X-linked adrenoleukodystrophy. Science. 326:818–823.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shiseki M, Nagashima M, Pedeux RM, et al:
p29ING4 and p28ING5 bind to p53 and p300, and enhance p53 activity.
Cancer Res. 63:2373–2378. 2003.PubMed/NCBI
|
|
4
|
Zhang X, Xu LS, Wang ZQ, et al: ING4
induces G2/M cell cycle arrest and enhances the chemosensitivity to
DNA-damage agents in HepG2 cells. FEBS Lett. 570:7–12. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie Y, Zhang H, Sheng W, Xiang J, Ye Z and
Yang J: Adenovirus-mediated ING4 expression suppresses lung
carcinoma cell growth via induction of cell cycle alteration and
apoptosis and inhibition of tumor invasion and angiogenesis. Cancer
Lett. 271:105–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai L, Li X, Zheng S, et al: Inhibitor of
growth 4 is involved in melanomagenesis and induces growth
suppression and apoptosis in melanoma cell line M14. Melanoma Res.
19:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie YF, Sheng W, Xiang J, Zhang H, Ye Z
and Yang J: Adenovirus-mediated ING4 expression suppresses
pancreatic carcinoma cell growth via induction of cell-cycle
alteration, apoptosis, and inhibition of tumor angiogenesis. Cancer
Biother Radiopharm. 24:261–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Colla S, Tagliaferri S, Morandi F, et al:
The new tumor-suppressor gene inhibitor of growth family member 4
(ING4) regulates the production of proangiogenic molecules by
myeloma cells and suppresses hypoxia-inducible factor-1 alpha
(HIF-1alpha) activity: involvement in myeloma-induced angiogenesis.
Blood. 110:4464–4475. 2007. View Article : Google Scholar
|
|
9
|
Li Z, Xie Y, Sheng W, Miao J, Xiang J and
Yang J: Tumor-suppressive effect of adenovirus-mediated inhibitor
of growth 4 gene transfer in breast carcinoma cells in vitro and in
vivo. Cancer Biother Radiopharm. 25:427–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang H, Lin JJ, Su ZZ, Goldstein NI and
Fisher PB: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.
|
|
11
|
Sauane M, Gopalkrishnan RV, Sarkar D, et
al: MDA-7/IL-24: novel cancer growth suppressing and apoptosis
inducing cytokine. Cytokine Growth Factor Rev. 14:35–51. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fisher PB: Is mda-7/IL-24 a ‘magic bullet’
for cancer? Cancer Res. 65:10128–10138. 2005.
|
|
13
|
Saeki T, Mhashilkar A, Swanson X, et al:
Inhibition of human lung cancer growth following
adenovirus-mediated mda-7 gene expression in vivo. Oncogene.
21:4558–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramesh R, Mhashilkar AM, Tanaka F, et al:
Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a
novel ligand that regulates angiogenesis via the IL-22 receptor.
Cancer Res. 63:5105–5113. 2003.PubMed/NCBI
|
|
15
|
Nishikawa T, Ramesh R, Munshi A, Chada S
and Meyn RE: Adenovirus-mediated mda-7 (IL-24) gene therapy
suppresses angiogenesis and sensitizes NSCLC xenograft tumors to
radiation. Mol Ther. 9:818–828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inoue S, Branch CD, Gallick GE, Chada S
and Ramesh R: Inhibition of Src kinase activity by Ad-mda7
suppresses vascular endothelial growth factor expression in
prostate carcinoma cells. Mol Ther. 12:707–715. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramesh R, Ito I, Gopalan B, Saito Y,
Mhashilkar AM and Chada S: Ectopic production of MDA-7/IL-24
inhibits invasion and migration of human lung cancer cells. Mol
Ther. 9:510–518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pei Z, Chu L, Zou W, et al: An oncolytic
adenoviral vector of Smac increases antitumor activity of TRAIL
against HCC in human cells and in mice. Hepatology. 39:1371–1381.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen Y, Muramatsu SI, Ikeguchi K, et al:
Triple transduction with adeno-associated virus vectors expressing
tyrosine hydroxylase, aromatic-L-amino-acid decarboxylase, and GTP
cyclohydrolase I for gene therapy of Parkinson’s disease. Hum Gene
Ther. 11:1509–1519. 2000.
|
|
20
|
Ngoi SM, Chien AC and Lee CG: Exploiting
internal ribosome entry sites in gene therapy vector design. Curr
Gene Ther. 4:15–31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheng WH, Xie YF, Miao JC, et al: The
anti-tumor effect by adenovirus-mediated ING4 and IL-24
co-expression on hepatocellular carcinoma in vitro. Chin J
Microbiol Immunol. 30:695–703. 2010.
|
|
22
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar
|
|
23
|
Wang W, Qin SK, Chen BA and Chen HY:
Experimental study on antitumor effect of arsenic trioxide in
combination with cisplatin or doxorubicin on hepatocellular
carcinoma. World J Gastroenterol. 7:702–705. 2001.PubMed/NCBI
|
|
24
|
Garkavtsev I, Kozin SV, Chernova O, et al:
The candidate tumour suppressor protein ING4 regulates brain tumour
growth and angiogenesis. Nature. 428:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson DR: Viral-mediated gene transfer
for cancer treatment. Curr Pharm Biotechnol. 3:151–164. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meijerink JP, Smetsers TF, Slöetjes AW,
Linders EH and Mensink EJ: Bax mutations in cell lines derived from
hematological malignances. Leukemia. 9:1828–1832. 1995.PubMed/NCBI
|
|
27
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adida C, Crotty PL, McGrath J, Berrebi D,
Diebold J and Altieri DC: Developmentally regulated expression of
the novel cancer anti-apoptosis gene survivin in human and mouse
differentiation. Am J Pathol. 152:43–49. 1998.PubMed/NCBI
|
|
29
|
AItieri DC: The molecular basis and
potential role of survivin in cancer diagnosis and therapy. Trends
Mol Med. 7:542–547. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Niculescu AB III, Chen X, Smeets M, Hengst
L, Prives C and Reed SI: Effects of p21(Cip1/Waf1) at both the G1/S
and the G2/M cell cycle transitions: pRb is a critical determinant
in blocking DNA replication and in preventing endoreduplication.
Mol Cell Biol. 18:629–643. 1998.PubMed/NCBI
|
|
32
|
Smits VA, Klompmaker R, Vallenius T,
Rijksen G, Mäkela TP and Medema RH: p21 inhibits Thr161
phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. J
Biol Chem. 275:30638–30643. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toyoshima H and Hunter T: p27, a novel
inhibitor of G1 cyclin-Cdk protein kinase activity, is related to
p21. Cell. 78:67–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petrova TV, Makinen T and Alitalo K:
Signaling via vascular endothelial growth factor receptors. Exp
Cell Res. 253:117–130. 1999. View Article : Google Scholar : PubMed/NCBI
|