Introduction
Gastric cancer is the second most common cause of
cancer mortality worldwide (1–3).
Although the molecular mechanisms of gastric cancer are still
unclear, some molecular changes in the development of tumor were
characterized. In recent studies, TFF1 is a spotlight molecule in
gastric cancer. TFF1 is a 60-amino acid, highly conserved protein
with a clover leaf-like structure formed by cysteine disulphide
bonds and belongs to the trefoil factor family (4–6). TFF1
was found abundantly expressed in normal gastric mucosa. As a
secreted protein, TFF1 protein was synthesized and secreted by the
gastric epithelial cells and expressed in the upper part of the
pits. In ulceration or inflammatory diseases, TFF1 induced
epithelial restitution after injury and protects the integrity of
the epithelial barrier (7–10). TFF1 was also involved in gastric
carcinogenesis. In most gastric adenocarcinomas, the expression of
TFF1 was downregulated or absent comparing to abundant in normal
gastric mucosa (11,12). A previous study found that the
TFF1-deficient mice were dysfunctional and showed severe
hyperplasia and dysplasia, and all homozygous mutant mice developed
antropyloric adenoma (13).
Moreover, TFF1 demonstrated inhibition of cell growth and arrest of
cell difference in gastric cancer (14,15).
Thus, TFF1 is hypothesized as a tumor suppressor in gastric cancer.
TFF1 was also involved in other human cancers such as breast,
pancreas and prostate cancer, but TFF1 stimulates cancer cell
poliferation and migration in these cancers (16–18).
Since the biological effects are not clear in different cancers,
the role of TFF1 should be further addressed in gastric cancer.
GKN1, which contains a conserved BRICHOS domain, is
a secreted protein belonging to gastrokine family (19). It is abundantly expressed in gastric
epithelium (19,20). GKN1 plays a protective role in
gastric mucosa but is downregulated in gastric cancer (21,22).
In other words, GKN1 and TFF1 were similar in both the expressed
location and physical functions. GKN2, a number of gastrokine
family, also contains a BRICHOS domain and is a downstream gene of
GKN1 (23). Several studies found
that GKN2 combined with TFF1 as a heterodimer to maintain the
stabilization of mucosa (24–26).
GKN1 and GKN2 showed remarkable similarity in protein level
(24). Thus, it is reasonable to
speculate that there may be some relationships between GKN1 and
TFF1 in protein or functional level.
In the present study, we investigated the role of
TFF1 and the relationships between TFF1 and GKN1 in gastric
cancer.
Materials and methods
Tissue specimens
Normal gastric mucosa tissues were collected from 20
recruited healthy volunteers by endoscopy biopsy. Tissues of
gastric dysplasia were collected from 40 patients by endoscopy
biopsy. And tissues of gastric tumors and their corresponding
adjacent non-tumor tissues were collected from 39 gastric cancer
patients who underwent surgery (Table
I). None of the gastric cancer patients received preoperative
chemotherapy or radiotherapy. Written informed consent was signed
by all participants, and the research was approved by our
Institutional Review Board. All tissue specimens were diagnosed by
pathologists and confirmed by an experienced pathologist (27).
 | Table IStudy population. |
Table I
Study population.
| Histological
type | No. of patients | Gender | Age (years) Mean ±
SD |
|---|
|
|---|
| Male | Female |
|---|
| Normal gastric
mucosa | 20 | 10 | 10 | 44.6±12.7 |
| Gastric
dysplasia | 40 | 30 | 10 | 64.0±11.4 |
| Gastric cancer | 39 | 23 | 16 | 53.0±10.0 |
Gastric cancer cell lines
Seven gastric cancer cell lines, MKN28, MKN45, AGS,
NCI-N-87, SNU 1, SNU16 and KATO, were obtained from the Riken Cell
Bank (Tsukuba, Japan) or the American Type Culture Collection
(Manassas, VA, USA). Cells were cultured in RPMI-1640 medium
containing 10% fetal bovine serum (Hyclone, Logan, USA), and
maintained at 37°C in a humidified 5% CO2
atmosphere.
RNA isolation and RT-PCR
Gastric cancer tissue specimens and gastric cancer
cell lines were homogenized with an ultrasound homogenizer. Total
RNA from tissues and tumor cells was isolated using the Qiagen
RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the
manufacturer’s instructions. After quantification, RNA was reverse
transcribed into cDNA using ReverTra Ace™ Kit (Toyobo, Osaka,
Japan). The newly synthesized cDNA was then amplified by PCR with
specific primers for the TFF1 gene (5′-CACCATGGAGAACAAGGTGA-3′ and
5′-GGGACGTCGATGGTATTAGG-3′) or β-actin. PCR products were
subsequently electrophoresed on a 1.5% agarose gel.
Immunohistochemistry
Paraffin sections (4 μm thick) were prepared. In
short, paraffin sections were deparaffinised and rehydrated. After
antigen retrieval with citrate, endogenous peroxidase was quenched
with H2O2. The sections were then incubated
with mouse anti-human TFF1 (1:200) (Abnova, Taipei, R.O.C.) at room
temperature for 2 h. Then, a 2-step detection method was used
according to the manufacturer’s instructions (Envision™ Detection
kit, Gene Tech, Shanghai, China) by incubation of the tissue with
the ChemMate™ EnVision™/HRP for 30 min at room temperature. The
reaction was visualized by the CheMate™ DAB plus chromogen. Lastly,
the sections were counterstained with hematoxylin solution.
Negative controls were performed by staining with PBS.
Immunostaining was assessed by an experienced pathologist who was
blinded to the clinical data of the patients.
Construction of stable gene
transfection
TFF1 and GKN1 cDNA was amplified from total RNA of
the normal gastric mucosa using PCR. GKN1 CDS fragments with
SalI and BamHI restriction sites and TFF1 CDS
fragments with KpnI and BglI restriction sites were
then solely or simultaneously inserted into the pBudCE4.1 vector
(Invitrogen, Carlsbad, CA, USA), which contains two promoters for
independent expression of two recombinant proteins, using a DNA
ligation kit (Takara, Dalian, China). After transformation into
DH5α E. coli competent cells, the plasmid was amplified and
the contructs were verified by sequencing. To generate gastric
cancer cells expressing TFF1 or co-expressing TFF1 and GKN1
(TFF1-GKN1), gastric cancer AGS cells were grown to 50–75%
confluency in a 6-well plate, and subjected to the
Lipofectamine-mediated transfection (Invitrogen) according to the
manufacturer’s protocol. The TFF1 and TFF1-GKN1 transfected gastric
cancer cells were then selected in medium containing Zeocin
(Invitrogen). After the transfected cells formed individual cell
colonies, stable cells were obtained and then confirmed for TFF1 or
GKN1 expression by RT-PCR, western blotting and immunoblot
analyses. Vector was also transfected into AGS cells as control
cells.
Co-IP and western blotting
Total cellular protein was extracted from normal
gastric mucosa tissues, control cells and TFF1-GKN1 co-transfected
cells using a lysis buffer containing protease inhibitor cocktail
(Roche, Mannheim, Germany). An equal amount of protein (500 μg) was
incubated with a 1:200–1:500 dilution of anti-TFF1 antibody for 1.5
h at 4°C followed by addition of protein A/G Plus agarose beads
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) and incubation for
an additional 1.5 h at the same conditions. The
protein/antibody/beads mix was then washed three times with PBS
buffer, resuspended in SDS sample buffer, and denatured at 95°C for
5 min. All samples were resolved by 10% SDS-PAGE, and
electroblotted onto PVDF membranes. Membranes were blocked in 5%
non-fat milk for 2 h, and then incubated for 2 h with a 1:500
dilution of anti-GKN1 antibody (Abnova). After washed with PBS
buffer three times and incubation for 2 h with the appropriate
secondary antibody, enhanced chemiluminescence (Pierce, Rockford,
IL, USA) was used for protein visualization.
Cell viability (MTT) assay
To detect changes in tumor cell viability after TFF1
or TFF1-GKN1 transfection, a total of 1×104
trypsin-dispersed cells was seeded into each well of a 96-well
plate, and cultured for 24 or 48 h. Next, 20 μl of MTT (5 g/l) was
added to each well and incubated for additional 4 h at 37°C.
Culture medium was then replaced with 200 μl of dimethyl sulfoxide
(DMSO) and the absorbance rate was determined using an ELISA reader
at 490 nm. Cell growth inhibition rate was calculated as (the value
of experimental group OD/the value of control group OD) × 100%.
Annexin V apoptosis assay
To detect tumor cell apoptosis, the TFF1 or
TFF1-GKN1 transfected AGS cells were seeded into 60-mm diameter
culture plates, and cultured for 24 and 48 h. The apoptotic rates
were analyzed by flow cytometry using an annexin V-FITC/PI kit
(Keygen, Nanjing, China). Staining was performed according to the
manufacturer’s instructions, and flow cytometry was conducted with
a flow cytometer (Beckman-Coulter, Brea, CA, USA). Cells with
annexin V (−) and PI (−) were deemed viable cells. Cells with
annexin V (+) and PI (−) were deemed early apoptotic cells. Cells
with both annexin V (+) and PI (+) were deemed late apoptotic
cells.
Cell cycle analysis
To analyze cell cycle distribution, transfected
cells were grown and treated with 25 μM molomoucine (Santa Cruz)
for 1 h, and then incubated in regular culture medium without
molomoucine for an additional 1 h (15). Cells were then collected and
subjected to cell cycle analysis by flow cytometry.
Statistical analysis
All quantitative data were expressed as mean ± SD
and analyzed by Student’s t-tests. The differential expression of
TFF1 among different groups was determined by Kruskal-Wallis test.
All statistical analyses were performed using the SPSS statistical
software package (version 11.0, SPSS Inc. Chicago, IL, USA).
P<0.05 was considered statistically significant.
Results
Expression of TFF1 in cancer cell lines
and gastric tissue specimens
Results of RT-PCR analysis showed that TFF1 mRNA had
low expression in gastric cancer KATO3, MKN45, lower expressed in
AGS and SNU16 cells, and was lost in N87, MKN28 and SNU1 cells
(Fig. 1A). In 39 gastric cancer
tissues, TFF1 mRNA was only weakly expressed in 15 tissue specimens
and absent in the remaining 24 tissues (Fig. 1B). However, TFF1 mRNA was abundant
in all of the 39 corresponding adjacent non-cancer tissues as well
as in normal gastric epithelial cells obtained from 20 healthy
volunteers.
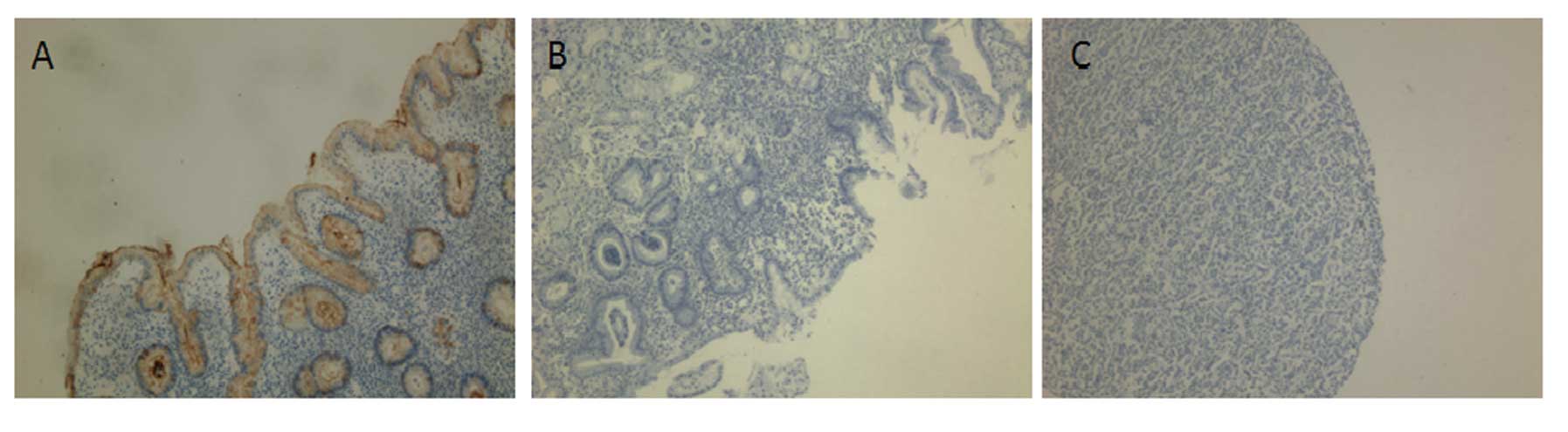
Next, we immunohistochemically stained TFF1 in the
tissue sections from patients. We found that TFF1 protein
expression was significantly downregulated or even lost in
dysplastic and cancer tissues specimens. In contrasts, the TFF1
protein was strongly expressed in the normal mucosa (Fig. 2). This reduction in protein
expression was statistically significant (P<0.05) (Table II).
 | Table IITFF1 expression detected by
immunohistochemistry in gastric tissues. |
Table II
TFF1 expression detected by
immunohistochemistry in gastric tissues.
| Histological
type | No. of patients | − | + | ++ | +++ | P-valuea |
|---|
| Normal gastric
mucosa | 20 | 0 | 0 | 0 | 20 | |
| Dysplastic
lesion | 40 | 22 | 18 | 0 | 0 | <0.05 |
| Gastric cancer | 39 | 27 | 12 | 0 | 0 | <0.05 |
Identification of TFF1 and GKN1 protein
interaction by Co-IP
To determined protein interaction between TFF1 and
GKN1, we successfully generated AGS cells that stably co-expressed
TFF1 and GKN1; expression was confirmed by RT-PCR, western blotting
and immunohistochemistry. In cells, both GKN1 and TFF1 showed
granular cytoplasmic distribution. In normal gastric mucosa and
co-transfected AGS cells, the protein interaction between GKN1 and
TFF1 were investigated by Co-IP analysis. The negative results of
Co-IP demonstrate that there was no direct molecular interaction
between GKN1 and TFF1 in protein level.
Effect of TFF1 or TFF1-GKN1 on AGS cell
proliferation
In the previous experiments, we confirmed TFF1 was
downregulated in gastric cancer. Then effects of TFF1 on cancer
cells were determined. The MTT results showed that TFF1
significantly decreased the proliferation of AGS cells as compared
to the vector-transfected cells in both 24- and 48-h cultures
(P<0.05), while the proliferation between the TFF1 transfected
cells and the TFF1-GKN1 co-transfected cells was not statistically
significant in either 24-or 48-h cultures (P>0.05) (Fig. 3).
Effect of TFF1 and TFF1-GKN1 on AGS cell
apoptosis
The cell apoptosis of the transfected cells was also
investigated. The flow cytometry results demonstrated that cell
apoptosis rate was not statistically significant between TFF1
transfected cells (2.73±0.30%) and vector-transfected cells
(2.76±0.38%) in 24 h (P>0.05), while TFF1-GKN1 co-transfected
cells showed an increased apoptosis rate (4.61±0.42%) comparing to
TFF1 transfected cells or vector-transfected cells (P<0.05)
(Fig. 4).
Cell cycle redistribution of transfected
cells
We then investigated the effects of TFF1 and
TFF1-GKN1 on cell cycle redistribution. Olomoucine, a purine
derivative and a cyclin-dependent kinase (CDK) inhibitor, was used
to enrich parental AGS cells in the G1 phase. Specifically, cells
were arrested in the cell cycle by 1 h olomoucine treatment and
continued to be incubated for another 1 h without olomoucine. The
cell cycle distribution of TFF1 transfected cells changed from (G1
40.4%, S 36.1%) to (G1 57.0%, S 21.7%). The cell cycle distribution
of TFF1-GKN1 co-transfected cells changed from (G1 42.7%, S 37.0%)
to (G1 45.8%, S 28.5%) (Fig. 5).
These data demonstrated that TFF1 was superior to TFF1-GKN1 in
arresting AGS cells in the G1-S transition phase.
Discussion
In the present study, we have shown that TFF1 mRNA
was downregulated or even absent in gastric cancer cell lines,
gastric dysplasia and cancer tissues, but TFF1 protein was abundant
in the corresponding adjacent non-cancer tissues as well as in
normal gastric mucosa. These results suggested that downregulation
of TFF1 is a frequent event in gastric precancer or cancer, and the
presence of TFF1 may protect gastric mucosa from neoplasia.
The effects of TFF1 on gastric cancer cells were
investigated. Our MTT data showed that endogenous TFF1 protein
reduced cell proliferation. It was consistent with the result of a
previous study that exogenous TFF1 protein could reduce gastric
cancer cell proliferation in a dose-dependent manner (14). The following data of flow cytometry
assay showed that TFF1 was unable to induce apoptosis in cancer
cells, but TFF1 participated in cell differentiation by delaying
G1-S cell phase transition. It indicated that the ability of cell
viability suppression was due to the regulation of cell
differentiation but not the induction of apoptosis. These data were
consistent with the previous studies (14,15,28).
Taken together, the results suggested that TFF1 may play an
important role as a tumor suppressor in gastric cancer.
The protein interaction and functional relationships
between TFF1 and GKN1 were further addressed. We successfully
cloned and co-transfected TFF1 and GKN1 into gastric cancer AGS
cells and the cells stably expressed both TFF1 and GKN1 protein.
Both TFF1 and GKN1 showed granular cytoplasmic distribution in the
the co-transfected cells. The expression locations of TFF1 and GKN1
in gastric mucosa are similar. In gastric mucosa, GKN1 plays an
important role in maintaining mucosal integrity and mediating
repair after injury. In gastric cancer, GKN1 showed downregulated
expression and inhibited cell growth. In view of the similar
expression and biological functions, it was reasonable to speculate
that there is some possible relationships between TFF1 and GKN1.
GKN2 is a downstream gene of GKN1 and belongs to gastroinkase
family (23). Recent studies have
shown that GKN2 may interact with TFF1 to be a heterodimer. The
ligament between GKN2 and TFF1 in the heterodimer is an
intermolecular disulfide bond between cysteine residues in the
carboxy-terminus of TFF1 and in the BRICHOS domain of GKN2
(24,26,29,30).
However, the relationship between TFF1 and GKN1 has not been
reported. To determine whether TFF1 conjoin to GKN1 by a
biochemistry connection between these two molecules, we used Co-IP
assay to detect the TFF1-GKN1 co-transfected AGS cells and normal
gastric tissues which all abundantly expressed both TFF1 and GKN1
protein. The negative result of Co-IP identified that there was no
directly protein-protein interactions between TFF1 and GKN1 to be a
protein complex. GKN1 and GKN2 have the same intron and exon
structures, and are located in upstream and downstream on the same
chromosomes of genomes. GKN1 has a significant homology to GKN2 in
protein level (24). Specially,
both GKN1 and GKN2 contain the conserved BRICHOS domain which
contains a pair of conserved cysteine residues (19). The most striking difference between
GKN2 and GKN1 was that GKN2 contains an additional cysteine residue
at position 38, which locates within the BRICHOS domain. Thus, this
additional cysteine residue of GKN2 interacted with the cysteine 58
of TFF1 in the carboxy-terminus to form intermolecular disulfide
bonds and stabilizing the interaction between the two molecules
(GKN2 and TFF1). GKN1 does not have this additional cysteine
residue, therefore GKN1 was unable to interact with TFF1 in protein
level.
Although TFF1 did not interact with GKN1 to form a
heterodimer, the expression and biological functions in gastric
cancer of these two molecules were similar. We hypothesized that
these two molecules may cooperate or perform synergistic effects in
gastric cancer, but TFF1-GKN1 did not show more cells viability
inhibition than TFF1 by MTT assay. GKN1 was demonstrated apoptosis
induction in gastric cancer cells in a previous study (31). Our results show that TFF1
participated in cell differentiation by delaying G1-S phase
transition. The co-transfected cells with presence of TFF1 and GKN1
were observed with a lower rate in holding cells in the G1-S
transition phase compared to TFF1 transfected cells. In normal
gastric mucosa, GKN1 was mitogenic and motogenic for facilitating
restitution and proliferation after gastric mucosal injury. Thus,
GKN1 may weaken the effect of TFF1 in delaying G1-S phase
transition in the co-transfected cells.
In conclusion, TFF1 may be a tumor suppressor in
gastric cancer and the inhibition of cancer cell growth may mainly
be due to delaying G1-S phase transition of cells. In gastric
cancer, TFF1 and GKN1 were unable to interact in protein level and
cooperate in functional level.
Acknowledgements
This study was supported in part by grants from the
National Natural Science Foundation of China (nos. 81072048 and
30871145), the Natural Science Foundation of Guangdong Province
(no. 7001641), the Junior Teacher Cultivation Project of Sun
Yat-sen University (nos. 09ykpy22 and 10ykjc23).
References
|
1
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000. The global picture. Eur J Cancer. 37(Suppl
8): S4–S66. 2001.PubMed/NCBI
|
|
2
|
Jemal A, Thomas A, Murray T and Thun M:
Cancer statistics, 2002. CA Cancer J Clin. 52:23–47. 2002.
View Article : Google Scholar
|
|
3
|
Krejs GJ: Gastric cancer: epidemiology and
risk factors. Dig Dis. 28:600–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thim L: Trefoil peptides: from structure
to function. Cell Mol Life Sci. 53:888–903. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoffmann W, Jagla W and Wiede A: Molecular
medicine of TFF-peptides: from gut to brain. Histol Histopathol.
16:319–334. 2001.PubMed/NCBI
|
|
6
|
Wright NA, Hoffmann W, Otto WR, Rio MC and
Thim L: Rolling in the clover: trefoil factor family (TFF)-domain
peptides, cell migration and cancer. FEBS Lett. 408:121–123. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rio MC, Bellocq JP, Daniel JY, et al:
Breast cancer-associated pS2 protein: synthesis and secretion by
normal stomach mucosa. Science. 241:705–708. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rio MC, Chenard MP, Wolf C, et al:
Induction of pS2 and hSP genes as markers of mucosal ulceration of
the digestive tract. Gastroenterology. 100:375–379. 1991.PubMed/NCBI
|
|
9
|
Wright NA, Poulsom R, Stamp G, et al:
Trefoil peptide gene expression in gastrointestinal epithelial
cells in inflammatory bowel disease. Gastroenterology. 104:12–20.
1993.PubMed/NCBI
|
|
10
|
Kjellev S: The trefoil factor family -
small peptides with multiple functionalities. Cell Mol Life Sci.
66:1350–1369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Henry JA, Bennett MK, Piggott NH, Levett
DL, May FE and Westley BR: Expression of the pNR-2/pS2 protein in
diverse human epithelial tumours. Br J Cancer. 64:677–682. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Machado JC, Carneiro F, Blin N and
Sobrinho-Simoes M: Pattern of pS2 protein expression in
premalignant and malignant lesions of gastric mucosa. Eur J Cancer
Prev. 5:169–179. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lefebvre O, Chenard MP, Masson R, et al:
Gastric mucosa abnormalities and tumorigenesis in mice lacking the
pS2 trefoil protein. Science. 274:259–262. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calnan DP, Westley BR, May FE, Floyd DN,
Marchbank T and Playford RJ: The trefoil peptide TFF1 inhibits the
growth of the human gastric adenocarcinoma cell line AGS. J Pathol.
188:312–317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bossenmeyer-Pourie C, Kannan R, Ribieras
S, et al: The trefoil factor 1 participates in gastrointestinal
cell differentiation by delaying G1-S phase transition and reducing
apoptosis. J Cell Biol. 157:761–770. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prest SJ, May FE and Westley BR: The
estrogen-regulated protein, TFF1, stimulates migration of human
breast cancer cells. FASEB J. 16:592–594. 2002.PubMed/NCBI
|
|
17
|
Ather MH, Abbas F, Faruqui N, Israr M and
Pervez S: Expression of pS2 in prostate cancer correlates with
grade and Chromogranin A expression but not with stage. BMC Urol.
4:142004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arumugam T, Brandt W, Ramachandran V, et
al: Trefoil factor 1 stimulates both pancreatic cancer and stellate
cells and increases metastasis. Pancreas. 40:815–822. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanchez-Pulido L, Devos D and Valencia A:
BRICHOS: a conserved domain in proteins associated with dementia,
respiratory distress and cancer. Trends Biochem Sci. 27:329–332.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshikawa Y, Mukai H, Hino F, Asada K and
Kato I: Isolation of two novel genes, down-regulated in gastric
cancer. Jpn J Cancer Res. 91:459–463. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toback FG, Walsh-Reitz MM, Musch MW, et
al: Peptide fragments of AMP-18, a novel secreted gastric antrum
mucosal protein, are mitogenic and motogenic. Am J Physiol
Gastrointest Liver Physiol. 285:G344–G353. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walsh-Reitz MM, Huang EF, Musch MW, et al:
AMP-18 protects barrier function of colonic epithelial cells: role
of tight junction proteins. Am J Physiol Gastrointest Liver
Physiol. 289:G163–G171. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baus-Loncar M, Lubka M, Pusch CM, Otto WR,
Poulsom R and Blin N: Cytokine regulation of the trefoil factor
family binding protein GKN2 (GDDR/TFIZ1/blottin) in human
gastrointestinal epithelial cells. Cell Physiol Biochem.
20:193–204. 2007.
|
|
24
|
Westley BR, Griffin SM and May FE:
Interaction between TFF1, a gastric tumor suppressor trefoil
protein, and TFIZ1, a brichos domain-containing protein with
homology to SP-C. Biochemistry. 44:7967–7975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Otto WR and Thim L: Trefoil factor
family-interacting proteins. Cell Mol Life Sci. 62:2939–2946. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kouznetsova I, Laubinger W, Kalbacher H,
et al: Biosynthesis of gastrokine-2 in the human gastric mucosa:
restricted spatial expression along the antral gland axis and
differential interaction with TFF1, TFF2 and mucins. Cell Physiol
Biochem. 20:899–908. 2007. View Article : Google Scholar
|
|
27
|
Stolte M and Meining A: The updated Sydney
system: classification and grading of gastritis as the basis of
diagnosis and treatment. Can J Gastroenterol. 15:591–598.
2001.PubMed/NCBI
|
|
28
|
Beckler AD, Roche JK, Harper JC, et al:
Decreased abundance of trefoil factor 1 transcript in the majority
of gastric carcinomas. Cancer. 98:2184–2191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moss SF, Lee JW, Sabo E, et al: Decreased
expression of gastrokine 1 and the trefoil factor interacting
protein TFIZ1/GKN2 in gastric cancer: influence of tumor histology
and relationship to prognosis. Clin Cancer Res. 14:4161–4167. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
May FE, Griffin SM and Westley BR: The
trefoil factor interacting protein TFIZ1 binds the trefoil protein
TFF1 preferentially in normal gastric mucosal cells but the
co-expression of these proteins is deregulated in gastric cancer.
Int J Biochem Cell Biol. 41:632–640. 2009. View Article : Google Scholar
|
|
31
|
Rippa E, La Monica G, Allocca R, Romano
MF, De Palma M and Arcari P: Overexpression of gastrokine 1 in
gastric cancer cells induces fas-mediated apoptosis. J Cell
Physiol. 226:2571–2578. 2011. View Article : Google Scholar : PubMed/NCBI
|