Introduction
Colorectal carcinoma (CRC) is a serious global
health problem, with over one million new cases and half a million
mortalities worldwide each year (1). The pathogenesis of CRC is complex,
with the involvement of multiple cellular transduction pathways
including IL-6/STAT3 signaling. Interleukin-6 (IL-6) is an
important pro-inflammatory cytokine that has been shown to play a
potential role in CRC. Elevated IL-6 levels have been detected in
the serum (2) and cancer tissue
(3) in CRC patients. In addition,
IL-6 levels are correlated with tumor size and are commonly
associated with the disease severity (2,4). IL-6
has also been shown to directly stimulate the proliferation of some
colon cancer cell lines in vitro (5). Recent studies indicated that IL-6
signal transduction in CRC is remarkably not mediated by the
membrane-bound receptor for IL-6 (IL-6R), but the soluble form of
the IL-6R (sIL-6R), a process called IL-6 trans signaling (6–8). The
IL-6/sIL-6R complex in turn binds to a common signal transducing
receptor gp130, promoting dimerization of gp130 and then resulting
in activation of the associated Janus kinases (JAKs). Activated
JAKs phosphorylate gp130, leading to the recruitment and activation
of signal transducer and activator of transcription 3 (STAT3)
(9). STAT3 is an important
transcription factor that plays an essential role in cell survival
and proliferation (10,11). Following activation via
phosphorylation at tyrosine 705 by JAKs, STAT3 proteins in the
cytoplasm dimerize and translocate to the nucleus where they
regulate the expression of various critical genes involved in cell
proliferation and survival (12–14).
Constitutive activation of STAT3 has been found in many types of
human cancer and generally suggests poor prognosis (15–18).
IL-6 signal transduction is regulated in a variety
of ways. Suppressor of cytokine signaling 3 (SOCS3) is regarded as
a key negative regulator of the IL-6/STAT3 pathway. SOCS3 can be
rapidly induced by IL-6 stimulation but it then limits
IL-6-mediated STAT3 phosphorylation/activation through
competitively binding to gp130 and JAKs (9), creating a negative feedback loop of
IL-6/JAK/STAT3 signal transduction cascade (19–22).
Reduced or silenced SOCS3 has been found in many human types of
cancer including CRC (23–25), and restoring SOCS3 expression in
cancer cells inhibited IL-6-mediated STAT3 activation, induced
tumor cell apoptosis and decreased cell proliferation (23,26).
Therefore, suppression of the IL-6/STAT3 pathway via modulation of
SOCS3 has been a promising strategy for anticancer therapies.
Despite recent advances in CRC chemotherapy,
5-fluorouracil (5-FU)-based regimens continue to be the
international standard chemotherapy for patients with advanced CRC
(27). However, due to drug
resistance and the unacceptable level of toxicity to normal cells,
systemic chemotherapy using 5-FU-based regimens produces objective
response rates of less than 40% (28–30).
These problems highlight the urgent need for the development of
novel cancer chemotherapies. Natural products, such as traditional
Chinese herbal medicines (TCMs), have received attention as they
have relatively few side-effects and have long been used clinically
as significant alternative remedies for a variety of diseases
(31–33). Pien Tze Huang (PZH) is a well-known
TCM formula that was first prescribed 450 years ago in the Ming
Dynasty. The main ingredients of PZH include Moschus, Calculus
Bovis, Snake Gall and Radix Notoginseng. These products
together confer PZH properties of heat-clearing, detoxification,
promotion of blood circulation and removal of blood stasis
(34). PZH has been used in China
and Southeast Asia for centuries as a folk remedy for various types
of cancer (35,36), since in the TCM system accumulation
of toxic dampness and heat is one of the major causative factors in
the pathogenesis of cancer and, therefore, clearing heat and
detoxification is a principle of anticancer treatment. We
previously reported that PZH can inhibit colon cancer cell growth
in vitro via promotion of apoptosis (37). In addition, using a CRC mouse
xenograft model we found that PZH can suppress tumor growth in
vivo without apparent adverse effects; PZH treatment also
reduces the phosphorylation level of STAT3 in tumor tissues
(38). To further elucidate the
mechanism of the tumoricidal activity of PZH, herein we
investigated its effects on the IL-6-mediated activities in human
carcinoma HT-29 cells, such as cell proliferation and apoptosis,
phosphorylation level and transcriptional activity of STAT3, as
well as the expression of several IL-6/STAT3 signaling target genes
including SOCS3.
Materials and methods
Materials and reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA,
Lipofectamine™ LTX with PLUS™ reagent, TRIzol reagent were
purchased from Invitrogen (Carlsbad, CA, USA). Bcl-2, cyclin D1,
SOCS3 and phospho-STAT3 (Tyr705) antibodies, horseradish
peroxidase (HRP)-conjugated secondary antibodies were obtained from
Cell Signaling (Beverly, MA, USA). SuperScript II reverse
transcriptase and Dual-Luciferase Reporter Assay System were
obtained from Promega (Madison, WI, USA). The Hoechst staining kit
was obtained from the Beyotime Institute of Biotechnology (Jiangsu,
China). Cignal STAT3 Reporter (luc) kit was obtained from
SABiosciences (Qiagen, Hilden, Germany). All the other chemicals,
unless otherwise stated, were obtained from Sigma Chemicals (St.
Louis, MO, USA).
Preparation of PZH
PZH was obtained from and authenticated by the sole
manufacturer Zhangzhou Pien Tze Huang Pharmaceutical Co. Ltd.,
China (Chinese FDA approval no. Z35020242). Stock solution of PZH
was prepared immediately prior to use by dissolving the PZH power
in phosphate-buffered saline (PBS) to a concentration of 20 mg/ml.
The working concentrations of PZH were made by diluting the stock
solution in the culture medium.
Cell culture
Human colon carcinoma HT-29 cells were obtained from
the American Type Culture Collection (ATCC, Manassas, VA, USA).
Cells were grown in DMEM containing 10% (v/v) FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin in a 37°C humidified
incubator with 5% CO2.
Treatment of PZH and IL-6
HT-29 cells were first grown in complete DMEM (10%
FBS) until ~50% confluency and then continuously cultured in
FBS-free medium overnight. The medium was replaced with DMEM
complete with 10% FBS and cells were pre-treated with various
concentrations of PZH for 1 h followed by stimulation with 10 ng/ml
of IL-6 for the indicated periods of time in different experiments
as described below.
Evaluation of cell viability by MTT
assay
Viability of HT-29 cells was examined by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay. HT-29 cells were seeded into 96-well plates at
a density of 1×104 cells/well in 0.1 ml medium. Cells
were treated with PZH and/or IL-6 as described above for 24 h. MTT
(100 μl) (0.5 mg/ml in PBS) was added to each well, and the samples
were incubated for an additional 4 h at 37°C. The purple-blue MTT
formazan precipitate was dissolved in 100 μl DMSO. The absorbance
was measured at 570 nm using an ELISA reader (Model ELX800; BioTek,
Winooski, VT, USA).
Colony formation
HT-29 cells were seeded into 6-well plates at a
density of 2×105 cells/well in 2 ml medium. After
treatment with PZH and/or IL-6 as described above for 24 h, cells
were harvested and diluted in 2 ml fresh medium without PZH and
IL-6, and then reseeded into 6-well plates at a density of
1×103 cells/well. Following incubation for 8 days in a
37°C humidified incubator with 5% CO2, formed colonies
were fixed with 10% formaldehyde, stained with 0.01% crystal violet
and counted. Cell survival was calculated by normalizing the
survival of the control cells as 100%.
Detection of apoptosis with Hoechst
staining
HT-29 cells were seeded into 12-well plates at a
density of 1×105 cells/well in 1 ml medium. After
treatment with PZH and/or IL-6 as described above for 24 h, cell
apoptosis was evaluated by Hoechst staining kit according to the
manufacturer’s instructions. Briefly, at the end of treatment,
cells were fixed with 4% polyoxymethylene and then incubated in
Hoechst solution for 5 min in the dark. The staining images were
recorded using a phase-contrast fluorescent microscope (Olympus,
Japan). The images were captured at a magnification of ×400.
RT-PCR analysis
HT-29 cells were seeded into 6-well plates at a
density of 2×105 cells/well in 2 ml medium. After cells
were treated with PZH and/or IL-6 as described above for 24 h,
total-RNA was isolated with TRIzol reagent. Oligo(dT)-primed RNA (1
μg) was reverse-transcribed with SuperScript II reverse
transcriptase according to the manufacturer’s instructions. The
obtained cDNA was used to determine the mRNA amount of Bcl-2 and
cyclin D1 by PCR. GAPDH was used as an internal control.
Western blot analysis
HT-29 cells were seeded into 6-well plates at a
density of 2×105 cells/well in 2 ml medium. Cells were
treated with PZH and/or IL-6 as described above. IL-6 stimulation
was performed for 15 min for pSTAT3 detection, or 24 h for
examination of SOCS3, Bcl-2 and cyclin D1 protein expression.
Treated cells were lysed with mammalian cell lysis buffer
containing protease and phosphatase inhibitor cocktails. The
lysates were resolved in 12% SDS-PAGE gels and electroblotted. The
PVDF membranes were blocked with 5% skimmed milk and probed with
primary antibodies against phosphor-specific STAT3
(Tyr705), SOCS3, Bcl-2, cyclin D1 and β-actin (1:1,000)
overnight at 4°C and then with the appropriate HRP-conjugated
secondary antibody followed by enhanced chemiluminescence
detection.
Luciferase gene reporter assay
HT-29 cells were seeded into 96-well plates at a
density of 1×104 cells/well in 0.1 ml complete DMEM
until ~50% confluency and then continuously cultured in FBS- and
antibiotics-free medium overnight. Cells were transfected with a
mixture of inducible STAT3-responsive firefly luciferase construct
and constitutively expressing Renilla luciferase construct using
Lipofectamine LTX with PLUS™ reagent. Six hours after transfection
the medium was changed back into DMEM complete with FBS, penicillin
and streptomycin. After 24 h of transfection, cells were treated
with various concentrations of PZH for 1 h followed by IL-6 for
another 24 h. Cell extracts were prepared and analyzed using
Promega Dual Luciferase Reporter Assay System according to the
manufacturer’s instructions. The measured firefly luciferase
activity was normalized to the activity of Renilla luciferase in
the same well.
Statistical analysis
Data were analyzed using the SPSS package for
Windows (Version 11.5). Statistical analysis of the data was
performed with the Student’s t-test and one-way ANOVA. Differences
with P<0.05 were considered statistically significant.
Results
PZH inhibits IL-6-mediated STAT3
activation in HT-29 cells
Several cultured human cancer cell lines including
HT-29 do not express constitutively phosphorylated STAT3 in
vitro; we therefore stimulated STAT3 activation with IL-6 in
HT-29 cells. We first determined STAT3 activation by performing
western blotting to examine its phosphorylation level using an
antibody that recognizes phosphorylated STAT3 (pSTAT3) at
Tyr705. As shown in Fig.
1, stimulation with 10 ng/ml of IL-6 for 15 min significantly
increased the level of pSTAT3 in HT-29 cells, which, however, was
profoundly inhibited by PZH in a dose-dependent manner. The levels
of non-phosphorylated STAT3 remained unchanged after the treatment
with IL-6 and/or PZH. To further confirm the inhibitory effect of
PZH on the activation of STAT3, we performed Dual Luciferase
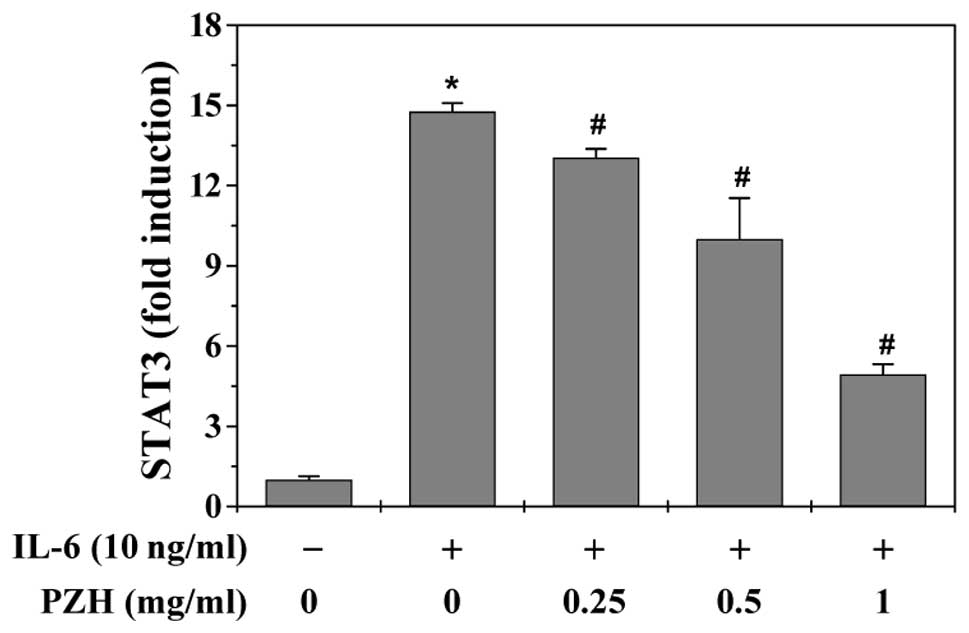
Reporter Assay to examine STAT3 transcriptional activity. Results
in Fig. 2 showed that PZH
significantly and dose-dependently inhibited IL-6-stimulated
increase of STAT3 transcriptional activity. Taken together, our
data suggest that PZH is potent in inhibiting IL-6-mediated STAT3
activation in human colon carcinoma cells.
PZH inhibits HT-29 cell
proliferation
The effect of PZH on HT-29 cell viability in the
presence of IL-6 was determined by MTT assay. As shown in Fig. 3A, although IL-6 stimulation
increased the viability of HT-29 cells to 115% compared to control
cells (P<0.05), treatment with 0.25–1 mg/ml of PZH for 24 h
decreased the viability of IL-6-stimulated cells from 60 to 22%
(P<0.05 vs. PZH-untreated cells). To further verify these
results, we examined the effect of PZH on HT-29 cell survival using
a colony formation assay. As shown in Fig. 3B, treatment with 0.25, 0.5 and 1
mg/ml of PZH for 24 h dose-dependently reduced the survival rate of
IL-6-stimulated cells by 27, 73 and 81% (P<0.05). Collectively,
these data demonstrate that PZH inhibits HT-29 cell proliferation
in the presence of IL-6 stimulation.
PZH induces HT-29 cell apoptosis
Cell apoptosis was evaluated by observing nuclear
morphological changes by staining the cell nuclei with DNA-binding
dye Hoechst. As shown in Fig. 4,
PZH-treated cells showed condensed chromatin and fragmented nuclear
morphology that are typical apoptotic morphological features,
whereas the untreated cell nuclei showed homogenous staining and
were less intense than PZH-treated cells, suggesting that PZH
promotes HT-29 cell apoptosis in the presence of IL-6
stimulation.
PZH downregulates the expression of Bcl-2
and cyclin D1 and upregulates SOCS3 expression in HT-29 cells
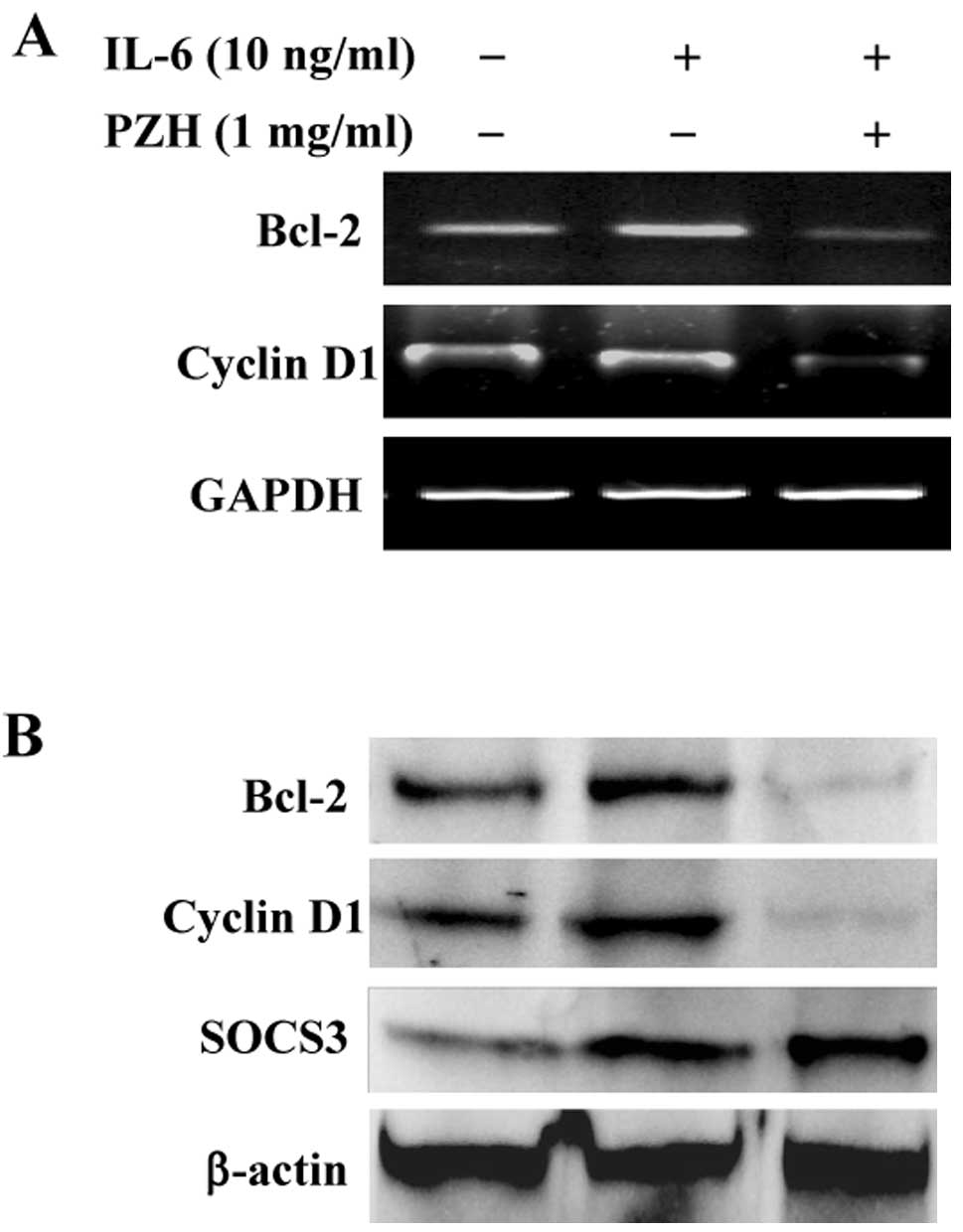
To further investigate the underlying mechanism of
PZH’s activities, we performed RT-PCR and western blot analyses to
examine the effect of PZH on the expression of the
pro-proliferative cyclin D1 and the anti-apoptotic Bcl-2, two
important target genes of the STAT3 signaling pathway. The results
in Fig. 5 show that the mRNA and
protein expression of cyclin D1 and Bcl-2 was clearly increased by
IL-6 stimulation. However, PZH treatment profoundly inhibited
IL-6-induced upregulation of cyclin D1 and Bcl-2 expression, at
both the transcriptional and translational levels.
We next investigated the effect of PZH on another
STAT3 transcriptional target, the SOCS3 protein, a critical
negative feedback inhibitor of the IL-6/STAT3 pathway. As shown in
Fig. 5B, IL-6 stimulation induced
SOCS3 expression, which is consistent with previous studies
(39,40). Notably, PZH treatment further
increased the protein expression of SOCS3, suggesting that PZH
suppresses the IL-6/STAT3 pathway in HT-29 cells partially via
promoting SOCS3 expression.
Discussion
Colorectal cancer (CRC) is a complex and
heterogeneous tumor involving multiple cellular signaling
transduction pathways. It is noteworthy that these signaling
pathways usually have functional redundancy. In addition, there is
crosstalk between these pathways, forming a complicated and robust
cellular signal transduction network that is regulated by
compensatory mechanisms. Therefore, specific inhibitors that target
only one single pathway might not always be effective on the
complex tumor systems; also, the long-term use of many
single-target-based agents will often generate unsatisfactory drug
resistance and side-effects, which is possibly one of the major
reasons why overall CRC patient response to chemotherapy is less
than 40% despite advances in this area. These problems highlight
the urgent need for the development of novel cancer chemotherapies.
Natural products, such as traditional Chinese herbal medicines
(TCMs), have relatively fewer side-effects compared to modern
chemotherapeutics and have long been used clinically for cancer
treatment. Pien Tze Huang (PZH), a well-known and important TCM
formula, has been demonstrated to be clinically effective in
treating various types of cancer including CRC. However, the mode
of action for its antitumor effect is largely unknown.
IL-6/STAT3 is one of the most critical cellular
signal transduction pathways known to malfunction in CRC. IL-6
transduces its signal through a common signaling receptor gp130,
eventually resulting in the activation of STAT3. The transcription
factor STAT3 is an oncogenic protein that is constitutively
activated in most tumor cells but not in normal cells (1–14).
Activation of STAT3 is mediated by phosphorylation at tyrosine 705,
leading to its homodimerization, nuclear translocation and DNA
binding, which in turn upregulates the expression of various
critical genes involved in cell proliferation and survival, such as
the pro-proliferative cyclin D1 and the anti-apoptotic Bcl-2.
Markedly, as another target gene of STAT3 signaling, SOCS3 can be
quickly induced by IL-6 stimulation but it then strongly inhibits
IL-6-mediated STAT3 activation, functioning as a negative feedback
regulator of the IL-6/STAT3 pathway. Aberrant activation of STAT3
and/or reduced expression of SOCS3 facilitate unregulated increase
in cell proliferation and reduction in cell apoptosis resulting in
cancer development. Therefore, modulation of IL-6/STAT3/SOCS3
signaling has been a promising target for the development of
anticancer therapies.
In the present study, we stimulated the human colon
carcinoma HT-29 cells with IL-6 and found that STAT3 was quickly
activated upon IL-6 stimulation, leading to a significant increase
in its phosphorylation level and transcriptional activity. However,
the IL-6-mediated STAT3 activation could be profoundly inhibited by
PZH treatment in a dose-dependent manner. Consequently, PZH
treatment significantly inhibited IL-6-induced upregulation of
cyclin D1 and Bcl-2, two key target genes of the STAT3 pathway.
Moreover, the inhibitory effect of PZH on the IL-6-mediated STAT3
activation and cyclin D1/Bcl-2 expression resulted in the
suppression of HT-29 cell proliferation and the induction of cell
apoptosis. Furthermore, PZH treatment increased the expression of
SOCS3. In conclusion, our data demonstrate that PZH could
effectively inhibit proliferation and promote apoptosis of human
colon carcinoma cells via modulation of the IL-6/STAT3 signaling
pathway and its target genes.
Several TCM formulas including PZH are composed of
many natural products, each of which contains numerous chemical
compounds. TCM formulas are thus considered to be multi-component
and multi-target agents that exert their therapeutic function in a
more holistic manner. However, it remains unknown whether PZH can
affect other cancer-related cellular signal transduction pathways,
such as Hedgehog, Ras/ERK, PI3K/Akt and Wnt signalings, as well as
STAT3. This issue could be addressed in future studies to fully
elucidate the molecular mechanism by which PZH is involved in
cancer treatment and in order to develop better multi-target drugs
for cancer therapy.
Acknowledgements
This study was sponsored by the National Natural
Science Foundation of China (81073097), the Developmental Fund of
Chen Keji Integrative Medicine (CKJ 2011001), and the China
Postdoctoral Science Foundation (2012M511437).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
PZH
|
Pien Tze Huang
|
|
IL-6
|
interleukin-6
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
SOCS3
|
suppressor of cytokine signaling 3
|
|
TCM
|
traditional Chinese medicine
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Belluco C, Nitti D, Frantz M, Toppan P,
Basso D, Plebani M, Lise M and Jessup JM: Interleukin-6 blood level
is associated with circulating carcinoembryonic antigen and
prognosis in patients with colorectal cancer. Ann Surg Oncol.
7:133–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Komoda H, Tanaka Y, Honda M, Matsuo Y,
Hazama K and Takao T: Interleukin-6 levels in colorectal cancer
tissues. World J Surg. 22:895–898. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galizia G, Orditura M, Romano C, Lieto E,
Castellano P, Pelosio L, Imperatore V, Catalano G, Pignatelli C and
De Vita F: Prognostic significance of circulating IL-10 and IL-6
serum levels in colon cancer patients undergoing surgery. Clin
Immunol. 102:169–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schneider MR, Hoeflich A, Fischer JR, Wolf
E, Sordat B and Lahm H: Interleukin-6 stimulates clonogenic growth
of primary and metastatic human colon carcinoma cells. Cancer Lett.
151:31–38. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Becker C, Fantini MC, Schramm C, Lehr HA,
Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al:
TGF-beta suppresses tumor progression in colon cancer by inhibition
of IL-6 trans-signaling. Immunity. 2:491–501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Becker C, Fantini MC, Wirtz S, Nikolaev A,
Lehr HA, Galle PR, Rose-John S and Neurath MF: IL-6 signaling
promotes tumor growth in colorectal cancer. Cell Cycle. 4:217–220.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dowdall JF, Winter DC, Andrews E, Laug WE,
Wang JH and Redmond HP: Soluble interleukin 6 receptor (sIL-6R)
mediates colonic tumor cell adherence to the vascular endothelium:
a mechanism for metastatic initiation? J Surg Res. 107:1–6. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, Gupta SR, Tharakan ST, Koca C, Dey S and Sung B: Signal
transducer and activator of transcription-3, inflammation, and
cancer: how intimate is the relationship? Ann NY Acad Sci.
1171:59–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zushi S, Shinomura Y, Kiyohara T, Miyazaki
Y, Kondo S, Sugimachi M, Higashimoto Y, Kanayama S and Matsuzawa Y:
STAT3 mediates the survival signal in oncogenic ras-transfected
intestinal epithelial cells. Int J Cancer. 78:326–330. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masuda M, Suzui M, Yasumatu R, Nakashima
T, Kuratomi Y, Azuma K, Tomita K, Komiyama S and Weinstein IB:
Constitutive activation of signal transducers and activators of
transcription 3 correlates with cyclin D1 overexpression and may
provide a novel prognostic marker in head and neck squamous cell
carcinoma. Cancer Res. 62:3351–3355. 2002.
|
|
15
|
Bromberg J and Wang TC: Inflammation and
cancer: IL-6 and STAT3 complete the link. Cancer Cell. 15:79–80.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kusaba T, Nakayama T, Yamazumi K, Yakata
Y, Yoshizaki A, Inoue K, Nagayasu T and Sekine I: Activation of
STAT3 is a marker of poor prognosis in human colorectal cancer.
Oncol Rep. 15:1445–1451. 2006.PubMed/NCBI
|
|
17
|
Lin Q, Lai R, Chirieac LR, Li C, Thomazy
VA, Grammatikakis I, Rassidakis GZ, Zhang W, Fujio Y, Kunisada K,
Hamilton SR and Amin HM: Constitutive activation of JAK3/STAT3 in
colon carcinoma tumors and cell lines: inhibition of JAK3/STAT3
signaling induces apoptosis and cell cycle arrest of colon
carcinoma cells. Am J Pathol. 167:969–980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong H, Zhang Z, Tian X, Sun D, Liang Q,
Zhang Y, Lu R, Chen Y and Fang J: Inhibition of JAK1, 2/STAT3
signaling induces apoptosis, cell cycle arrest, and reduces tumor
cell invasion in colorectal cancer cells. Neoplasia. 10:287–297.
2008.PubMed/NCBI
|
|
19
|
Endo TA, Masuhara M, Yokouchi M, Suzuki R,
Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H,
et al: A new protein containing an SH2 domain that inhibits JAK
kinases. Nature. 387:921–924. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hilton DJ, Richardson RT, Alexander WS,
Viney EM, Willson TA, Sprigg NS, Starr R, Nicholson SE, Metcalf D
and Nicola NA: Twenty proteins containing a C-terminal SOCS box
form five structural classes. Proc Natl Acad Sci USA. 95:114–119.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naka T, Narazaki M, Hirata M, Matsumoto T,
Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, et
al: Structure and function of a new STAT-induced STAT inhibitor.
Nature. 387:924–929. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Starr R, Willson TA, Viney EM, Murray LJ,
Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA
and Hilton DJ: A family of cytokine-inducible inhibitors of
signalling. Nature. 387:917–921. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
He B, You L, Uematsu K, Zang K, Xu Z, Lee
AY, Costello JF, McCormick F and Jablons DM: SOCS-3 is frequently
silenced by hypermethylation and suppresses cell growth in human
lung cancer. Proc Natl Acad Sci USA. 100:14133–14138. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oshimo Y, Kuraoka K, Nakayama H, Kitadai
Y, Yoshida K, Chayama K and Yasui W: Epigenetic inactivation of
SOCS-1 by CpG island hypermethylation in human gastric carcinoma.
Int J Cancer. 112:1003–1009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sutherland KD, Lindeman GJ, Choong DY,
Wittlin S, Brentzell L, Phillips W, Campbell IG and Visvader JE:
Differential hypermethylation of SOCS genes in ovarian and breast
carcinomas. Oncogene. 23:7726–7733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rigby RJ, Simmons JG, Greenhalgh CJ,
Alexander WS and Lund PK: Suppressor of cytokine signaling 3
(SOCS3) limits damage-induced crypt hyper-proliferation and
inflammation-associated tumorigenesis in the colon. Oncogene.
26:4833–4841. 2007. View Article : Google Scholar
|
|
27
|
Gustin DM and Brenner DE: Chemoprevention
of colon cancer: current status and future prospects. Cancer
Metastasis Rev. 21:323–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gorlick R and Bertino JR: Drug resistance
in colon cancer. Semin Oncol. 26:606–611. 1999.
|
|
29
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. Biochim Biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
30
|
Boose G and Stopper H: Genotoxicity of
several clinically used topoisomerase II inhibitors. Toxicol Lett.
116:7–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Newman DJ, Cragg GM and Snader KM: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin JM, Chen YQ, Wei LH, Chen XZ, Xu W,
Hong ZF, Sferra TJ and Peng J: Hedyotis Diffusa Willd
extract induces apoptosis via activation of the
mitochondrion-dependent pathway in human colon carcinoma cells. Int
J Oncol. 37:1331–1338. 2010.
|
|
34
|
Chinese Pharmacopoeia Commission.
Pharmacopoeia of the Peoples Republic of China. 1. Chinese Medical
Science and Technology Press; Beijing: pp. 573–575. 2010
|
|
35
|
Xu YY and Yu EX: Clinical analysis of the
effect of Pien Tze Huang in treatment of 42 patients with moderate
or advanced liver cancer. Shanghai J Tradit Chin Med. 12:4–5.
1994.
|
|
36
|
Gu ZX: Therapeutical observation of
advanced colon cancer. Chin Tradit Patent Med. 15:231993.
|
|
37
|
Lin JM, Wei LH, Chen YQ, Liu XX, Hong ZF,
Sferra TJ and Peng J: Pien Tze Huang-induced apoptosis in human
colon cancer HT-29 cells is associated with regulation of the Bcl-2
family and activation of caspase 3. Chin J Integr Med. 17:685–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhuang QC, Hong F, Shen AL, Zheng LP, Zeng
JW, Lin W, Chen YQ, Sferra TJ, Hong ZF and Peng J: Pien Tze Huang
inhibits tumor cell proliferation and promotes apoptosis via
suppressing the STAT3 pathway in a colorectal cancer mouse model.
Int J Oncol. 40:1569–1574. 2012.PubMed/NCBI
|
|
39
|
Schmitz J, Weissenbach M, Haan S, Heinrich
PC and Schaper F: SOCS3 exerts its inhibitory function on
interleukin-6 signal transduction through the SHP2 recruitment site
of gp130. J Biol Chem. 275:12848–12856. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sommer U, Schmid C, Sobota RM, Lehmann U,
Stevenson NJ, Johnston JA, Schaper F, Heinrich PC and Haan S:
Mechanisms of SOCS3 phosphorylation upon interleukin-6 stimulation.
Contributions of Src- and receptor-tyrosine kinases. J Biol Chem.
280:31478–31488. 2005. View Article : Google Scholar : PubMed/NCBI
|