Introduction
Gene therapy has been used to treat human cancers in
clinical trials and is considered to have therapeutic potential to
treat local-regional cancers. Our group previously cloned the
reduced expression in immortalized cells (REIC) gene and reported
that its expression is downregulated in a variety of cancer cell
lines and tumors (1–5). The REIC gene is identical to the
Dickkopf-3 (Dkk-3) gene (1), a
member of the Dickkopf (Dkk) gene family known to interfere with
Wnt signaling via Wnt-receptors (6). The REIC/Dkk-3 gene has been previously
reported to play a distinct role in the induction of apoptosis and
the inhibition of metastasis (7,8). The
REIC/Dkk-3 gene induces potent anti-tumor effects in vivo
and cancer-specific apoptosis in a variety of cancer types when
expressed by adenovirus-mediated gene transfer (9–12).
Therefore, REIC/Dkk-3 is one of the most attractive tumor
suppressor genes for cancer gene therapy. Based on this background,
we are now promoting the transfer of in situ REIC/Dkk-3 gene
therapy from the lab to the clinic for use in treating patients
with cancer.
The therapeutic effects of adenoviral vectors
encoding the REIC/Dkk-3 gene (Ad-REIC) were previously evaluated in
a variety of in vivo tumor models using prostate, breast,
and mesothelioma cancer cells (3,4,8–11);
however, the toxicity of Ad-REIC treatment remains unknown. An
important component in the safety evaluation of gene therapy agents
is the determination of the biodistribution and dissemination of
the agent in vivo following administration by appropriate
routes. Because locoregional treatment for prostate cancer is
achieved with intraprostatic administration of vectors, adenoviral
vectors have been used in several clinical trials of gene therapy
(13–15). However, the systemic biodistribution
of viral vectors after intraprostatic administration is not fully
elucidated. To determine the vector biodistribution of Ad-REIC
following intraprostatic administration, and as a necessary
preclinical study prior to clinical use as a treatment for prostate
cancer, we herein examine the biodistribution and safety of
intraprostatic injection of Ad-REIC vectors. The use of mice in
this kind of preclinical evaluation has many advantages (16); therefore, vector-specific DNA
polymerase chain reaction (PCR) was used to track the distribution
of the vectors from the prostate to other organs in mice. In a
comparative study, we also determined vector distribution following
the intravenous systemic administration of Ad-REIC.
Materials and methods
Cell cultures
Normal human prostate epithelial cells (PrEC) and
hepatocytes (HC) were purchased from Lonza (Basel, Switzerland) and
cultivated using the medium recommended by the supplier. Human
cancer cell lines (LNCaP, PC3, HEP3B, HEPG2) were provided by the
American Type Culture Collection (Rockville, MD). Dulbecco’s
modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) and
RPMI-1640 medium (Nissui, Tokyo, Japan) were used as the cultures
for the cancer cell lines. The cell lines were grown in media
supplemented with 10% fetal bovine serum (FBS; Biowest, Nuaille,
France), as previously described (5).
Adenovirus vectors carrying REIC/Dkk-3
(Ad-REIC)
The full-length cDNA of the human REIC/Dkk-3 gene
was inserted into the cosmid vector, pAxCAwt, and then transferred
into an adenoviral vector using the COS-TPC method (Takara Bio,
Shiga, Japan) (3). An adenoviral
vector carrying the LacZ gene (Ad-LacZ) was used as the control
vector. The adenoviral vectors consisted of replication-defective
adenovirus of serotype 5 that contained the indicated genes under
the control of the CMV early enhancer/chicken β actin (CAG)
promoter.
Western blot analyses
The total protein of in vitro treated cells
and mouse prostatic tissue was extracted with a lysis buffer (50 mM
HEPES, pH 7.4, 250 mM NaCl, 1 mM EDTA, 1% NP-40, 1 mM DTT, 1 mM
PMSF, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 2 mM
Na3VO4, 1 mM NaF, 10 mM β-GP). After
centrifugation was completed, the volume of the supernatants was
adjusted in each sample to achieve equal protein concentrations,
and the samples were diluted with the same volume of 2X SDS sample
buffer and heated for 5 min at 95˚C. The samples (10 μg of protein)
were then separated on 7.5% SDS-PAGE gels and electroblotted onto
polyvinylidene fluoride (PVDF) membranes. The blots were blocked
for 1 h with 10% non-fat milk powder, 6% gycine, and 0.1% Tween-20
in Tris-buffered saline (TBS) at room temperature. The REIC protein
was then identified with the use of a primary antibody: rabbit
anti-human REIC/Dkk-3 polyclonal antibody raised in our laboratory
(3,5). After being extensively washed with
0.1% Tween-20 in TBS (T-TBS), the blots were exposed to horseradish
peroxidase-conjugated secondary antibodies. After being extensively
washed with T-TBS, the membranes were developed using the enhanced
chemiluminescence detection method (ECL kit, Amersham Pharmacia
Biotech, Chandler, AZ).
Apoptosis assays
To examine the induction of in vitro
apoptosis following the treatments, the cells were seeded in
flat-bottom 6-well plates and incubated for 24 h. The cells were
then treated with either the Ad-CAG-LacZ or Ad-CAG-REIC at the
indicated multiplicity of infection (MOI) in the complete medium
(500 μl) for 1 h. Fresh medium was added to bring the total to 2
ml. After an additional 72 h of incubation, Hoechst 33342 stock
solution was added to the medium at a concentration of 2 μg/ml and
the cells were incubated in the dark for 10 min. Hoechst 33342 is
an intercalating dye that allows a determination of the variation
in the total chromatin quantity and the degree of chromatin
condensation to be made (17,18).
Using fluorescence microscopy, apoptotic cells were identified by
the presence of highly condensed or fragmented nuclei. Apoptotic
cells were counted in five different fields under microscopic
observation, and 100 cells were judged in each field.
Animal experiments
An analysis of the biodistribution of Ad-REIC and a
histological evaluation were completed following two doses of
vector injection into the dorsolateral prostate (left lobe) and one
dose of intravenous injection into the tail vein of each mouse. The
treatment groups were classified as follows: control group (group
1), intraprostatic injection of the vehicle [phosphate buffered
saline (PBS)]; intraprostatic injection of the vector (group 2),
intraprostatic injection of 2.5×108 viral particles (vp)
of Ad-REIC in 20 μl vehicles; intraprostatic injection (group 3),
intraprostatic injection of 2.0×1010 vp of Ad-REIC in 20
μl vehicles; intravenous injection via the tail vein (group 4):
intravenous injection of 2.5×108 vp of Ad-REIC in 100 μl
vehicles. A total of 27 mice (C57BL/6 male mice, 6–8 weeks of age)
were treated in order to obtain 19 evaluable animals, one for the
control and two for each of the Ad-REIC treatment groups for each
time-point. The first day of vector administration was designated
as day 0, and the mice were sacrificed on days 1, 7 and 28. The
physical appearance, activity levels, and mortality patterns of all
of the animals were observed daily. When the mice were sacrificed,
the following organs were collected for biodistribution and
histological analysis: brain, lung, thymus, heart, liver, pancreas,
spleen, kidney, stomach, colon, urinary bladder, dorsolateral
prostate (injection site in groups 1–3), testis, femoris muscle,
adrenal gland, and whole blood. To complete the DNA-PCR assays of
the biodistribution analyses, whole blood was firstly drawn from
the inferior vena cava of each animal under deep anesthesia with
sodium pentobarbital. The blood samples were collected in a plastic
tube and preserved with ethylenediaminetetra-acetic acid (EDTA).
The organs were then dissected and stored at −80˚C. Scalpels and
forceps were changed after each organ dissection or incision to
avoid cross-contamination of the tissues. To complete the
histological analysis of the potential side effects of Ad-REIC
treatment, the tissues were fixed in formalin and embedded to
create paraffin sections. The sections (5 μm) were stained with
hematoxylin and eosin and examined for histopathological and
cytotoxic changes.
DNA-PCR (polymerase chain reaction) for
the analyses of the biodistribution of Ad-REIC
DNA-PCR was performed on each mouse organ to detect
the presence of vector DNA derived from the Ad-REIC vectors and to
determine the biodistribution of Ad-REIC. The organs were weighed
and each (~25 mg) was suspended in 180 μl of ATL buffer (QIAamp DNA
Mini kit, Qiagen) with 20 μl of proteinase K. A 200-μl aliquot of
each organ was incubated at 56˚C until the tissue was completely
lysed and then was placed at room temperature. AL buffer (200 μl)
was added to each sample and the samples were incubated at 70˚C for
10 min. 100% ethanol (200 μl) was added to each sample and the
samples were mixed thoroughly and then placed into QIAamp
spin-columns. After several washing steps were completed, the DNA
was eluted with 200 μl of AE buffer. Each DNA sample was used in a
PCR that amplified a 621-bp fragment of the REIC gene. The
reactions contained 300 ng of sample DNA, 10 pmol of each primer
(REIC-F: 5′-ATGCAGCGGCTTGGGGCCACCCTGCT GTGC-3′; REIC-R:
5′-GATGGTCCCATTGCTGCCCCTGGT GGCCAT-3′), 8 mmol of each
deoxynucleoside triphosphate, 2X GC buffer, and 1 U of LA
Taq (Takara Bio) in a volume of 20 μl. The reactions were
incubated at 98˚C for 30 sec, then 30 cycles of 10 sec at 98˚C and
30 sec at 72˚C were performed. Following the final cycle, a 5-min
incubation at 72˚C was performed. After loading dye was added to
the reactions, the samples were electrophoresed in a 1.0% agarose
gel containing 0.5 μg/ml ethidium bromide and visualized with an
ultraviolet transilluminator. A sample containing an Ad-REIC vector
was assayed as a positive control. A sample without any vector was
assayed as a negative control in order to detect any
contamination.
Statistical analyses
The data are expressed as the means ± the standard
deviations (SD). An unpaired Student’s t-test was used to compare
the data between the two groups. Differences were considered to be
statistically significant at p<0.05.
Results
In vitro REIC protein expression and
apoptosis induction with Ad-REIC treatment
To confirm whether protein expression and apoptotic
effects were induced by Ad-REIC, various human cancer cell lines
and normal human cells were treated with Ad-REIC. Western blot
analysis demonstrated a significant expression of REIC protein in
PC3 human prostate cancer cells and human hepatocytes on day 1
(Fig. 1A). In an apoptosis assay
using the Hoechst 33342 stain, significant apoptosis induction was
observed in Ad-REIC treated prostate cancer cells (LNCaP, PC3) and
hepatoma cells (HEP3B, HEPG2) in comparison to the control Ad-LacZ
treated cells; however, no apoptosis was observed in normal cells
(PrEC, HC) (Fig. 1B). The mean rate
of apoptosis at 100 MOI in the cancer cells (LNCaP, PC3, HEP3B,
HEPG2) was 31.0, 67.0, 30.0 and 31.8%, respectively.
The biodistribution of Ad-REIC in mice
after intraprostatic and intravenous injection of the vectors
Human REIC gene detection was performed using
DNA-PCR to identify the presence of Ad-REIC vectors in various
tissues, including the brain, lung, thymus, heart, liver, pancreas,
spleen, kidney, stomach, colon, urinary bladder, prostate
(injection site in groups 1–3), testis, femoris muscle, adrenal
gland, and whole blood. Total DNA was extracted from each tissue
and analyzed for the presence of REIC DNA (621-bp fragment) using
PCR. The PCR bands were confirmed with electrophoresis. The
analysis used to detect REIC DNA in a given tissue is
semi-quantitative and the assay provides a gross indication of the
relative distribution of the vector Ad-REIC DNA. The sensitivity of
the DNA-PCR was established by adding various amounts of Ad-REIC
viral particles into the buffer (ranging from 1×1010
vp/ml to 1×104 vp/ml. The limit of detection for this
method was demonstrated to be 1×107 vp/ml; however,
trace levels were also visible at 1×106 vp/ml (data not
shown).
In the control group (group 1), no bands of vector
DNA derived from Ad-REIC were detected, demonstrating that the
assay did not react with the mouse REIC gene and no interference
from human REIC DNA occurred in the mice. On day 1 after a dose of
2.5×108 vp was injected into the prostate of the mice
(group 2), vector Ad-REIC DNA was detected in the prostate
(injection site), with trace amounts found in the brain, kidney,
colon, and urinary bladder. Bands of vector DNA were also detected
in the prostate on days 7 and 28. On day 1, the group that received
the higher dose of 2.0×1010 vp of Ad-REIC (group 3)
showed detectable levels of vector DNA in the lung, heart, liver,
pancreas, spleen, kidney, stomach, colon, urinary bladder, prostate
(injection site), testis, femoris muscle, and adrenal gland. On
days 1, 7 and 28, the most pronounced levels of vector DNA were
observed in the colon, urinary bladder, and prostate. On day 28,
vector Ad-REIC DNA was still detectable in the colon, urinary
bladder, and prostate.
The intravenous injection of Ad-REIC at a dose of
2.5×108 vp (group 4) resulted in detectable levels of
vector Ad-REIC DNA in the liver, spleen, colon, and whole blood on
day 1. No detectable DNA vectors were present by day 7. A summary
of the biodistribution of the Ad-REIC vectors following
intraprostatic and intravenous administration is shown in Table I.
 | Table IA summary of the biodistribution of
the Ad-REIC vectors following intraprostatic or intravenous
administration. |
Table I
A summary of the biodistribution of
the Ad-REIC vectors following intraprostatic or intravenous
administration.
| Intraprostate (lower
dose) | Intraprostate (higher
dose) | Intravenous |
|---|
| Brain | + | − | − |
| Lung | − | + | − |
| Thymus | − | + | − |
| Heart | − | + | − |
| Liver | − | + | + |
| Pancreas | − | + | − |
| Spleen | − | + | + |
| Kidney | + | + | − |
| Stomach | − | + | − |
| Colon | + | + | + |
| Urinary bladder | + | + | − |
| Prostate (injected
site) | + | + | − |
| Testis | − | + | − |
| Femoris muscle | − | + | − |
| Adrenal gland | − | + | − |
| Whole blood | − | − | + |
Toxicological examinations
As a preliminary experiment, the expression of REIC
protein following intraprostatic Ad-REIC injection was examined in
normal mouse prostate using western blot analysis. On day 3, an
elevated expression of intraprostatic REIC protein, in comparison
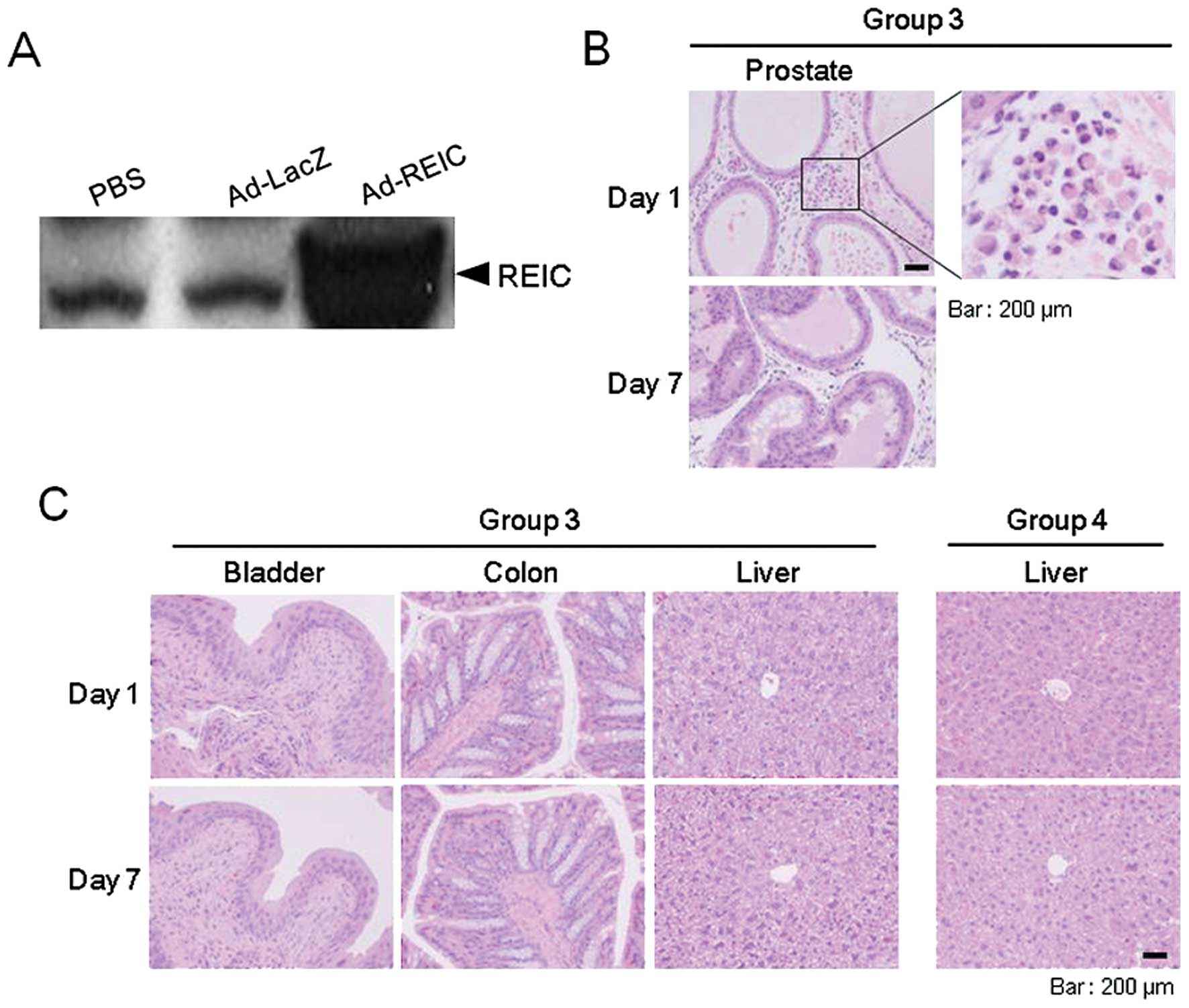
to the control treatment, was verified (Fig. 5A). In all of the experiments, the
physical appearance, activity, and mortality of the mice (groups
1–4) were evaluated daily. No adverse findings or signs of toxicity
were noted and no animal deaths occurred in this study. At
necropsy, no inflammation-related findings, such as the presence of
abscesses or abundant ascites, were detected in any of the
animals.
To examine the toxicology of the Ad-REIC treatment,
histological analyses were completed on sections of the brain,
lung, thymus, heart, liver, pancreas, spleen, kidney, stomach,
colon, urinary bladder, dorsolateral prostate (injection site in
groups 1–3), testis, femoris muscle, adrenal gland, and whole
blood. We first analyzed the local effects of Ad-REIC injection
using a histopathological analysis of the dorsolateral prostate,
the vector injection site. In the Ad-REIC injected prostate of
group 3, infiltrations of inflammatory cells were observed in the
interstitial areas on day 1; however, no such infiltrations were
observed on day 7 (Fig. 5B).
Similar findings were also observed in the Ad-LacZ injected
prostate; however, we were unable to detect any histological
effects in the prostatic sections of the PBS treated group (group
1) (data not shown). Since the biodistribution of the Ad-REIC
vectors following intraprostatic injection with the high dose
(group 3) was observed to be widespread, we next investigated
whether systemic toxicity resulted from Ad-REIC treatment in this
group. As for tissues other than the prostate, no histopathological
abnormalities were observed in the sections obtained from any
treatment group at any time-point (Fig.
5C).
The liver is the primary target organ for adenoviral
vectors following intravenous administration (19). We therefore examined the liver
tissue of group 4. None of these mice showed any histopathological
liver abnormalities, such as cytotoxicity or inflammation, during
the experiment (Fig. 5C).
Discussion
The adenoviral vectors carrying the human REIC/Dkk-3
gene (Ad-REIC) selectively induce apoptosis in prostate cancer
cells and other cancer cells through the activation of
c-Jun-NH2-kinase (JNK) and c-Jun (3,4,11). It
is also reported that the adenovirus-mediated expression of the
REIC/Dkk-3 gene efficiently induces endoplasmic reticulum (ER)
stress-mediated apoptosis in a manner specific to cancer cells
(4,20,21).
The present study demonstrated that Ad-REIC treatment induces
significant apoptosis in human prostate cancer cells and hepatoma
cell lines; however, Ad-REIC treatment does not induce apoptosis in
normal human cells. Therefore, the adenoviral vector agent,
Ad-REIC, appears to be effective against human cancer cells and is
attractive in terms of cancer-specific targeting. In the experiment
evaluating the biodistribution of Ad-REIC vectors, we also examined
the in vivo toxicity of the vectors using a
non-tumor-bearing mouse model. The aim was to examine the safety of
Ad-REIC vectors and to ensure that overexpression of the REIC/Dkk3
gene via intraprostatic injection does not result in systemic
toxicity. The experiment was also designed as a preclinical study
to support the future clinical use of Ad-REIC. No deaths or signs
of toxicity or unusual behavior were observed in the treated mice
in any group.
In this study, we used two doses (2.5×108
vp and 2.0×1010 vp) for the Ad-REIC treatment in mice.
In the clinical trial planned for the patients with prostate
cancer, intraprostatic injection of 1.0×1012 viral
particles (vp) of Ad-REIC was scheduled for one treatment. In this
study, the lower dose (a dose of 2.5×108 vp administered
via intraprostatic or tail vein injection in groups 2 and 4,
respectively) was calculated based on the comparison of mouse and
human body weights (25 g versus 100 kg). In this experiment, the
equivalent of a 1×1012 vp human dose/prostate was
considered to be a 2.5×108 vp dose in a mouse. The
selection of the higher dose (group 3) was based on previous
clinical studies of prostate cancer gene therapy in which
adenovirus-mediated gene delivery was performed using a dose
between 1010 and 1011 viral
particles/prostate (13–15). The higher dose of
2.0×1010 vp is 80-fold higher than the lower dose of
2.5×108 vp which is equivalent to the highest human dose
of 1×1012 vp/prostate anticipated to be used in the
planned clinical protocol.
This study demonstrates that Ad-REIC injected into
the prostate of mice disseminates to various tissues, even at the
lower dose of 2.5×108 vp. In the organs of the mice in
group 3, which were treated with the higher dose of
2.0×1010 vp, the PCR bands of vector DNA were found to
be stronger in the widespread organs, including the prostate. These
findings suggest that Ad-REIC injected into the prostate passes
into the general circulation and disseminates to various tissues,
leading to the sporadic appearance of vector sequences in other
distant organs. At the higher dose, vector Ad-REIC DNA was still
detectable in the colon, urinary bladder, and prostate on day 28.
The colon and urinary bladder are located near the prostate and
this may explain the tendency of Ad-REIC to disseminate to these
organs after intraprostatic administration. On the other hand, in
the intravenous injection via the tail vein group (group 4),
Ad-REIC vectors tended to disseminate to the liver and spleen on
day 1, and vector DNA disappeared from all organs by day 28. Since
the liver and spleen are organs of the reticuloendothelial system,
intravenously injected Ad-REIC might be trapped more frequently in
these organs. These results support the idea that the
biodistribution and clearance time of Ad-REIC are dependent on the
dose and route of vector administration.
Based on these biodistribution studies, we
demonstrated that Ad-REIC is widely distributed in the bodies of
mice after intraprostatic or intravenous vector administration. At
the dose of 2.0×1010 vp used in group 3, residual DNA
from Ad-REIC was detected in various organs and the injected
prostate until day 28. A dose of 2.0×1010 vp is very
high relative to the body weight of a mouse. Even at the high dose
used in group 3, no significant histological damage or definite
toxicity were observed in any organs examined at any time-point.
Regarding the inflammatory cells infiltrated in the prostatic
interstitial spaces in the mice in group 3, this finding was also
observed in the mice in the Ad-LacZ treatment group. It seems that
adenoviral vectors are directly involved in the inflammatory
reactions. Across the multiple therapeutic and pharmacological
studies previously conducted in mice in our laboratory, no overt
signs of gross toxicity or significant findings that would raise
concerns regarding unanticipated toxicological effects of Ad-REIC
were observed (3,4,8,9).
Therefore, even in clinical situations, the risk of significant
adverse effects and toxicity to other organs after intraprostatic
Ad-REIC administration might be limited.
Our results show that adenoviral vectors encoding
the REIC/Dkk-3 gene have no significant toxicity when injected into
the prostate of normal mice at the indicated doses. It seems that
Ad-REIC and its gene product, REIC protein, do not induce systemic
adverse effects under these conditions. Adenovirus-mediated in
situ REIC/Dkk-3 gene therapy is considered to be safe for use
as a treatment for human prostate cancer. We believe that the
present study provides valuable information for future clinical
trials with Ad-REIC and other adenovirus-mediated gene therapies
used to treat human prostate cancer.
Acknowledgements
This study was supported by a scientific research
grant (KAKENHI 23390382) from the Ministry of Education, Culture,
Sports, Science, and Technology of Japan.
References
|
1
|
Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y
and Namba M: A REIC gene shows downregulation in human immortalized
cells and human tumor-derived cell lines. Biochem Biophys Res
Commun. 268:20–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsieh SY, Hsieh PS, Chiu CT and Chen WY:
Dickkopf-3/REIC functions as a suppressor gene of tumor growth.
Oncogene. 23:9183–9189. 2004.PubMed/NCBI
|
|
3
|
Abarzua F, Sakaguchi M, Takaishi M, Nasu
Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H and Huh NH:
Adenovirus-mediated overexpression of REIC/Dkk-3 selectively
induces apoptosis in human prostate cancer cells through activation
of c-Jun-NH2-kinase. Cancer Res. 65:9617–9622. 2005. View Article : Google Scholar
|
|
4
|
Kashiwakura Y, Ochiai K, Watanabe M,
Abarzua F, Sakaguchi M, Takaoka M, Tanimoto R, Nasu Y, Huh NH and
Kumon H: Downregulation of inhibition of differentiation-1 via
activation of activating transcription factor 3 and Smad regulates
REIC/Dickkopf-3-induced apoptosis. Cancer Res. 68:8333–8341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang K, Watanabe M, Kashiwakura Y, Li SA,
Edamura K, Huang P, Yamaguchi K, Nasu Y, Kobayashi Y, Sakaguchi M,
et al: Expression pattern of REIC/Dkk-3 in various cell types and
the implications of the soluble form in prostatic acinar
development. Int J Oncol. 37:1495–1501. 2010.PubMed/NCBI
|
|
6
|
Mao B, Wu W, Davidson G, Marhold J, Li M,
Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al: Kremen
proteins are Dickkopf receptors that regulate Wnt/beta-catenin
signaling. Nature. 417:664–667. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abarzua F, Sakaguchi M, Tanimoto R,
Sonegawa H, Li DW, Edamura K, Kobayashi T, Watanabe M, Kashiwakura
Y, Kaku H, et al: Heat shock proteins play a crucial role in
tumor-specific apoptosis by REIC/Dkk-3. Int J Mol Med. 20:37–43.
2007.PubMed/NCBI
|
|
8
|
Edamura K, Nasu Y, Takaishi M, Kobayashi
T, Abarzua F, Sakaguchi M, Kashiwakura Y, Ebara S, Saika T,
Watanabe M, et al: Adenovirus-mediated REIC/Dkk-3 gene transfer
inhibits tumor growth and metastasis in an orthotopic prostate
cancer model. Cancer Gene Ther. 14:765–772. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe M, Kashiwakura Y, Huang P, Ochiai
K, Futami J, Li SA, Takaoka M, Nasu Y, Sakaguchi M, Huh NH and
Kumon H: Immunological aspects of REIC/Dkk-3 in monocyte
differentiation and tumor regression. Int J Oncol. 34:657–663.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanimoto R, Abarzua F, Sakaguchi M,
Takaishi M, Nasu Y, Kumon H and Huh NH: REIC/Dkk-3 as a potential
gene therapeutic agent against human testicular cancer. Int J Mol
Med. 19:363–368. 2007.PubMed/NCBI
|
|
11
|
Kawasaki K, Watanabe M, Sakaguchi M,
Ogasawara Y, Ochiai K, Nasu Y, Doihara H, Kashiwakura Y, Huh NH,
Kumon H and Date H: REIC/Dkk-3 overexpression downregulates
P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces
apoptosis in breast cancer. Cancer Gene Ther. 16:65–72. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin Y, Murata H, Sakaguchi M, Kataoka K,
Watanabe M, Nasu Y, Kumon H and Huh NH: Partial sensitization of
human bladder cancer cells to a gene-therapeutic adenovirus
carrying REIC/Dkk-3 by downregulation of BRPK/PINK1. Oncol Rep.
27:695–699. 2012.PubMed/NCBI
|
|
13
|
Freytag SO, Khil M, Stricker H, Peabody J,
Menon M, DePeralta-Venturina M, Nafziger D, Pegg J, Paielli D,
Brown S, et al: Phase I study of replication-competent
adenovirus-mediated double suicide gene therapy for the treatment
of locally recurrent prostate cancer. Cancer Res. 62:4968–4976.
2002.PubMed/NCBI
|
|
14
|
Kubo H, Gardner TA, Wada Y, Koeneman KS,
Gotoh A, Yang L, Kao C, Lim SD, Amin MB, Yang H, et al: Phase I
dose escalation clinical trial of adenovirus vector carrying
osteocalcin promoter-driven herpes simplex virus thymidine kinase
in localized and metastatic hormone-refractory prostate cancer. Hum
Gene Ther. 14:227–241. 2003. View Article : Google Scholar
|
|
15
|
van der Linden RR, Haagmans BL,
Mongiat-Artus P, van Doornum GJ, Kraaij R, Kadmon D,
Aguilar-Cordova E, Osterhaus AD, van der Kwast TH and Bangma CH:
Virus specific immune responses after human neoadjuvant
adenovirus-mediated suicide gene therapy for prostate cancer. Eur
Urol. 48:153–161. 2005.PubMed/NCBI
|
|
16
|
Su C, Cao H, Tan S, Huang Y, Jia X, Jiang
L, Wang K, Chen Y, Long J, Liu X, et al: Toxicology profiles of a
novel p53-armed replication-competent oncolytic adenovirus in
rodents, felids, and nonhuman primates. Toxicol Sci. 106:242–250.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Belloc F, Dumain P, Boisseau MR,
Jalloustre C, Reiffers J, Bernard P and Lacombe F: A flow
cytometric method using Hoechst 33342 and propidium iodide for
simultaneous cell cycle analysis and apoptosis determination in
unfixed cells. Cytometry. 17:59–65. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maciorowski Z, Delic J, Padoy E,
Klijanienko J, Dubray B, Cosset JM, Dumont J, Magdelénat H and
Vielh P: Comparative analysis of apoptosis measured by Hoechst and
flow cytometry in non-Hodgkin’s lymphomas. Cytometry. 32:44–50.
1998.PubMed/NCBI
|
|
19
|
Muruve DA, Barnes MJ, Stillman IE and
Libermann TA: Adenoviral gene therapy leads to rapid induction of
multiple chemokines and acute neutrophil-dependent hepatic injury
in vivo. Hum Gene Ther. 10:965–976. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakaguchi M, Kataoka K, Abarzua F,
Tanimoto R, Watanabe M, Murata H, Than SS, Kurose K, Kashiwakura Y,
Ochiai K, et al: Overexpression of REIC/Dkk-3 in normal fibroblasts
suppresses tumor growth via induction of interleukin-7. J Biol
Chem. 284:14236–14244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ochiai K, Watanabe M, Ueki H, Huang P,
Fujii Y, Nasu Y, Noguchi H, Hirata T, Sakaguchi M, Huh NH,
Kashiwakura Y, Kaku H and Kumon H: Tumor suppressor REIC/Dkk-3
interacts with the dynein light chain, Tctex-1. Biochem Biophys Res
Commun. 412:391–395. 2011. View Article : Google Scholar : PubMed/NCBI
|