Introduction
Angiogenesis, the process of new blood vessel
formation from an existing network of vasculature, plays a key role
in the growth and metastatic spread of solid tumors (1,2).
Emerging evidence underscores the potential role of bone marrow
(BM) vascularization, indicative of angiogenesis, in the
progression and chemosensitivity of hematological malignancies,
including acute myeloid leukemia (AML) (3,4).
Moreover, the BM microenvironment has been demonstrated to regulate
the angiogenic switch and give rise to novel vascularization by
controlling the balance between pro- and anti-angiogenic factors
(5,6). Among them, the vascular endothelial
growth factor (VEGF) and angiopoietin (Ang) are 2 of the most
important families involved in the regulation of angiogenesis
(7).
The VEGF family is essential for the initiation of
vascular development, while the Ang family is responsible for
vascular maturation and stabilization (8). VEGF-A (from here on termed VEGF) is
one of the most prominent angiogenic factors and it induces a
pro-angiogenic and permeability-enhancing signal via the tyrosine
kinase receptor, VEGFR-2 (9,10).
However, signaling through VEGFR-1 and the regulation of
angiogenesis by this receptor is more complex (9,10).
Additionally, Ang-1 and -2 can bind to the Tie2 receptor, although
they have opposite effects on Tie2 activation (11,12).
Ang-1 functions as a stabilizing signal for mature vasculature,
whereas Ang-2 may be regarded as a regulator of vessel plasticity
(11). Dysregulation of the VEGF
and Ang families is suggested to have a major impact on leukemic
growth and has been established as a crucial step in the
development of AML (13,14). Indeed, elevated levels of VEGF and
Ang-2 have been reported in patients with de novo AML and
appear to be negative prognostic factors in AML (15–18).
The Notch pathway is an evolutionarily conserved
intercellular pathway affecting a myriad of cellular activities,
such as cell survival, proliferation, migration and invasion
(19), and thereby may contribute
to leukemogenesis (20,21). Recently, the Notch pathway,
particularly the vascular-specific δ-like ligand 4 (Dll4), has been
identified as another pivotal pathway in the regulation of vascular
development (22). The activation
of the Notch pathway is initiated by ligand binding to Notch
receptors mainly between adjacent cells, and there seems to be an
important Notch cross-signaling between tumor and endothelial cells
that promotes angiogenesis (23).
Haploinsufficiency of Dll4 is essential for embryonic vascular
development, and Dll4-mediated Notch signaling has a unique role in
regulating endothelial cell proliferation and differentiation
(24,25). Studies have demonstrated that the
Notch pathway displays either increased or decreased angiogenic
processes, such as endothelial proliferation, migration and tube
formation in vitro, depending on the cell type and context
(22,23). Furthermore, the prognostic impact of
the Notch pathway in different malignancies has demonstrated a
diverging pattern (26), and thus
exploring the specific prognostic impact in AML angiogenesis is of
substantial interest.
Previous studies have shown that VEGF and Ang act
coordinately during vascular growth and remodeling (27). Moreover, the VEGF pathway acts as a
potent upstream activating stimulus for angiogenesis, while the
Notch/Dll4 pathway contributes to guide cell fate decisions that
shape the activation appropriately (28,29).
In addition, Notch1 signaling activates the Ang pathway, presenting
that angiogenesis of endothelial cells is mediated in part through
a Notch-dependent Ang-1/Tie2 pathway (30). In turn, Ang-1/Tie2 signaling
potentiates basal Notch1 signaling controlling vascular quiescence
by upregulating Dll4 (31). Since
BM neovascularization depends on the mutual and coordinated
interaction of various angiogenic factors from endothelial cells
and leukemia cells (4,6,32), and
many of these factors that are present in significant amounts in
the BM microenvironment may function in a synchronized fashion, it
will be of interest to comprehensively analyze the correlation
among these factors and further assess the clinical
implications.
In this study, we investigated the expression of 8
angiogenesis-related factors (Dll4, Notch1, VEGF, VEGFR-1, VEGFR-2,
Ang-1, Ang-2 and Tie2) in BM mononuclear cells of adult patients
with untreated AML. Furthermore, we assessed the prognostic impact
of Dll4 and Notch1 expression levels and their association with the
VEGF and Ang families.
Materials and methods
Patient samples
Sixty adult patients with untreated AML (31 males
and 29 females; median age, 45 years; range, 14–73 years) were
enrolled in this study. Diagnoses were established according to the
French-American-British (FAB) criteria (33). All the patients with non-M3 AML
subtypes underwent standard induction chemotherapy with one of the
anthracyclines (doxorubicin or idarubicin) for 3 days and
cytarabine for 7 days. The patients with acute promyelocytic
leukemia (subtype M3) received all-trans retinoic acid with or
without concurrent induction chemotherapy. After the patients
achieved complete remission (CR), they underwent consolidation
chemotherapy with a conventional dose of cytarabine and one
anthracycline or with a high dose of cytarabine. The control group
consisted of 40 healthy adult donors (22 males and 18 females;
median age, 44 years; range, 24–68 years), with normal BM
morphology as demonstrated by cytological and histological
analyses. Chromosomal analyses for newly diagnosed patients were
conducted on BM cells after 1–3 days of unstimulated culture and
karyotyped according to the International System for Human
Cytogenetic Nomenclature (ISCN). The median follow-up duration was
25 months. This study protocol was approved by the Medical Ethics
Committee of the Affiliated Qilu Hospital of Shandong University,
Jinan, China, and written informed consent was obtained from all
patients. The clinical information for the 60 AML patients is
presented in Table I.
 | Table IClinical features for AML
patients. |
Table I
Clinical features for AML
patients.
| Features | Patients |
|---|
| No. | 60 |
| Median age (range,
years) | 45 (14–73) |
| Gender |
| Male | 31 |
| Female | 29 |
| FAB
classification |
| M0 | 2 |
| M1 | 3 |
| M2 | 12 |
| M3 | 12 |
| M4 | 10 |
| M5 | 16 |
| M6 | 5 |
| Median hemoglobin
(range, g/l) | 75.5
(31.0–122.0) |
| Median WBC (range,
109/l) | 16.0
(0.3–110.3) |
| Median PLT (range,
109/l) | 43.5
(3.0–836.0) |
| Median BM blasts
(range, %) | 70 (40–98) |
| Karyotype |
| Favorable:
t(8;21), t(15;17), inv(16) | 16 |
| Intermediate:
normal, +8, +22, other | 39 |
| Unfavorable: −7,
−5, complex | 5 |
| Median follow-up
(range, months) | 25.0
(7.0–45.9) |
Real-time reverse transcription-PCR
(RT-PCR) analysis
Total RNA was isolated using TRIzol (Invitrogen,
Carlsbad, CA, USA) and cDNA was prepared using M-MLV Reverse
Transcriptase (Promega, Madison, WI, USA) according to the
manufacturer's instructions. Real-time RT-PCR was carried out using
an ABI Prism 7500 sequence detection system (Applied Biosystems,
Foster City, CA, USA) and performed with SYBR-Green PCR Master Mix
(Toyobo, Osaka, Japan) in a 20 μl reaction volume as described
previously (34). The specificity
of the desired products was documented with melting curves analysis
and examined using electrophoresis on a 2% agarose gel. Each sample
was tested twice, while PCR-grade water instead of template cDNA
was used as the negative controls. A comparative CT method
(2−ΔΔCT) was used to analyze the relative changes in
gene expression. The results were expressed relative to the number
of GAPDH transcripts used as the internal control. The sequences of
primers of different angiogenic factors are listed in Table II.
 | Table IIPrimer sequences used for real-time
RT-PCR. |
Table II
Primer sequences used for real-time
RT-PCR.
| ID | Genes | Primers
(5′-3′) | Size |
|---|
| NM_017617.3 | Notch1 | F:
TCAGCGGGATCCACTGTGAG | 104 |
| | R:
ACACAGGCAGGTGAACGAGTTG | |
| NM_019074.3 | Dll4 | F:
GCCAACTATGCTTGTGAATGTCC | 161 |
| | R:
CAGTAGGTGCCCGTGAATCC | |
| NM_001025366.2 | VEGF | F:
GAGCCTTGCCTTGCTGCTCTAC | 148 |
| | R:
CACCAGGGTCTCGATTGGATG | |
| NM_002019.4 | VEGFR-1 | F:
CTGGACTGACAGCAAACCCAAG | 117 |
| | R:
CCACAGCTGGAATGGCAGAA | |
| NM_002253.2 | VEGFR-2 | F:
AGCCAGCTCTGGATTTGTGGA | 133 |
| | R:
CATGCCCTTAGCCACTTGGAA | |
| NM_001146.3 | Ang-1 | F:
CCTGATCTTACACGGTGCTGATT | 146 |
| | R:
GTCCCGCAGTATAGAACATTCCA | |
| NM_001147.2 | Ang-2 | F:
AAGAGATCAAGGCCTACTGTGACA | 70 |
| | R:
TCCTCACGTCGCTGAATAATTG | |
| NM_000459.3 | Tie2 | F:
CTGTGAAGGGCGAGTTCGA | 76 |
| | R:
TGGTAGGAAGGAAGCTTGTTGAC | |
| NM_002046.3 | GAPDH | F:
GCACCGTCAAGGCTGAGAAC | 138 |
| | R:
TGGTGAAGACGCCAGTGGA | |
Western blot analysis for Dll4 and Notch1
protein
The cells were lysed in lysis buffer [50 mmol/l Tris
(pH 7.5), 100 mmol/l NaCl, 1 mmol/l EDTA, 0.5% NP40, 0.5% Triton
X-100, 2.5 mmol/l sodium orthovanadate, 10 μl/ml protease inhibitor
cocktail, 1 mmol/l phenylmethylsulfonyl fluoride] for 30 min at
4°C. Total proteins were fractionated using SDS-PAGE and were then
transferred onto nitrocellulose membranes. The membranes were then
incubated with the appropriate primary antibodies (Dll4 and
Notch1), followed by incubation with a secondary HRP-conjugated
antibody (Jingmei Co., Ltd., Beijing, China). The probed proteins
were detected using enhanced chemiluminescent reagents (Amersham
Pharmacia Biotech, Piscataway, NJ, USA). The two-dimensional
optical densities of proteins on the film were quantified and
analyzed using the Quantity One software (Bio-Rad, Hercules, CA,
USA).
Statistical analysis
The Mann-Whitney U test, analysis of variance
(ANOVA) and the Kruskal-Wallis test were used to analyze the
differences in the expression of angiogenic factors between the AML
and control groups, as well as differences in age, gender, FAB
subtype and karyotype subgroups of the AML patients. Spearman's
test was used to evaluate the correlation between the individual
expression of the genes studied, and the association of gene
expression with clinical features. Furthermore, overall survival
(OS) was defined as the number of days from the date of the first
diagnosis to death from any cause. Patients were categorized into
high and low angiogenic factors expressing subgroups using the
median value as the cut-off. Kaplan-Meier estimation was applied to
plot survival curves, and a log-rank test was used to compare
survival between groups. Univariate Cox proportional hazards
regression models were used to evaluate the predictive effect of
each factor alone on survival. Factors statistically significant in
the univariate models at a 5% level were included in the
multivariate models. Subsequently, multivariate models were reduced
one factor at a time such that all factors remaining in the model
were statistically significant at a 5% significance level.
Multivariate Cox models estimated the hazard ratio in terms of
relative risk (RR) for death, and 95% confidence interval (CI) to
determine independent risk factors associated with survival.
Two-sided P<0.05 were considered statistically significant. All
statistical analyses were performed with the SPSS 13 software (SPSS
Inc., Chicago, IL, USA).
Results
Aberrant expression profile of angiogenic
factors in AML patients
As a ratio with the expression of the housekeeping
gene GAPDH, BM levels of the 8 angiogenesis-related factors were
compared between the untreated AML patients and the healthy
controls. In spite of the wide range of individual values, median
levels of Notch1 (P=0.012), Dll4 (P=0.001), VEGF (P=0.005), VEGFR-2
(P=0.009) and Ang-2 (P=0.006) were significantly elevated in the
AML patients compared with the controls, while the median levels of
VEGFR-1 (P=0.439), Ang-1 (P=0.123) and Tie2 (P=0.733) were similar
in AML and control patients (Fig.
1A).
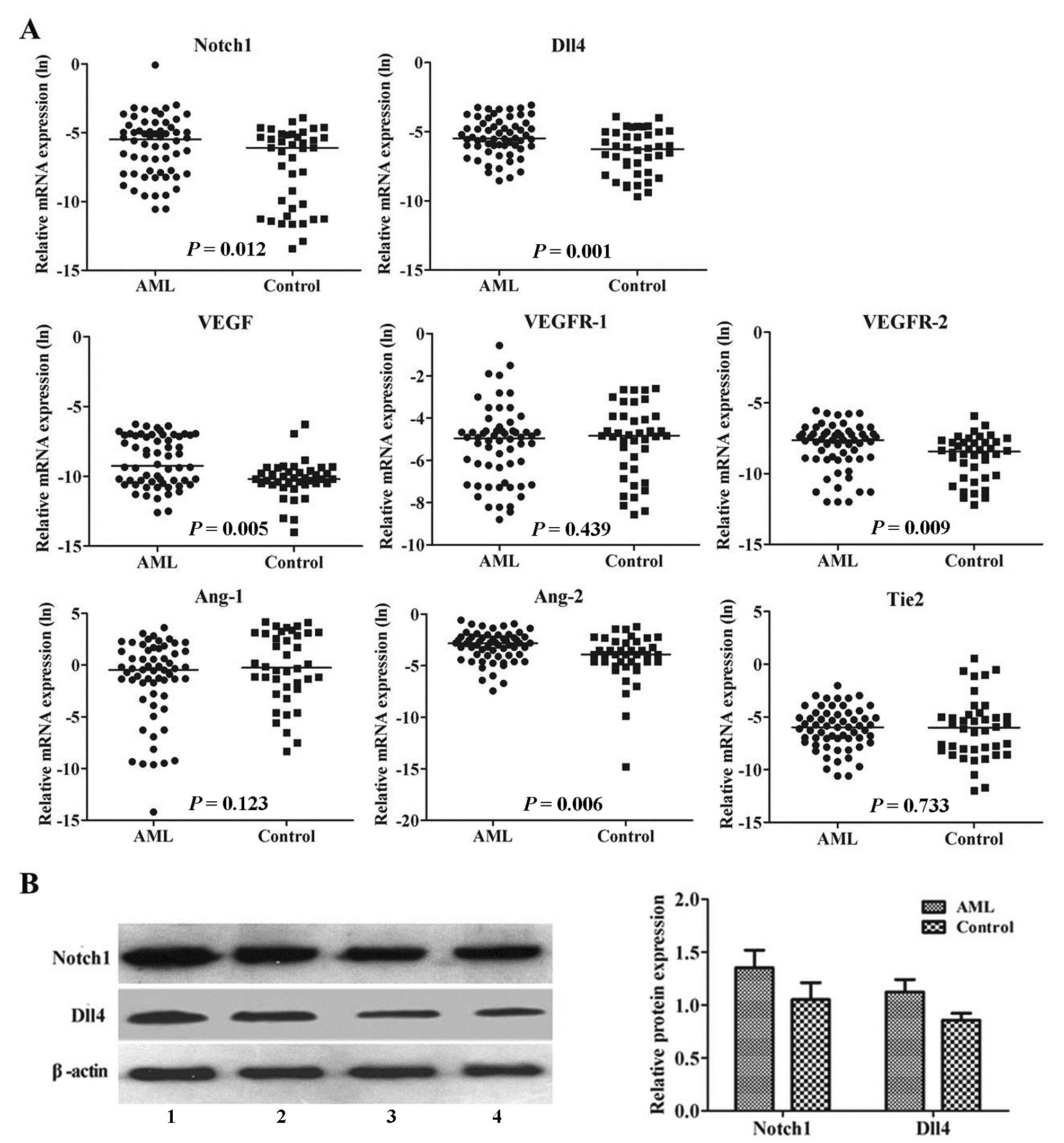 | Figure 1Aberrant expression profile of
angiogenic factors in AML patients. (A) Real-time RT-PCR analysis
of Notch1, Dll4, VEGF, VEGFR-1, VEGFR-2, Ang-1, Ang-2 and Tie2
expression in 60 untreated AML patients and 40 healthy controls.
The mRNA expressions of Notch1, Dll4, VEGF, VEGFR-2 and Ang-2 were
significantly upregulated in BM leukemia cells from AML patients,
compared to the controls. GAPDH was used to normalize the mRNA
level. Solid points indicate individual values and horizontal lines
represent the group median. Data were analyzed using the
Mann-Whitney U test. (B) Western blot analysis of Notch1 and Dll4
expression in 24 untreated AML patients and 8 healthy controls.
Left panel, representative photomicrographs of western blot
analysis of Notch1 and Dll4. Lane 1, AML patient no. 1; lane 2, AML
patient no. 2; lane 3, control no. 7; and lane 4, control no. 8.
β-actin was used as the sample loading control. Right panel, the
protein expressions of Notch1 and Dll4 were significantly higher in
BM leukemia cells from AML patients compared to the controls
(P<0.001). Columns indicate the mean from 2 independent
experiments and bars represent SD. Data were analyzed using the
Student's t-test. |
It is important to note that the active functional
form of Notch1 is the Notch1 intracellular domain. Therefore, we
focused our study on the Notch1 intracellular domain at the protein
level and Notch1 in the figure legends indicates the active
functional form of Notch1. In concordance with the real-time RT-PCR
data, western blot analysis showed that the protein expression
levels of Notch1 and Dll4 were upregulated in the untreated AML
patients compared to the controls (P<0.001, Fig. 1B). Taken together, these data
indicate that the Notch/Dll4 pathway is aberrantly activated in
AML.
It has been reported that BM vascularization is
regulated by the relative balance between angiogenic activators and
inhibitors (4,35). Therefore, we investigated the
correlation between the angiogenic factors studied in AML patients.
Significantly positive correlations were observed between Notch1
and Dll4 (r=0.326; P=0.013), VEGF and VEGFR-2 (r=0.354; P=0.007)
and Ang-1 and Tie2 (r=0.463; P=0.001). Furthermore, Dll4 was
closely associated with VEGF (r=0.663; P=0.001) and Notch1
correlated well with Ang-2 (r=0.324; P=0.012).
VEGF receptors expressed in leukemic cells are
functional and convey distinct signals, such as increasing
proliferation, metalloproteinase activation and trans-membrane
migration (9). The degree of Tie2
receptor activation depends on the balance between agonistic and
antagonistic ligands (11). Thus,
we further calculated the VEGFR-2:VEGFR-1 and Ang-2:Ang-1
expression ratio in normal and leukemic cells. The median
VEGFR-2:VEGFR-1 expression ratio was 3.61-fold higher in the AML
patients than in the controls (P=0.04), suggesting that the pathway
enhanced by the VEGF/VEGFR-2 interaction may be essential for the
pathogenesis of AML. The median Ang-2:Ang-1 expression ratio was
3.18-fold higher in the AML patients than in the controls
(P=0.015), indicating a dominant influence of Ang-2 in the
neoplastic BM and Ang-1 in the normal BM microenvironment.
Correlation of angiogenic factor
expression with clinical features
The association between BM levels of these 8
angiogenic factors in the 60 AML patients and clinical features
were investigated, and significant differences were not observed in
the gender and FAB subtypes (Table
III). However, the Tie-2 level was higher in AML patients
<45 years old than those >45 years old (P=0.03), and the
levels of Dll4 and VEGF were significantly different among the
karyotype subgroups (P=0.001). The BM of the AML patients studied
was highly infiltrated by leukemic blasts. The median (range)
percentage of blasts was 70% (range 40–98%). Significantly positive
associations of the percentage of leukemic blast infiltration were
observed with the individual expression of Notch1 (r=0.428;
P=0.001), Dll4 (r=0.288; P=0.026), VEGF (r=0.428; P=0.001) and
Ang-2 (r=0.474; P<0.001).
 | Table IIIExpression of angiogenic factors in
the different subgroups. |
Table III
Expression of angiogenic factors in
the different subgroups.
| Features | No. | Notch1 | Dll4 | VEGF | VEGFR-1 | VEGFR-2 | Ang-1 | Ang-2 | Tie2 |
|---|
| Age (years) |
| <45 | 30 | 0.62±1.36 | 0.39±1.26 | 0.85±4.94 | 0.66±5.22 | 0.45±1.08 | 0.87±7.82 | 0.55±0.78 | 0.50±2.60 |
| ≥45 | 30 | 0.16±16.53 | 0.48±1.06 | 1.51±5.17 | 0.79±10.3 | 0.50±0.64 | 0.31±4.34 | 0.60±1.35 | 0.13±1.01 |
| P-value | | 0.109 | 0.631 | 0.584 | 0.971 | 0.953 | 0.191 | 0.652 | 0.030a |
| Gender |
| Male | 31 | 0.47±16.22 | 0.52±1.20 | 0.86±5.33 | 0.74±10.7 | 0.43±0.88 | 0.91±7.82 | 0.63±1.19 | 0.26±1.18 |
| Female | 29 | 0.37±0.95 | 0.40±1.11 | 1.96±4.74 | 0.54±3.64 | 0.49±0.91 | 0.26±3.91 | 0.57±1.04 | 0.19±2.64 |
| P-value | | 0.383 | 0.717 | 0.912 | 0.549 | 0.510 | 0.061 | 0.935 | 0.745 |
| FAB subtypes |
| M0 | 2 | 4.35±0.91 | 0.26±0.19 | 0.54±0.39 | 1.10±3.68 | 1.16±0.61 | 0.46±0.63 | 0.87±0.33 | 0.19±0.04 |
| M1 | 3 | 2.60±1.90 | 1.25±1.10 | 1.06±8.11 | 0.08±0.42 | 0.12±0.32 | 0.02±0.51 | 2.48±1.40 | 0.12±0.31 |
| M2 | 12 | 0.76±26.03 | 0.70±1.28 | 3.25±4.19 | 0.63±6.18 | 0.48±1.04 | 2.47±11.3 | 0.51±0.53 | 0.39±1.14 |
| M3 | 12 | 0.20±0.51 | 0.26±0.53 | 0.25±2.47 | 0.36±5.48 | 0.30±0.43 | 2.20±4.69 | 0.45±1.26 | 0.36±3.75 |
| M4 | 10 | 0.10±0.48 | 0.31±1.04 | 2.78±5.27 | 0.85±1.76 | 0.49±1.16 | 0.01±0.25 | 0.66±1.17 | 0.09±1.62 |
| M5 | 16 | 0.47±1.19 | 0.48±1.52 | 5.25±6.22 | 0.73±0.53 | 0.58±0.82 | 1.75±4.53 | 0.45±1.34 | 0.25±1.32 |
| M6 | 5 | 0.70±1.06 | 0.98±0.81 | 0.36±4.09 | 0.34±2.56 | 0.61±1.29 | 0.34±3.14 | 0.62±0.99 | 0.32±0.38 |
| P-value | | 0.661 | 0.474 | 0.258 | 0.53 | 0.725 | 0.140 | 0.676 | 0.595 |
| Karyotype |
| Favorable | 16 | 0.11±0.25 | 0.25±0.29 | 0.29±0.73 | 0.50±6.32 | 0.36±0.73 | 2.79±4.27 | 0.35±0.76 | 0.22±3.31 |
| Intermediate | 39 | 0.52±14.47 | 0.45±1.15 | 3.60±4.98 | 0.74±9.24 | 0.61±0.97 | 0.49±7.23 | 0.63±1.01 | 0.26±1.29 |
| Unfavorable | 5 | 2.20±1.37 | 2.00±1.34 | 11.36±6.0 | 0.63±0.34 | 0.52±0.55 | 0.62±5.23 | 0.92±2.21 | 0.29±0.81 |
| P-value | | 0.676 | 0.001a | 0.001a | 0.835 | 0.510 | 0.917 | 0.092 | 0.319 |
Association between angiogenic factor
expression and clinical outcome
We subsequently investigated the correlation between
pre-therapeutic angiogenic factor expression and prognosis in AML.
The median OS was 25 months (range, 7.0–45.9 months). Univariate
analysis of the factors associated with OS revealed a significantly
shorter survival in the patients with the unfavorable karyotype,
higher Notch1 expression, higher Dll4 expression, higher VEGF
expression or higher Ang-2 expression (Table IV). Variables, such as age, gender,
hemoglobin levels, white blood cell (WBC) counts, platelet counts,
VEGFR-1, VEGFR-2, Ang-1 and Tie2 expression, as well as
VEGFR-2:VEGFR-1 and Ang-2:Ang-1 expression ratio had no impact. Cox
proportional hazards multivariate analysis of the univariate
predictors identified karyotype (RR, 3.19; 95% CI, 1.18–8.63;
P=0.22) and gene expression of Notch1 (RR, 5.04; 95% CI,
1.62–15.69; P=0.005), Dll4 (RR, 8.09; 95% CI, 1.72–37.95; P=0.008),
VEGF (RR, 5.25; 95% CI, 1.17–23.48; P=0.03) and Ang-2 (RR, 11.25;
95% CI, 3.09–41.01; P=0.001) as prognostic factors for OS. The
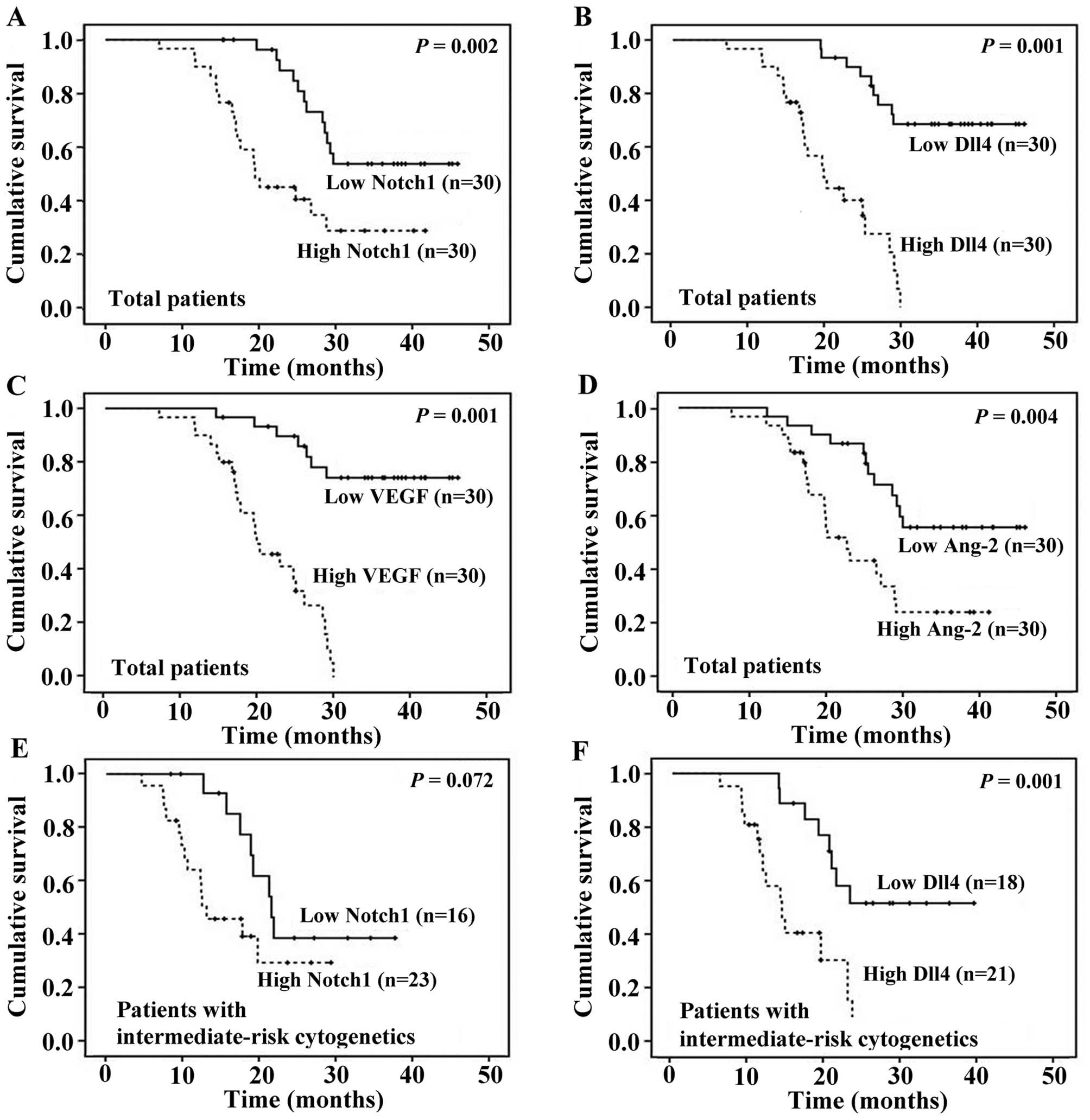
Kaplan-Meier curves for OS stratified according to Notch1, Dll4,
VEGF and Ang-2 expression in BM leukemia cells are shown in
Fig. 2A-D.
 | Table IVUnivariate analysis of the impact of
variables on overall survival. |
Table IV
Univariate analysis of the impact of
variables on overall survival.
| Variablesa | No. of
patients | Median (months ±
SD) | P-value |
|---|
| Age (years) | | | 0.837 |
| <45 | 30 | 25.5±10.2 | |
| ≥45 | 30 | 24.6±9.8 | |
| Gender | | | 0.532 |
| Female | 31 | 26.8±11.0 | |
| Male | 29 | 24.8±8.7 | |
| Karyotype | | | 0.001 |
| Favorable | 16 | 37.9±7.1 | |
| Intermediate | 39 | 22.7±8.0 | |
| Unfavorable | 5 | 13.7±3.9 | |
| Notch1 | | | 0.004 |
| Low | 30 | 29.5±9.1 | |
| High | 30 | 19.5±8.5 | |
| Dll4 | | 0.001 | |
| Low | 30 | 34.5±8.0 | |
| High | 30 | 17.4±5.8 | |
| VEGF | | | 0.001 |
| Low | 30 | 34.5±9.0 | |
| High | 30 | 19.4±5.9 | |
| VEGFR-1 | | 0.090 | |
| Low | 30 | 26.5±10.2 | |
| High | 30 | 24.6±9.8 | |
| VEGFR-2 | | | 0.149 |
| Low | 30 | 28.7±9.4 | |
| High | 30 | 22.0±10.2 | |
| Ang-1 | | | 0.865 |
| Low | 30 | 23.6±9.7 | |
| High | 30 | 25.9±10.3 | |
| Ang-2 | | | 0.005 |
| Low | 30 | 29.5±9.3 | |
| High | 30 | 19.5±8.7 | |
| Tie2 | | | 0.955 |
| Low | 30 | 26.1±9.8 | |
| High | 30 | 24.8±10.2 | |
In order to further illustrate the impact of Notch1
and Dll4 expression on OS, the Kaplan-Meier survival curves of 3
cytogenetic-risk groups were analyzed. Dll4 expression had a
similar impact on OS in all the patients and those with the
intermediate-risk karyotype, whereas Notch1 expression lost its
predictive value for survival in patients with the
intermediate-risk karyotype (Fig. 2E
and F). However, there was no survival difference between
patients with a low and high expression of Notch1 or Dll4 in the
favorable-risk group and unfavorable-risk group.
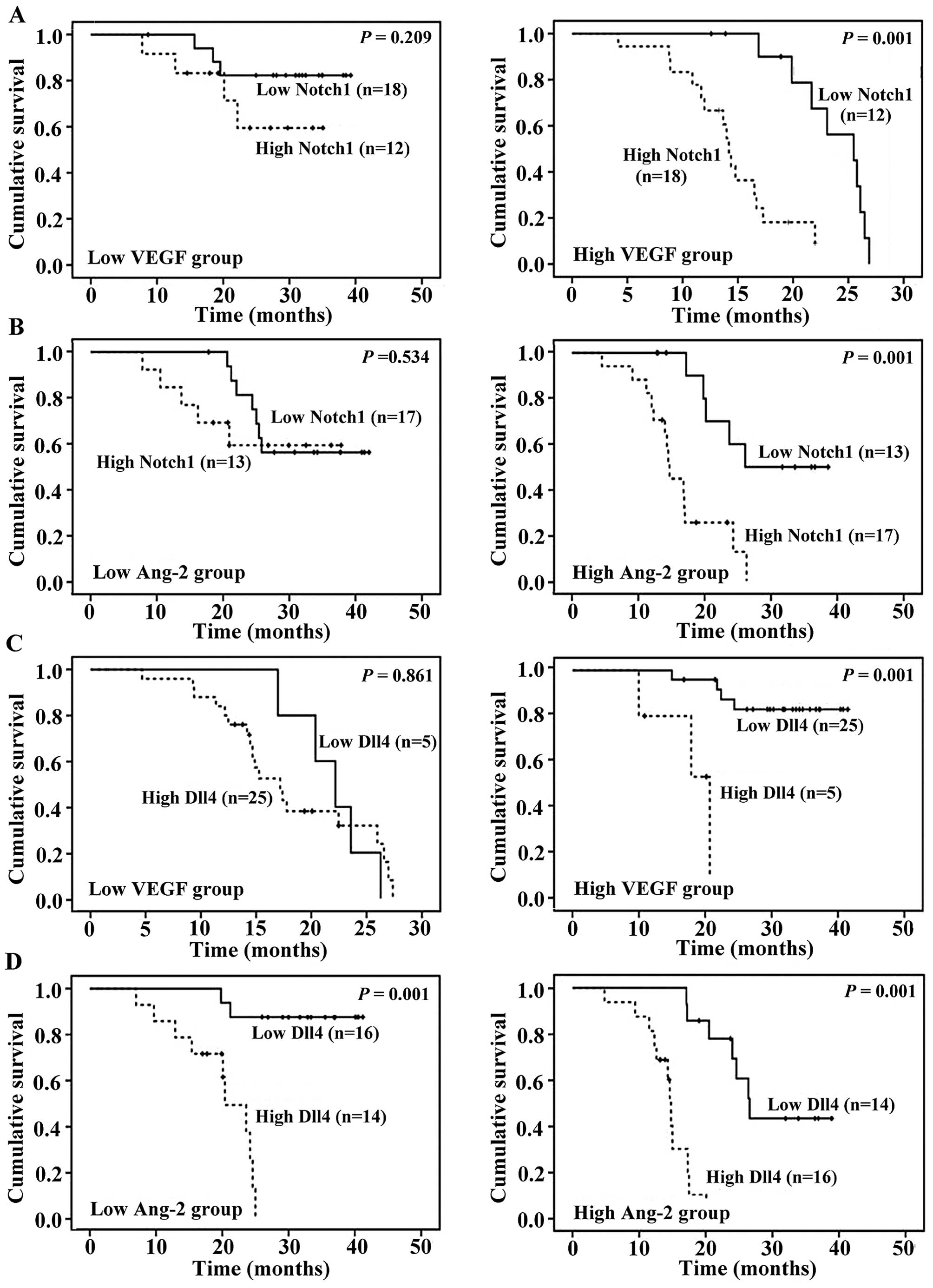
Finally, we performed subgroup analyses to
investigate potential interactions between the Notch/Dll4 pathway
and other angiogenic factors on OS. Patients were divided into 2
groups with a low or high expression (respectively, below or above
the median level) of VEGF and Ang-2. Survival estimates were
calculated for each group according to the Notch1 and Dll4
expression levels. The survival difference between patients with a
low and high Notch1 expression was pronounced in the subgroups with
high VEGF levels (P=0.001) and high Ang-2 levels (P=0.001),
respectively, but not evident in the patients with low values
(Fig. 3A and B). However, the
outcome was insignificant in patients with a high compared to a low
Dll4 expression in the presence of high VEGF expression (P=0.001,
Fig. 3C). The impact of the Dll4
expression on OS was equally prominent in the subgroups of AML
patients expressing low and high levels of Ang-2 (P=0.001, Fig. 3D).
Discussion
During the past few years, it has been proposed that
AML may be an angiogenesis-dependent disease with progressive
recruitment of blood vessels in the BM microenvironment (35). Previous studies have demonstrated a
close correlation between leukemogenesis and dysregulation of
angiogenesis, including increased BM vascularity (3) and elevated levels of numerous
angiogenic factors (7,13). Our present study clearly
demonstrates an elevated expression of Notch1, Dll4, VEGF, VEGFR-2
and Ang-2 in the BM leukemia cells of patients with untreated AML,
compared with the controls. Cox regression analyses revealed that
the expression levels of Notch1, Dll4, VEGF and Ang-2 were
predictors of poor OS, independent of cytogenetics. Furthermore,
the prognostic relevance of Dll4 expression was more evident in
patients with the intermediate-risk karyotype (62.5% of patients
with low Dll4 expression vs. 33.3% with high level in this subgroup
survived, P=0.001). Therefore, the Dll4 expression may serve as a
potential biomarker for prognosis prediction, particularly in the
intermediate-risk cytogenetic group.
Aberrant Notch signaling contributes to the genesis
of diverse cancers (26). The fact
that constitutively active Notch1 transforms cells in vitro
and causes mice to develop leukemia indicates the Notch pathway
involvement in the development of leukemia (21). Conversely, Notch is a tumor
suppressor in certain epithelial cancers where its normal function
is to promote terminal differentiation (36). Our present study shows that patients
with untreated AML express higher levels of Notch1 and Dll4
compared to healthy controls, as assessed by real-time RT-PCR and
western blot analysis. Moreover, the high expression levels of
Notch1 and Dll4 represent a significant feature of AML patients.
Patients with high Notch1 and Dll4 expression levels show several
adverse prognostic indicators, such as high BM blast counts and
unfavorable karotype. Recent studies have shown that reciprocal
Notch signaling may be necessary for the proliferation and survival
of AML cells, possibly through the maintenance of c-Myc and Bcl2,
as well as the phosphorylation of Rb protein (37,38).
Furthermore, the activation of the Notch pathway has been reported
to be associated with an adverse outcome in various solid cancers,
such as lung (39), breast
(40) and bladder cancer (41). Consistent with these findings, our
study also demonstrates that there is an inverse correlation
between Notch1/Dll4 expression and OS in AML. Collectively, our
data provide evidence for the critical role of the Notch/Dll4
pathway activation in the pathogenesis and prognosis of AML.
Elegant genetic and molecular studies have suggested
that the Notch/Dll4 pathway interacts extensively with the VEGF
pathway in endothelial cells (42,43).
Indeed, Dll4 expression may be induced by VEGF and hypoxia
(42). Conversely, Dll4 is
considered as a selective inhibitor of VEGF biological activity by
downregulating VEGFR-2 (43).
Recently, emphasis has been placed on the interaction of the
Notch/Dll4 and VEGF pathways in tumor angiogenesis (28,29).
Our study shows that Dll4 positively correlates with VEGF in AML
patients. Moreover, the poor prognosis in patients with high Notch1
and Dll4 expression levels was only evident in the presence of
higher (not lower) levels of VEGF, suggesting that the angiogenic
effect of Notch/Dll4 pathway may be dependent on VEGF. Donnem et
al also reported that Notch1 and Dll4 were independent
prognostic factors for non-small cell lung cancer, and the
influence of Notch1 was enhanced in the presence of high VEGF
expression (39). Thus, our results
and published reports confirm the hypothesis of a cross-talk
between Notch/Dll4 and VEGF pathways in tumor angiogenesis.
Notch1 signaling has been shown to activate the Ang
pathway, and Ang-1/Tie2 signaling augments basal Notch signaling by
inducing Dll4 expression through the Akt-mediated activation of
β-catenin (30,31). The fact that Notch1 lost its impact
on survival in the patients with low Ang-2 expression, as revealed
in our study, suggests these 2 factors may function in a synergetic
fashion; however, the mechanism involved remains unclear. The grade
of Tie2 phosphorylation is determined by the balance between Ang-1
and its naturally occurring antagonist Ang-2 (11). Owing to low Ang-2 expression,
Ang-1-mediated Tie2 activation was relatively enhanced, resulting
in the activation of the Akt pathway, which is associated with
increased survival and the anti-apoptotic effect (44,45).
Therefore, the prognostic impact of Notch1 expression may be
lessened in the presence of low Ang-2 expression.
Previous studies have led to the proposal that
VEGFR-2 is the critical receptor for transmitting cellular signals
for the proliferation and differentiation of endothelial cells,
whereas VEGFR-1 may be more important for vascular remodeling and
monocyte migration (9).
Additionally, several studies have shown that Ang-1 mediates
vascular stability, while Ang-2 induces vascular instability by
overriding Ang-1-mediated Tie2 activation (11). Therefore, it is important to
elucidate the balance between VEGFR-1 and VEGFR-2, Ang-1 and Ang-2
in the BM microenvironment. In this study, we observed a difference
in the VEGFR-2:VEGFR-1 and Ang-2:Ang-1 expression ratio between AML
patients and healthy controls, indicating that the angiogenic
balance is altered in favor of VEGFR-2 and Ang-2 in AML. Indeed, a
high ratio of Ang-2:Ang-1 expression in association with high
levels of VEGF, bFGF and other mitogenic angiogenic factors
stimulates angiogenesis in a wide range of solid tumors (11). Thus, our data provide evidence that
the alteration of the angiogenic balance in favor of Ang-2 acting
in concert with VEGFR-2 may be essential for BM neovascularization
in AML. However, we could not predict the prognosis of the AML
patients based on the VEGFR-2:VEGFR-1 and Ang-2:Ang-1 expression
ratio.
In conclusion, BM angiogenesis in AML is a complex
process involving the interplay of various angiogenic factors. Most
investigators have attempted to elucidate the impact of a single
angiogenic factor on the pathogenesis or prognosis of hematologic
malignancies. Our present study not only investigated the
prognostic role of the angiogenesis-related Dll4 and Notch1, but
also assessed their association with the VEGF and Ang families. Our
results provide evidence that the activation of the Notch/Dll4
pathway may indicate an unfavorable prognosis in AML. Furthermore,
the role of the Notch/Dll4 pathway in AML angiogenesis and its
correlation with VEGF and Ang-2 establishes the Notch/Dll4 pathway
as a potentially important target of anti-angiogenic therapy.
However, further prospective and multi-centre studies as well as
in-depth studies are required to verify the role of Notch/Dll4
pathway in AML angiogenesis.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China [81070422, 30871088, 81070407,
81000223]; the Specialized Research Fund for the Doctoral Program
of Higher Education [20100131110060]; the Shandong Technological
Development Project [2009HD012, 2009GG20002020, BS2009SW014,
ZR2010HQ030] and the Independent Innovation Fund of Shandong
University [yzc10072, yzc10075].
References
|
1
|
Zhang Y, Tang H, Cai J, et al: Ovarian
cancer-associated fibroblasts contribute to epithelial ovarian
carcinoma metastasis by promoting angiogenesis, lymphangiogenesis
and tumor cell invasion. Cancer Lett. 303:47–55. 2011. View Article : Google Scholar
|
|
2
|
Hao CY: Angiogenesis blockade as therapy
for hepatocellular carcinoma: progress and challenges. J
Gastroenterol Hepatol. 26:4–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shih TT, Hou HA, Liu CY, et al: Bone
marrow angiogenesis magnetic resonance imaging in patients with
acute myeloid leukemia: peak enhancement ratio is an independent
predictor for overall survival. Blood. 113:3161–3167. 2009.
View Article : Google Scholar
|
|
4
|
Trujillo A, McGee C and Cogle CR:
Angiogenesis in acute myeloid leukemia and opportunities for novel
therapies. J Oncol. 2012:1286082012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Payne SJ and Jones L: Influence of the
tumor microenvironment on angiogenesis. Future Oncol. 7:395–408.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ayala F, Dewar R, Kieran M and Kalluri R:
Contribution of bone microenvironment to leukemogenesis and
leukemia progression. Leukemia. 23:2233–2241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hou HA, Chou WC, Lin LI, et al: Expression
of angiopoietins and vascular endothelial growth factors and their
clinical significance in acute myeloid leukemia. Leuk Res.
32:904–912. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wegiel B, Ekberg J, Talasila KM, Jalili S
and Persson JL: The role of VEGF and a functional link between VEGF
and p27Kip1 in acute myeloid leukemia. Leukemia.
23:251–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmidt T and Carmeliet P: Angiogenesis: a
target in solid tumors, also in leukemia? Hematology Am Soc Hematol
Educ Program. 2011:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tait CR and Jones PF: Angiopoietins in
tumours: the angiogenic switch. J Pathol. 204:1–10. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reikvam H, Hatfield KJ, Lassalle P,
Kittang AO, Ersvaer E and Bruserud O: Targeting the angiopoietin
(Ang)/Tie-2 pathway in the crosstalk between acute myeloid
leukaemia and endothelial cells: studies of Tie-2 blocking
antibodies, exogenous Ang-2 and inhibition of constitutive
agonistic Ang-1 release. Expert Opin Investig Drugs. 19:169–183.
2010. View Article : Google Scholar
|
|
13
|
Maffei R, Martinelli S, Castelli I, et al:
Increased angiogenesis induced by chronic lymphocytic leukemia B
cells is mediated by leukemia-derived Ang2 and VEGF. Leuk Res.
34:312–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Keith T, Araki Y, Ohyagi M, et al:
Regulation of angiogenesis in the bone marrow of myelodysplastic
syndromes transforming to overt leukaemia. Br J Haematol.
137:206–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aguayo A, Kantarjian HM, Estey EH, et al:
Plasma vascular endothelial growth factor levels have prognostic
significance in patients with acute myeloid leukemia but not in
patients with myelodysplastic syndromes. Cancer. 95:1923–1930.
2002. View Article : Google Scholar
|
|
16
|
de Bont ES, Fidler V, Meeuwsen T, Scherpen
F, Hahlen K and Kamps WA: Vascular endothelial growth factor
secretion is an independent prognostic factor for relapse-free
survival in pediatric acute myeloid leukemia patients. Clin Cancer
Res. 8:2856–2861. 2002.PubMed/NCBI
|
|
17
|
Loges S, Heil G, Bruweleit M, et al:
Analysis of concerted expression of angiogenic growth factors in
acute myeloid leukemia: expression of angiopoietin-2 represents an
independent prognostic factor for overall survival. J Clin Oncol.
23:1109–1117. 2005. View Article : Google Scholar
|
|
18
|
Schliemann C, Bieker R, Thoennissen N, et
al: Circulating angiopoietin-2 is a strong prognostic factor in
acute myeloid leukemia. Leukemia. 21:1901–1906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Cain-Hom C, Choy L, et al:
Therapeutic antibody targeting of individual Notch receptors.
Nature. 464:1052–1057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demarest RM, Dahmane N and Capobianco AJ:
Notch is oncogenic dominant in T-cell acute lymphoblastic leukemia.
Blood. 117:2901–2909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leong KG and Karsan A: Recent insights
into the role of Notch signaling in tumorigenesis. Blood.
107:2223–2233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Phng LK and Gerhardt H: Angiogenesis: a
team effort coordinated by notch. Dev Cell. 16:196–208. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li JL and Harris AL: Notch signaling from
tumor cells: a new mechanism of angiogenesis. Cancer Cell. 8:1–3.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ridgway J, Zhang G, Wu Y, et al:
Inhibition of Dll4 signalling inhibits tumour growth by
deregulating angiogenesis. Nature. 444:1083–1087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noguera-Troise I, Daly C, Papadopoulos NJ,
et al: Blockade of Dll4 inhibits tumour growth by promoting
non-productive angiogenesis. Nature. 444:1032–1037. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lobry C, Oh P and Aifantis I: Oncogenic
and tumor suppressor functions of Notch in cancer: it's NOTCH what
you think. J Exp Med. 208:1931–1935. 2011. View Article : Google Scholar
|
|
27
|
Lobov IB, Brooks PC and Lang RA:
Angiopoietin-2 displays VEGF-dependent modulation of capillary
structure and endothelial cell survival in vivo. Proc Natl Acad Sci
USA. 99:11205–11210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li JL and Harris AL: Crosstalk of VEGF and
Notch pathways in tumour angiogenesis: therapeutic implications.
Front Biosci. 14:3094–3110. 2009.PubMed/NCBI
|
|
29
|
Thurston G and Kitajewski J: VEGF and
Delta-Notch: interacting signalling pathways in tumour
angiogenesis. Br J Cancer. 99:1204–1209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morrow D, Cullen JP, Cahill PA and Redmond
EM: Ethanol stimulates endothelial cell angiogenic activity via a
Notch- and angiopoietin-1-dependent pathway. Cardiovasc Res.
79:313–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Fukuhara S, Sako K, et al:
Angiopoietin-1/Tie2 signal augments basal Notch signal controlling
vascular quiescence by inducing delta-like 4 expression through
AKT-mediated activation of beta-catenin. J Biol Chem.
286:8055–8066. 2011. View Article : Google Scholar
|
|
32
|
Kittang AO, Hatfield K, Sand K, Reikvam H
and Bruserud O: The chemokine network in acute myelogenous
leukemia: molecular mechanisms involved in leukemogenesis and
therapeutic implications. Curr Top Microbiol Immunol. 341:149–172.
2010.PubMed/NCBI
|
|
33
|
Muller-Berndorff H, Haas PS, Kunzmann R,
Schulte-Monting J and Lubbert M: Comparison of five prognostic
scoring systems, the French-American-British (FAB) and World Health
Organization (WHO) classifications in patients with myelodysplastic
syndromes: Results of a single-center analysis. Ann Hematol.
85:502–513. 2006. View Article : Google Scholar
|
|
34
|
Ji M, Li J, Yu H, et al: Simultaneous
targeting of MCL1 and ABCB1 as a novel strategy to overcome drug
resistance in human leukaemia. Br J Haematol. 145:648–656. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee CY, Tien HF, Hu CY, Chou WC and Lin
LI: Marrow angiogenesis-associated factors as prognostic biomarkers
in patients with acute myelogenous leukaemia. Br J Cancer.
97:877–882. 2007.PubMed/NCBI
|
|
36
|
Ghaleb AM, Aggarwal G, Bialkowska AB,
Nandan MO and Yang VW: Notch inhibits expression of the
Kruppel-like factor 4 tumor suppressor in the intestinal
epithelium. Mol Cancer Res. 6:1920–1927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li GH, Fan YZ, Liu XW, et al: Notch
signaling maintains proliferation and survival of the HL60 human
promyelocytic leukemia cell line and promotes the phosphorylation
of the Rb protein. Mol Cell Biochem. 340:7–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tohda S: Functional analysis of notch in
the pathophysiology of leukemia. Rinsho Byori. 57:351–356. 2009.(In
Japanese).
|
|
39
|
Donnem T, Andersen S, Al-Shibli K, Al-Saad
S, Busund LT and Bremnes RM: Prognostic impact of Notch ligands and
receptors in nonsmall cell lung cancer: coexpression of Notch-1 and
vascular endothelial growth factor-A predicts poor survival.
Cancer. 116:5676–5685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reedijk M, Odorcic S, Chang L, et al:
High-level coexpression of JAG1 and NOTCH1 is observed in human
breast cancer and is associated with poor overall survival. Cancer
Res. 65:8530–8537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi TP, Xu H, Wei JF, et al: Association
of low expression of notch-1 and jagged-1 in human papillary
bladder cancer and shorter survival. J Urol. 180:361–366. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Siekmann AF, Covassin L and Lawson ND:
Modulation of VEGF signalling output by the Notch pathway.
Bioessays. 30:303–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Williams CK, Li JL, Murga M, Harris AL and
Tosato G: Up-regulation of the Notch ligand Delta-like 4 inhibits
VEGF-induced endothelial cell function. Blood. 107:931–939. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wakabayashi M, Miwa H, Shikami M, et al:
Autocrine pathway of angiopoietins-Tie2 system in AML cells:
association with phosphatidyl-inositol 3 kinase. Hematol J.
5:353–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu Q, Simpson SE, Scialla TJ, Bagg A and
Carroll M: Survival of acute myeloid leukemia cells requires PI3
kinase activation. Blood. 102:972–980. 2003. View Article : Google Scholar : PubMed/NCBI
|