Introduction
Gastric cancer is the fourth most common cancer in
the world (1). Standard methods of
treatment, e.g. surgery, chemotherapy and radiotherapy have shown
mixed success for early-stage patients. However, the prognosis of
patients with advanced-stage gastric cancer is still extremely
poor. Currently, transfusion of lymphocytes or adoptive cellular
immunotherapy (ACI) have been used for the treatment of cancer both
in animal studies (2–4) and clinic trials (5–7). ACI
has also been used to treat gastric cancer with encouraging
results, indicating that ACI can improve the prognosis of some
patients, especially those who cannot tolerate surgery (8).
Cytokine-induced killer cells (CIKs) are commonly
used as immune effector cells to treat cancer (9–11). The
major component of the heterogeneous CIK cells, NKT cells,
expresses both T cell marker CD3 and NK cell marker CD56 and
exhibits non-major histocompatibility complex (MHC)-restricted
cytotoxicity. This represents an effective mechanism in the
treatment of cancer. Infusion of CIK cells is typically through an
intravenous pathway; however, little is known regarding the number
of cells that actually arrive at the tumor site with this method
and how long these cells could survive in vivo.
Apart from CIK cells, many other immune cells such
as lymphokine-activated killer (LAK) cells, tumor infiltrating
lymphocytes (TILs) and antigen-specific cytotoxic T lymphocytes
(CTLs) have been used in adoptive therapy (5). CTLs play a central role in antitumor
immunity (12,13) and have demonstrated therapeutic
efficacy of cancer immunotherapy both in vivo and in
vitro. However, the in vivo distribution of these cells
following various methods of injection is also unclear.
In this study, we infused PKH26-labeled human CIK
cells or CTLs into nude mice with established EGFP-positive human
gastric cancer through different pathways and sequentially examined
the tissue distribution of CIK cells and CTLs using live
fluorescence imaging. This study intended to identify the effective
ACI cell type as well as the effective route of immune cell
delivery to the tumor.
Materials and methods
Mice
Four-week-old female BALB/c nude (nu/nu) mice were
acquired from the Animal Center of the Academy of Military Medical
Sciences (Beijing, China). All animals were maintained in a
pathogen-free environment and all animal protocols followed the
experimental procedures of the National Institutes of Health Guide
for Care and Use of Laboratory Animals.
Cell line
The poorly differentiated human gastric
adenocarcinoma cell line, BGC823, was purchased from the Chinese
Academy of Medical Sciences (Beijing, China). These cells are kept
in our laboratory at the Institute of General Surgery, General
Hospital of PLA (Beijing, China) and maintained in Dulbecco's
modified Eagle's medium (DMEM) (Sigma, St. Louis, MO, USA).
DNA transfection and isolation of stable
EGFP-expressing cells
BGC823 tumor cells were transfected with the
pEGFP-C1 plasmid (Clontech, Mountain View, CA, USA) using the
Xfect™ transfection reagent (Clontech). The neomycin resistance
gene (neoR) in pEGFP-C1 plasmid allows stably transfected tumor
cells to be selected using G418. After transfection, the cells were
passaged at a ratio of 1:5 in selective medium that contained 200
μg/ml geneticin (G418; Sigma). The level of G418 was increased to
2,000 μg/ml in a stepwise manner (increased every day by 100
μg/ml). Cell clones expressing high levels of EGFP were isolated by
limit dilution in 96-well plates. The EGFP-expressing clones were
then amplified and transferred by conventional culture methods.
This resulted in the identification of a cell line with bright EGFP
fluorescence, designated as BGC823-EGFP that was used in this
study.
Tumor challenge
Nude mice were challenged with 1×107
BGC823-EGFP cells, subcutaneously. Whole-body images were taken
using the IVIS-200 Imaging System (Xenogen, Alameda, CA, USA) on
Day 10.
Dendritic cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) from
healthy donor were cultured with Cellix-901 medium (Beijing
XinMingLiTai Bio-technique, Co., Ltd., Beijing, China) for 2 h.
Adherent cells were collected and further cultured in Cellix-901
medium with 1,000 IU/ml rhIL-4 (Cell Genix) and 1,000 IU/ml
rhGM-CSF for 7 days to generate a dendritic cell (DC)-enriched cell
population. DCs were then pulsed with 3 μl/ml Pseudomonas
aeruginosa and 20 μg/ml BGC823 cell lysate followed by
incubation for 12 h before use for the next step. To prepare BGC823
cell lysate, BGC823 cells were digested with trypsin, washed in
normal saline and centrifuged at 450 × g for 5 min. The supernatant
was then discarded and cells were resuspended in sterile water, and
were frozen and thawed repeatedly 3 times. Lysate was obtained
after centrifugation and filtration.
CIK, CTL and CIK-CEA/CD3-bscAb cell
isolation and culture
Using a blood cell separator (Institute of
Biomedical Engineering, Chinese Academy of Medical Sciences),
2–4×109 PBMCs from healthy donors were obtained. To
prepare CIK, cell concentration was adjusted to 2×106
cells/ml in fresh, serum-free Cellix-601 medium (Beijing
XinMingLiTai Bio-technique, Co., Ltd.) with 2,000 U/ml rhIFN-γ and
incubated at 37°C in a humidified atmosphere of 50 ml/l
CO2. After 24 h, anti-CD3 (50 ng/ml) and rhIL-2 (1,000
U/ml) were added. On Days 0, 4, 7 and 10 of culture, cell densities
were determined and phenotypes were identified by flow cytometric
analysis (FACS) (Becton-Dickinson, USA). To make CTL cells, PBMCs
were incubated in tissue culture flasks with the Ag-pulsed DCs. To
generate CIK-CEA/CD3-bscAb cells, CEA/CD3-bispecific single chain
antibody (Beijing ABT Genetic Engineering Technology, Co., Ltd.,
Beijing, China) was added into the medium to bind CIK cells to
obtain CIK-CEA/CD3-bscAb cells.
Immune cell labeling and in vitro
assays
Immune cells were labeled with red fluorescent PKH26
(Sigma-Aldrich, Co., LLC.) following the kit instructions. CIK-,
CIK-CEA/CD3-bscAb- and CTL-mediated cell cytotoxicity was evaluated
using the lactate dehydrogenase (LDH) release assay according to
the manufacturer's protocol. Cytotoxicity was assessed as
[(Asample-Aspontaneous)/(Amaximum-Aspontaneous)]
×100%, (A, absorbance).
Tracing immune cells in vivo and
evaluating the inhibition of tumor growth
Seven days after BGC823-EGFP cells were implanted
into the nude mice, the tumor-bearing animals were separated into 3
groups. All mice were injected with 1×107 labeled CIKs,
intraperitoneally (i.p., Group 1) ; intravenously (i.v., Group 2)
or peritumorally (p.t., Group 3), respectively. The migration and
distribution of the infused CIK cells were then observed using the
IVIS-200 Imaging System (Xenogen) at 4, 24, 48 and 96 h after CIK
cells injection. The lung, liver, spleen, kidney, stomach and
intestine were collected on Days 2 and 8 for histopathological
analysis. Tumor size and weight were assessed on Day 35 to evaluate
the therapeutic efficacy of transferred CIK cells.
In separate experiments, we set up 3 groups to
compare the therapeutic efficacy of CIK, CIK-CEA/CD3-bscAb and CTL
cells. Each group of labeled immune cells was injected
peritumorally at 1×107 cells/mouse. Migration and
distribution of the infused immune cells were then observed at 4,
24, 48, 96 and 144 h after cell infusion.
Statistical analysis
Statistical analyses were performed with the SPSS
17.0 statistical software using the Student's t-test and ANOVA.
P-values of <0.05 were considered statistically significant
between the experimental groups.
Results
Antitumor effect of different immune
cells in vitro
Firstly, we detected the antitumor effect of
labeling on the immune cells. No difference was observed in cell
morphology after labeling with PKH26. Strong red fluorescence was
detected by flxuorescence microscopy (Fig. 1A) and FACS analysis showed that
99.37% of CIK cells were PKH26-positive (Fig. 1B). There was no significant
difference in the antitumor activity between CIK and CIK-PKH26
cells (Table I).
 | Table IIn vitro antitumor effects of
CIK cells before and after labeling with PKH26 (mean ± SD,
n=3). |
Table I
In vitro antitumor effects of
CIK cells before and after labeling with PKH26 (mean ± SD,
n=3).
| | 5 h | 10 h |
|---|
| |
|
|
|---|
| Groups | E/T ratio | LDH | Cytotoxicity,
% | LDH | Cytotoxicity,
% |
|---|
| CIK | 5:1 | 0.457±0.023 | 70.51±3.61 | 0.555±0.024 | 95.43±4.84 |
| 10:1 | 0.697±0.081 | 84.44±4.48 | 0.894±0.090 | 100 |
| CIK-PKH26 | 5:1 | 0.460±0.017 | 68.88±3.67 | 0.555±0.023 | 93.78±3.06 |
| 10:1 | 0.701±0.063 | 83.54±4.98 | 0.901±0.028 | 100 |
In addition to CIK cells, CIK-CEA/CD3-bscAb cells
and CTLs also showed strong antitumor activity against BGC823 cells
in vitro. At E:T cell ratios of 5:1 and 10:1 and at 5 and 10
h, the BGC823 cell killing activity was as follows: CTL >
CIK-CEA/CD3-bscAb > CIK cells (Table II).
 | Table IIAntitumor effect of different immune
cells in vitro (mean ± SD, %, n=3). |
Table II
Antitumor effect of different immune
cells in vitro (mean ± SD, %, n=3).
| 5 h | 10 h |
|---|
|
|
|
|---|
| Groups | 5:1 E/T ratio | 10:1 E/T ratio | 5:1 E/T ratio | 10:1 E/T ratio |
|---|
| CIK | 70.51±3.61 | 84.44±4.48 | 95.43±4.84 | 100 |
|
CIK-CEA/CD3-bscAb | 74.09±2.01 | 88.01±2.88 | 100c | 100 |
| CTL | 85.59±5.14a,b | 97.47±2.20a,b | 100c | 100 |
Establishment of human gastric cancer
(BGC823) in nude mice subcutaneously
Fluorescence imaging was employed to monitor the
growth of human gastric cancer in nude mice. First of all, we
successfully transfected BGC823 cells with EGFP (BGC823-EGFP cells)
and selected them with G418 (Fig.
2A). We then injected parental BGC823 and BGC823-EGFP cells
subcutaneously into the nude mice, respectively. The BGC823-EGFP
subcutaneous tumor was visible with the IVIS-200 Imaging System
within 10 days after tumor cell implantation (Fig. 2B).
EGFP transfection was found to have no effect on
BGC823 cell morphology and cell proliferation in vitro
(Fig. 2C) or tumor growth in
vivo (Fig. 2D). In addition, no
difference was observed in the histopathological analysis between
BGC823 and BGC823-EGFP tumors (Fig. 2E
and F).
Distribution and antitumor effect of CIK
cells infused via three different pathways
When CIK cells were i.p. injected (Fig. 3A), red fluorescent PKH26-labeled CIK
cells tended to gather first in the abdominal cavity. At 24 h
post-injection, cells began to spread, and a small amount of
accumulation could be observed in the tumor area before gradually
dissipating. On Day 5 post-injection, the red fluorescence was
nearly gone. In the i.v. group (Fig.
3B), CIK-PKH26 cells dispersed rapidly in the body, and a small
amount of accumulation could be observed in the tumor area 24 h
post-injection. On Day 5, the red fluorescence vanished. In
contrast, in the peritumoral injection group (Fig. 3C), CIK-PKH26 cells gathered around
the tumor after injection and dissipated very slowly. On Day 5, we
could still detect a small amount of red fluorescence around the
tumor.
On Day 2 post-injection via p.t. injection, labeled
CIK cells were found to infiltrate the tumor bed in high numbers,
although this infiltration was mainly localized around the tumor
(Fig. 4A), while in the i.p.
(Fig. 4B) and i.v. groups (Fig. 4C), the red fluorescent spots were
much fewer. In the i.p. group, the labeled CIK cells were detected
infiltrating multiple organs. The spleen was the most enriched in
CIK cells (Fig. 4D), followed by
the liver (Fig. 4E), kidney
(Fig. 4F), intestine (Fig. 4G), stomach (Fig. 4H) and lung (Fig. 4I). On Day 8 post-injection, we found
only a small quantity of red fluorescent spots in the liver and
spleen in the i.p. and i.v. groups; however, in the peritumoral
injection group, some labeled CIK cells were still found
infiltrating the tumor bed (Fig.
4J), liver (Fig. 4K) and spleen
(Fig. 4L).
The infusion of CIK cells via each of the three
pathways, particularly peritumorally, was found to inhibit
subcutaneous tumor growth in nude mice. Three weeks after CIK
transfusion, tumors of the peritumoral injection group were
significantly smaller than the normal saline (NS)-treated control
group. Although not statistically significant, tumors of the i.p.
and i.v. groups were also smaller. Four weeks after transfusion,
tumor weight in the peritumoral, i.v. and i.p. injection groups was
significantly lighter than the NS-treated control groups (Fig. 5).
Distribution and antitumor effect of
different immune cells infused via peritumoral injection
Following peritumoral injection, all of the immune
cells were gathered around the tumor. CIK cells were nearly gone 5
days post-injection (Fig. 6A),
followed by the dispersal of CTL cells within 7 days (Fig. 6B). However, CIK-CEA/CD3-bscAb cells
remained crowded around the tumor 7 days post-injection (Fig. 6C).
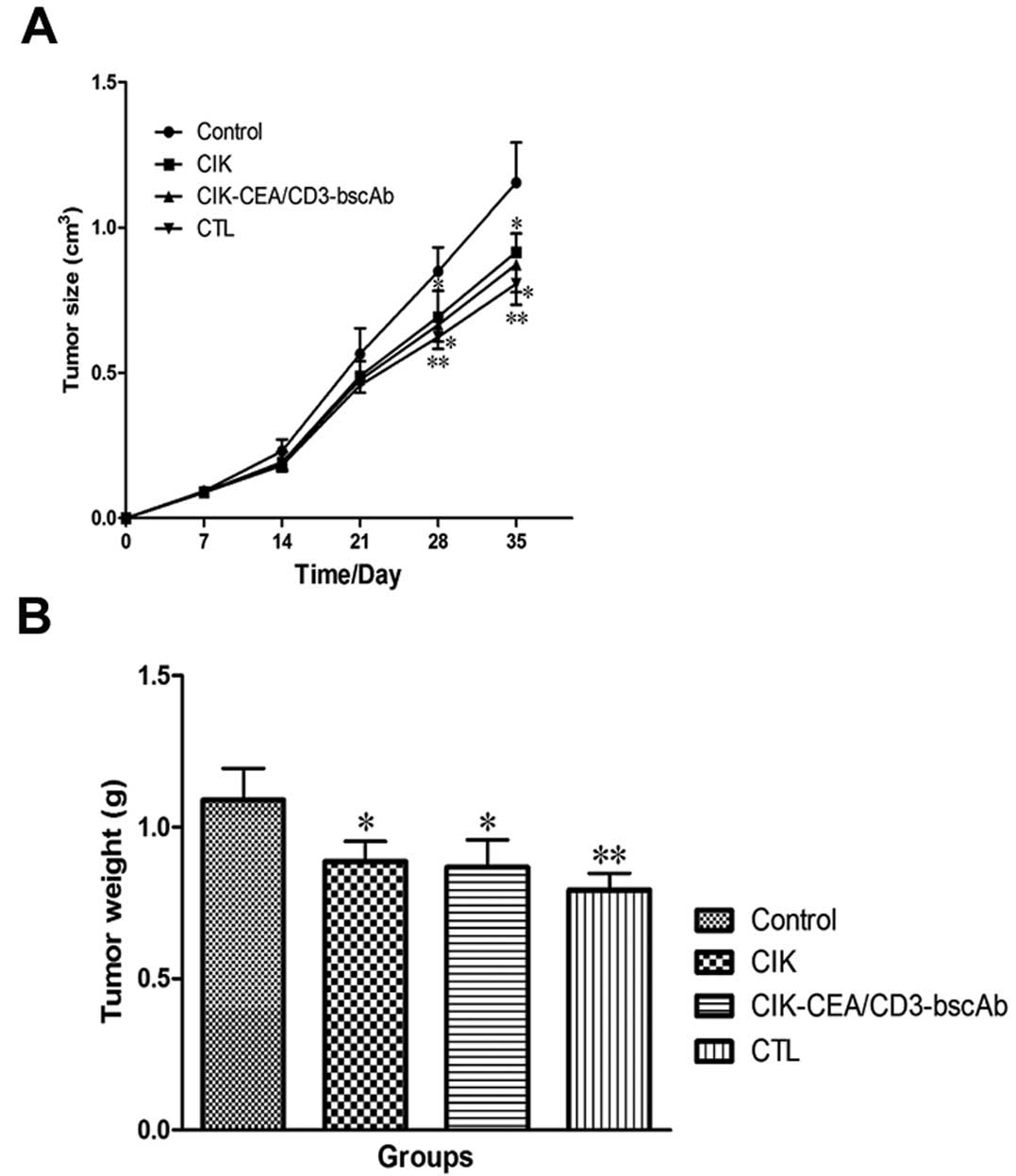
Peritumoral injection of three immune cell types,
particularly CTLs, was found to inhibit subcutaneous tumor growth
in nude mice (Fig. 7). Control
tumor weight was 1.09±0.10 g 35 days after implantation. CTL,
CIK-CEA/CD3-bscAb and CIK cells injected peritumorally 7 days after
tumor implantation reduced tumor weight by 27.29±5.05, 20.41±8.35
and 18.58±6.01%, respectively.
Discussion
Whole-body imaging has proven to be a useful
technology for the study of the dynamics of metastatic cancer.
Green fluorescent protein (GFP) expression in cancer cells can help
externally to image and follow the natural course or impediment of
tumor progression and metastasis (14). Tumor motility, progression and
metastasis can be visualized at the single-cell level in
vivo with GFP (15). EGFP is
the enhanced GFP, which is made for a heightened brightness
(16). In this study, we have
successfully established the stable EGFP-expressing human gastric
adenocarcinoma BGC823 cells (BGC823-EGFP). These cells form
subcutaneous tumors in nude mice. BGC823-EGFP cells have revealed
identical biological characteristics as parental BGC823 cells, and
can therefore be used to investigate tumor growth and metastasis
(15,17,18).
CIK cells are polyclonal T effector cells generated
when cultured under conditions of cytokine stimulation (19). The main effector cells of the
heterogeneous CIK cell family express NK and T cell markers CD56
and CD3, respectively, and are referred to as NK-like T (NKT)
cells. These cells possess non-MHC-restricted antitumor activity
which means they do not require prior specific sensitization to
induce the recognition of target cells (9,20,21).
Over the years, CIK cells have been used for their antitumor
activity against a variety of tumor targets (10,11,22).
In this study, we found that CIK cells killed 100% of BGC823 cells
within 10 h at the E:T cell ratio of 10:1 in vitro. CIK
cells also exhibited antitumor activity in vivo. In our
gastric cancer nude mouse model, infused CIKs inhibited
subcutaneous tumor growth. Importantly, we observed that CIK cells
infused via different pathways demonstrated different distribution
and tumor inhibitory effect.
CIK cells are usually infused intravenously in
adoptive cellular immunotherapy (ACI). Hazelrigg et al
(23) found that the immune cells
first arrived at the lungs after intravenous transfusion. In 2–6 h,
accumulation in the lungs reached a peak and dissipated, with
gradual accumulation in the liver, kidney and spleen. The overall
cell distribution tended to stabilize within 24 h. Skitzki et
al (24) reported that CIK
cells could widely immigrate into most organs after intravenous
transfusion; distribution was related to blood supply and immune
properties of the organs as well as the order in which cells
reached each organ. Furthermore, the in vivo cytotoxic
activity of adoptively transferred immune cells is mainly observed
in the initial peak period post-transfusion. These studies suggest
that immune cells can effectively reach most organs and tumor
tissue while maintaining their cytotoxic abilities. For leukemia,
lymphoma and other non-solid tumors, CIK cells can spread
throughout the body, including the bone marrow, via intravenous
transfusion to kill tumor cells (25,26).
However, for solid tumors, such as gastric cancer, the ability of
CIKs to target and accumulate in tumor tissue remains to be fully
defined.
In our study, we found that, after intravenous
transfusion, CIK cells dissipated with the blood circulation and
could indeed arrive at the tumor tissue. In comparison,
intraperitoneally infused CIK cells first gathered in the abdominal
cavity, then distribution followed the same course as the
intravenous group by 24 h. In contrast, peritumoral injection
resulted in the maintenance of CIK cells in the tumor tissue for
the maximum amount of time examined. In this case migration of
infused CIK cells to other organs, such as the liver and spleen,
was evident but not to the degree of the i.v. or i.p. groups. In
terms of tumor growth, peritumoral injection of CIK cells showed
improved inhibition. Together, these results indicate that immune
cells used for cancer adoptive immunotherapy, if injected directly
into the tumor area, may be able to achieve the maximum antitumor
effect.
In order to potentiate the antitumor activity of CIK
cells, many immunological manipulations have been developed. For
example stimulating factors such as Bacille Calmette-Guerin
(27) and virus vaccine (28) were added in cell culture to improve
cell proliferation. Interleukin-2 (IL-2) genes were transfected
into CIK cells to enhance their IL-2 production and potentiate
their cytotoxicity (29). DCs were
engineered to present tumor antigens to CIK cells with the hopes of
enhancing specific recognition of tumor cells and their subsequent
killing (30,31). Furthermore, bispecific antibodies
for CD3 on T and/or NKT cells and surface antigen on the target
cells were used to promote the engagement of CIK cells with target
cells (32,33). We have previously found that the
gastric adenocarcinoma BGC823 cells express surface
carcinoembryonic antigen (CEA). We therefore used
CEA/CD3-bispecific single chain antibody to coat CIK cells.
CEA/CD3-bscAb can pull together T lymphocytes and CEA-expressing
tumor cells (34). In this study,
CIK-CEA/CD3-bscAb cells showed stronger antitumor activity than CIK
cells against BGC823 cells in vitro. Use of CEA/CD3-bscAb to
arm CIK cells may represent a potential approach to enhance the
antitumor activity of CIK cells.
CTL cells are a subpopulation of T cells with
specific cytotoxicity. CD8+ CTLs are the most numerous
members of the CTL subgroup. After priming by antigen presented by
antigen presenting cells, CTLs can recognize and kill corresponding
target cells (35,36). Adoptive transfer of antitumor CTLs
has already been employed in clinical trials and has shown to be
effective in adoptive immunotherapy of ovarian cancer, melanoma,
breast cancer and renal carcinoma (12,37–40).
In this study, CTL cells were derived from PBMCs and stimulated by
DCs loaded with BGC823 tumor cell lysate. We found that the
antitumor activity against gastric cancer of the CTLs was much
stronger than CIK cells not stimulated with Ag-loaded DCs.
In summary, our results indicate that immune cells
such as CIKs and CTLs have strong and direct cytotoxic effects on
gastric cancer cells, which suggests that immunotherapy using these
immune cells, may become a novel treatment strategy for patients
with gastric cancer following surgery, radiotherapy and
chemotherapy.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (no. 60601018).
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Kim HM, Kang JS, Lim J, et al: Antitumor
activity of cytokine-induced killer cells in nude mouse xenograft
model. Arch Pharm Res. 32:781–787. 2009. View Article : Google Scholar
|
|
3
|
Klebanoff CA, Gattinoni L, Palmer DC, et
al: Determinants of successful CD8+ T-cell adoptive
immunotherapy for large established tumors in mice. Clin Cancer
Res. 17:5343–5352. 2011.PubMed/NCBI
|
|
4
|
Marcus A and Eshhar Z: Tumor-specific
allogeneic cells for cancer therapy. Expert Opin Biol Ther.
11:1551–1554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
June CH: Adoptive T cell therapy for
cancer in the clinic. J Clin Invest. 117:1466–1476. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Stasi A, Tey SK, Dotti G, et al:
Inducible apoptosis as a safety switch for adoptive cell therapy. N
Engl J Med. 365:1673–1683. 2011.PubMed/NCBI
|
|
7
|
Zhong JH, Ma L, Wu LC, et al: Adoptive
immunotherapy for postoperative hepatocellular carcinoma: a
systematic review. Int J Clin Pract. 66:21–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toh U, Fujii T, Mishima M, et al:
Conventional chemotherapy combined with the repetitive immune cell
transfer for patients with refractory advanced gastric cancer. Gan
To Kagaku Ryoho. 34:1931–1933. 2007.(In Japanese).
|
|
9
|
Linn YC and Hui KM: Cytokine-induced
NK-like T cells: from bench to bedside. J Biomed Biotechnol.
2010:4357452010.PubMed/NCBI
|
|
10
|
Sangiolo D: Cytokine induced killer cells
as promising immunotherapy for solid tumors. J Cancer. 2:363–368.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma Y, Zhang Z, Tang L, et al:
Cytokine-induced killer cells in the treatment of patients with
solid carcinomas: a systematic review and pooled analysis.
Cytotherapy. 14:483–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wright SE, Rewers-Felkins KA, Quinlin IS,
et al: Cytotoxic T-lymphocyte immunotherapy for ovarian cancer: a
pilot study. J Immunother. 35:196–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hickey MJ, Malone CC, Erickson KE, et al:
Implementing preclinical study findings to protocol design:
translational studies with alloreactive CTL for gliomas. Am J
Transl Res. 4:114–126. 2012.PubMed/NCBI
|
|
14
|
Yang M, Baranov E, Jiang P, et al:
Whole-body optical imaging of green fluorescent protein-expressing
tumors and metastases. Proc Natl Acad Sci USA. 97:1206–1211. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chishima T, Miyagi Y, Wang X, et al:
Cancer invasion and micrometastasis visualized in live tissue by
green fluorescent protein expression. Cancer Res. 57:2042–2047.
1997.PubMed/NCBI
|
|
16
|
Heim R, Cubitt AB and Tsien RY: Improved
green fluorescence. Nature. 373:663–664. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaneko K, Yano M, Tsujinaka T, et al:
Establishment of a visible peritoneal micrometastatic model from a
gastric adenocarcinoma cell line by green fluorescent protein. Int
J Oncol. 16:893–898. 2000.PubMed/NCBI
|
|
18
|
Kaneko K, Yano M, Yamano T, et al:
Detection of peritoneal micrometastases of gastric carcinoma with
green fluorescent protein and carcinoembryonic antigen promoter.
Cancer Res. 61:5570–5574. 2001.PubMed/NCBI
|
|
19
|
Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume
KG and Weissman IL: Use of a SCID mouse/human lymphoma model to
evaluate cytokine-induced killer cells with potent antitumor cell
activity. J Exp Med. 174:139–149. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Linn YC, Lau LC and Hui KM: Generation of
cytokine-induced killer cells from leukaemic samples with in vitro
cytotoxicity against autologous and allogeneic leukaemic blasts. Br
J Haematol. 116:78–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lopez RD, Waller EK, Lu PH and Negrin RS:
CD58/LFA-3 and IL-12 provided by activated monocytes are critical
in the in vitro expansion of CD56+ T cells. Cancer
Immunol Immunother. 49:629–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niam M, Linn YC, Fook Chong S, et al:
Clinical scale expansion of cytokine-induced killer cells is
feasible from healthy donors and patients with acute and chronic
myeloid leukemia at various stages of therapy. Exp Hematol.
39:897–903. 2011. View Article : Google Scholar
|
|
23
|
Hazelrigg MR, Hirsch JI and Merchant RE:
Distribution of adoptively transferred, tumor-sensitized
lymphocytes in the glioma-bearing rat. J Neurooncol. 60:143–150.
2002. View Article : Google Scholar
|
|
24
|
Skitzki J, Craig RA, Okuyama R, et al:
Donor cell cycling, trafficking, and accumulation during adoptive
immunotherapy for murine lung metastases. Cancer Res. 64:2183–2191.
2004. View Article : Google Scholar
|
|
25
|
Edinger M, Cao YA, Verneris MR, Bachmann
MH, Contag CH and Negrin RS: Revealing lymphoma growth and the
efficacy of immune cell therapies using in vivo bioluminescence
imaging. Blood. 101:640–648. 2003. View Article : Google Scholar
|
|
26
|
Nishimura R, Baker J, Beilhack A, et al:
In vivo trafficking and survival of cytokine-induced killer cells
resulting in minimal GVHD with retention of antitumor activity.
Blood. 112:2563–2574. 2008. View Article : Google Scholar
|
|
27
|
Brandau S and Bohle A: Activation of
natural killer cells by Bacillus Calmette-Guerin. Eur Urol.
39:518–524. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akagi J, Takai E, Tamori Y and Ogawa M:
CD3+CD56+CD8+ cells demonstrating
a suppressor T cell-like function in the peripheral blood of colon
cancer patients. Int J Oncol. 19:561–566. 2001.
|
|
29
|
Nagaraj S, Ziske C and Schmidt-Wolf IG:
Human cytokine-induced killer cells have enhanced in vitro
cytolytic activity via non-viral interleukin-2 gene transfer. Genet
Vaccines Ther. 2:122004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marten A, Renoth S, von Lilienfeld-Toal M,
et al: Enhanced lytic activity of cytokine-induced killer cells
against multiple myeloma cells after co-culture with
idiotype-pulsed dendritic cells. Haematologica. 86:1029–1037.
2001.
|
|
31
|
Ziske C, Marten A, Schottker B, et al:
Resistance of pancreatic carcinoma cells is reversed by coculturing
NK-like T cells with dendritic cells pulsed with tumor-derived RNA
and CA 19-9. Mol Ther. 3:54–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Spriel AB, van Ojik HH and van De
Winkel JG: Immunotherapeutic perspective for bispecific antibodies.
Immunol Today. 21:391–397. 2000.PubMed/NCBI
|
|
33
|
Flieger D, Kufer P, Beier I, Sauerbruch T
and Schmidt-Wolf IG: A bispecific single-chain antibody directed
against EpCAM/CD3 in combination with the cytokines interferon
alpha and interleukin-2 efficiently retargets T and
CD3+CD56+ natural-killer-like T lymphocytes
to EpCAM-expressing tumor cells. Cancer Immunol Immunother.
49:441–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lutterbuese R, Raum T, Kischel R, et al:
Potent control of tumor growth by CEA/CD3-bispecific single-chain
antibody constructs that are not competitively inhibited by soluble
CEA. J Immunother. 32:341–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Savage P, Millrain M, Dimakou S, Stebbing
J and Dyson J: Expansion of CD8+ cytotoxic T cells in
vitro and in vivo using MHC class I tetramers. Tumour Biol.
28:70–76. 2007.
|
|
36
|
Morishima N, Mizoguchi I, Okumura M, et
al: A pivotal role for interleukin-27 in CD8+ T cell
functions and generation of cytotoxic T lymphocytes. J Biomed
Biotechnol. 2010:6054832010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yee C, Thompson JA, Byrd D, et al:
Adoptive T cell therapy using antigen-specific CD8+ T
cell clones for the treatment of patients with metastatic melanoma:
in vivo persistence, migration, and antitumor effect of transferred
T cells. Proc Natl Acad Sci USA. 99:16168–16173. 2002.PubMed/NCBI
|
|
38
|
Mackensen A, Meidenbauer N, Vogl S, Laumer
M, Berger J and Andreesen R: Phase I study of adoptive T-cell
therapy using antigen-specific CD8+ T cells for the
treatment of patients with metastatic melanoma. J Clin Oncol.
24:5060–5069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Butler MO, Lee JS, Ansen S, et al:
Long-lived antitumor CD8+ lymphocytes for adoptive
therapy generated using an artificial antigen-presenting cell. Clin
Cancer Res. 13:1857–1867. 2007.PubMed/NCBI
|
|
40
|
Yamaguchi Y, Ohshita A, Hironaka K, et al:
Adoptive immunotherapy using autologous lymphocytes sensitized with
HLA class I-matched allogeneic tumor cells. Oncol Rep. 16:165–169.
2006.
|